- *Corresponding Author:
- B. S. Jayashree
Department of Chemistry, Manipal Institute of Technology, Manipal University, Manipal-576 104, India
E-mail: jayashree.sy@gmail.com
| Date of Submission | 16 August 2016 |
| Date of Revision | 29 February 2017 |
| Date of Acceptance | 18 August 2017 |
| Indian J Pharm Sci 2017;79(5):838-843 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A series of newer flavonols and their zinc complexes were synthesized and characterized by infrared, proton nuclear magnetic resonance, and mass spectrometry. The lipophilicity for the test compounds was determined by carrying out partition coefficient using n-octanol-water system. Evaluation of the oxidation potential for the test compounds was performed by cyclic voltammetry. Further, all the test compounds were screened for their anticancer activity against Vero and MCF-7 cell lines using quercetin as standard. Compound F4, a 2-hydroxy-3-(4-methylsulphanyl-phenyl)-4H-naphthalen-1-one was synthesized by using 4-(methylthio) benzaldehyde, exhibited an oxidation potential at 0.641V and 0.928V, while its zinc complex, F4M1 showed an oxidation potential at 0.59 V and 0.96 V along with their log p values at 2.71 and 3.9 respectively. Amongst the ten compounds tested for their cytotoxicity on MCF-7 cell lines, compound F4 exhibited cytotoxicity at 31.43 µM as compared with that of the standard quercetin with its IC50 at 26.5 µM. Thus, from our study it was found that, test compound F4 could become the promising anticancer molecule.
Keywords
Flavonols, complexes, anticancer activity, partition coefficient, cyclic voltammetry
Cancer is a complex disease and is a major health concern across the globe. Though, established treatment regime are available for treating cancer using chemotherapeutics agents, the toxicity associated with them has urged the need for the development of new anticancer agents [1]. The polyphenols, flavonoids and their synthetic analogues have been widely used in the treatment of ovarian, breast, cervical, pancreatic and prostate cancer [2]. A large number of epidemiological studies propose that high flavonoid consumption may be correlated with a reduction in the risk of cancer [3]. Hirano et al. [4] tested anticancer efficacy of 28 flavonoids on human acute myeloid leukemia cell line (HL-60), where, eight flavonoids showed considerable suppressive effects. Whereas, Kuntz et al. [5] studied more than 30 flavonoids for their effects on cell proliferation and potential cytotoxicity in human colon cancer cell lines both in Caco-2 and HT- 29 and found that, almost all the compounds exhibited antiproliferative activity without any cytotoxic effects. However, they could not correlate the antiproliferative effects with respect to their structure.
Metal complexes of chelating ligands have attracted considerable attention owing to their interesting physicochemical properties, diversified biological activities and as specific models of metallo-enzyme active sites [6,7]. Flavonoids are found to be promising metal chelators [8] with the coordination sites between the 5-hydroxy group of A ring and 4-carbonyl group of C ring and as well as 3-hydroxy and 4-carbonyl group of C ring. This work was supported based on the report that metal chelation could enhance the biological activity [9].
The new drug discovery of anticancer agents as metal complexes is quite a challenging task where, emphasis is made to synthesize metal complexes that are able to cleave both strands of DNA molecule [10]. A transition metal complex of flavone, namely, copper (II) complexes of trans-bis (3-aminoflavone-kappa 2N, O) bis (perchlorato-kappa O) copper (II), exhibiting potent antitumor activity was also reported by Rybarczyk-Pirek et al. [11]. Based on these reports, we have undertaken the synthesis and characterization of series of flavones and their zinc complexes and carried out the evaluation of their cytotoxicity on Vero and MCF-7 cell lines using 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) assay. Vero cells are normal monkey kidney epithelial cells. They are normally used when new compounds are tested for anticancer activity to negotiate the cytotoxic actions on normal cells. MCF-7 cells are highly metastatic human breast cancer cells. These cells can be cultured and sub-cultured, which can be used to screen anticancer activity.
All the chemicals required for the synthesis and purification of the intermediates and final compounds were purchased from Sigma-Aldrich (St. Louis, MO, USA). Melting points were determined using melting point apparatus from Shital Scientific Industries (Mumbai, India) and are uncorrected. The reactions were monitored by thin layer chromatography (TLC) plates, precoated with Kieselgel 60 F254, Merck (Billerica, MA, United States) and spots were detected using short and long-UV wavelength using UV chamber (Servewell Instruments Pvt. Ltd., Bengaluru, India). λmax for the synthesized compounds were established by UV/Vis spectrophotometer UV-1650 PC, Shimadzu (Japan). The infrared (IR) studies were determined using FTIR 8310, Shimadzu (Japan). Proton nuclear magnetic resonance (1HNMR) were recorded on Bruker AM, 400 MHz spectrometer, Bruker (Billerica, MA, United States) using tetramethylsilane (TMS) as internal standard. Mass spectral studies were done using gas chromatography-mass spectrometry (GCMS), GCMS QP5050, Shimadzu (Japan). The redox potentials of the test compounds were determined using electrochemical analyser, CHI600E, CH Instruments, Inc. (Austin, TX, United States).
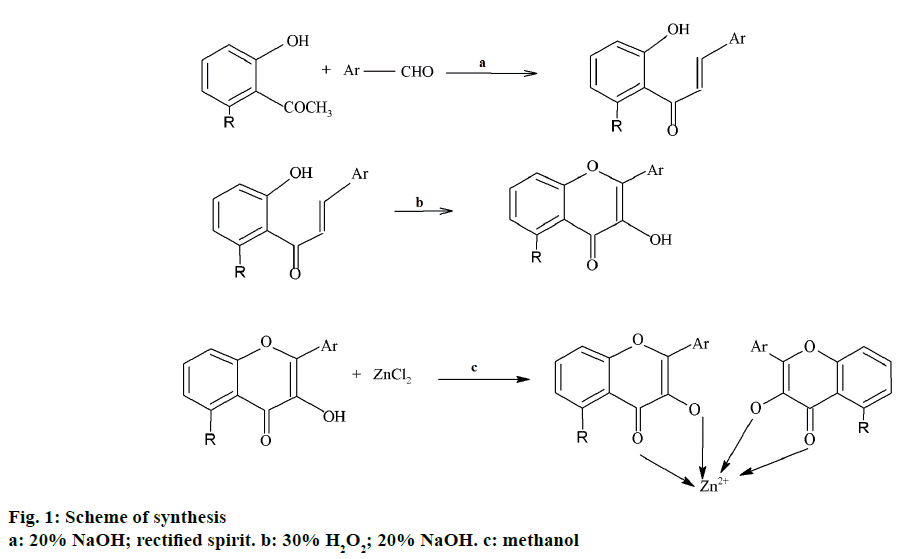
Conventionally, chalcones are prepared by Claisen- Schmidt condensation where, equimolar concentrations of aryl aldehydes are condensed with acetophenones in the presence of a base [12]. In the present work, a solution of 0.001 mol of 2-hydroxyacetophenone in 15 ml of methanol was thoroughly stirred with 2 ml of 20% alcoholic solution of sodium hydroxide. After stirring the reaction mixture for 30 min, 0.001 mol of substituted benzaldehyde was added in portions. Stirring was continued until the completion of the reaction. The reaction mixture was then suspended in ice cold water and the resulting solution was acidified with dilute hydrochloric acid. The product precipitated was collected by filtration and dried in air and stored in a desiccator.
To a solution of 0.005 mol of chalcone in 20 ml of methanol, 5 ml of 20% alcoholic sodium hydroxide was added and stirred. To this mixture, 3 ml of 30% hydrogen peroxide was added in portions with constant stirring. The stirring was continued till the completion of the reaction. The mixture was then suspended in ice cold water and was acidified with dilute hydrochloric acid to obtain product as precipitate. The product was then collected by filtration, washed with water, dried and was further purified by recrystallization using solvents such as ethanol (90%) and methanol (100%). The progress of the reactions was monitored by TLC using n-hexane:ethylacetate (7:3) as solvent system. The spots were observed under UV chamber.
A solution of flavone was prepared by heating 0.001 mol of the flavone in methanol by heating and to this to the hot solution, 0.001 mol of the anhydrous zinc chloride was added with constant stirring. Stirring was continued till the complex got precipitated and the precipitated complex was kept aside for the evaporation of the solvent, washed with water, dried and stored in a desiccator. Figure 1, represents the scheme followed for the synthesis. In the present study, shake flask method [13] was employed to determine partition coefficient using n-octanol and water system.
William et al. [14] reported that the electrochemical properties of the flavonoids might influence their biological activities. To support this, Aleksandra et al. [15] and Jesús et al. [16] compared their antioxidant activity with redox potential values.
Redox potentials of the test compounds were determined by using platinum wire and Ag/AgCl as auxiliary and reference electrodes, respectively. Glassy carbon electrode was used as working electrode and tetrabutylammonium perchlorate, 100 μM in DMSO was used as supporting electrolyte. Solutions for cyclic voltammetry were prepared by dissolving 1 mg of the test compounds in 1 ml of dimethyl sulfoxide (DMSO) and these stock solutions were further diluted to get a concentration of 10 μg×ml-1 of the supporting electrolyte solution. Cyclic voltammograms were obtained at a scan rate of 50 mVs-1 in the potential interval of −0.5 to +1.5 V versus Ag/AgCl.
Cytotoxicity screening for all the test compounds was performed by determining the cell viability using MTT assay [17] in different cell lines namely MCF-7 and Vero using quercetin as standard. Quercetin is 3,3′,4′,5,7- pentahydroxy-flavone and is one of the most abundant flavonoids found in fruits and vegetables. It has been reported to exhibit anticancer and antiinflammatory effects [18-20].
Dulbecco’s minimum essential media (DMEM), MTT, Quercetin and Trypsin-EDTA were obtained from HiMedia laboratories (Mumbai) and foetal bovine serum (FBS) from Gibco Sera, Life technologies (USA). Human breast adenocarcinoma cells (MCF- 7) and African green monkey kidney epithelial cells (Vero) cell lines procured from National Centre for Cell Science, Pune, India and maintained in our tissue culture lab. The assay was done in triplicates and the results were expressed in terms of IC50.
Cells were routinely grown in the 25 cm2 culture flasks with perforated screw-caps containing DMEM supplemented with 8% FBS and 50 μg×ml-1 gentamycin sulfate at 37° with 5% carbon dioxide (CO2) humidified air in the CO2 incubator. A known number of cells (5×103 cells/100 μl of medium in a well) were seeded in 96 well plates and incubated for 24 h for attachment. Desired concentrations of sample were prepared in 0.1% (v/v) DMSO in the media prior to the experiment. The reactant mixtures were diluted with media and cells were treated with different concentration ranges of the drug (25-200 μM). In control samples, equivalent amount of DMSO was maintained to determine its cytotoxic effect. After 48 h, the media was removed and the cells were incubated with 100 μl MTT reagent (1 mg×ml-1, in phosphate buffer) for 4 h at 37°, the yellow formazan so produced was then solubilised and converted to purple complex by the addition of 100 μl DMSO. The suspension was placed on a microvibrator for 5 min, absorbance was recorded at 540 nm by ELISA reader and the percentage cytotoxicity was calculated from the determination of cell viability.
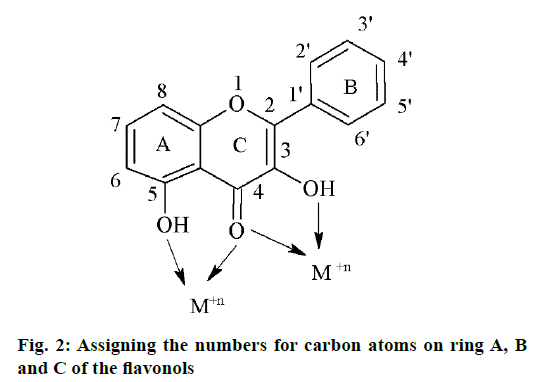
Five flavonols were synthesized by standard methods and their zinc complexes were also prepared. The compounds were characterized by spectral analysis using IR, NMR and MS techniques. The flavonols showed a characteristic absorption in the range of 3250-3350 cm-1 for the IR spectra corresponding to the OH group present at the 3rd position of ring A of flavones as shown in Figure 2. Further, compounds were showing strong absorption in the range of 1606-1616 cm-1 corresponding to C=O stretch. The IR spectra of the zinc complexes of corresponding flavones showed a shift of 2-6 cm-1 corresponding to –OH bond and 2-3 cm-1 corresponding to C=O bond showing the involvement of these two sites in the formation of coordinate bond with the metal. Similarly, in the 1H NMR spectra of the flavones, the peak corresponding to the –OH at 3rd position of A ring was in the range of δ 9.1-9.8 and was absent for the complexes. In case of compound F5, 2-(4-benzyloxy-phenyl)-3,6- dihydroxy-chromen-4-one, the peak corresponding to -OH group of the C ring showed a signal at δ of 5.1 whereas, the absence of the corresponding signal in the complex suggested that, the –OH group of C ring may be responsible in the formation of coordinate bond formation with the zinc metal (Table 1).
| Compound Code | R | Ar |
|---|---|---|
| F1 | H | 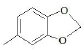 |
| F2 | H | 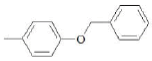 |
| F3 | H | 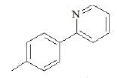 |
| F4 | H | 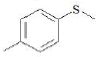 |
| F5 | OH | 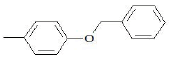 |
Table 1: Substitutions of flavones at various positions
F1, yield: 78%, melting point: 222-224°, electrospray ionization mass spectrometry (ESI-MS) m/z: 223.05 [M+H]+, IR (KBr, cm-1) 3252 (b, OH), 1610 (C=O), 1563, 1482 (C=C-C aromatic ring stretch), 1351 (aromatic C-H stretch), 1260 and 1200 cm-1, (aromatic C-H bend); 1H NMR (DMSO, δ in ppm): 9.6 (1H, s, 3-OH), 7.1-8.2 (7H, m, Ar-H), 6.15 (2H, s, -CH2- of benzodioxo ring). F1M1, yield: 60%, melting point: 210°, IR (KBr, cm-1) 3250 (b, OH), 1609 (C=O), 1562, 1482 (C=C-C aromatic ring stretch), 1349 (aromatic C-H stretch), 1260 and 1200 cm-1 (aromatic C-H bend); 1H NMR (DMSO, δ in ppm): 6.9-8.2 (7H, m, Ar-H), 6.0 (2H, s, -CH2- of benzodioxo ring).
F2, yield: 84%, melting point: 168-170°, ESI-MS m/z: 345.11 [M+H]+, IR (KBr, cm-1) 3281 (b, OH), 1606 (C=O), 1565, 1478 (C=C-C aromatic ring stretch), 1346 (aromatic C-H stretch), 1246 and 1209 cm-1 (aromatic C-H bend); 1H NMR (CDCl3, δ in ppm): 9.15 (1H, s, 3-OH), 6.95-8.0 (13H, m, Ar-H), 4.98 (2H, s, -CH2- of benzyloxy benzene ring). F2M1, yield: 68%, melting point: 210°, IR (KBr, cm-1) 3287 (b, OH), 1607 (C=O), 1566, 1476 (C=C-C aromatic ring stretch), 1345 (aromatic C-H stretch), 1246 and 1211 cm-1 (aromatic C-H bend); 1H NMR (CDCl3, δ in ppm): 7.0- 8.2 (13H, m, Ar-H), 5.04 (2H, s, -CH2- of benzyloxy benzene ring).
F3, yield: 60%, melting point: 222-224°, ESI-MS m/z: 302.1 [M+H]+, IR (KBr, cm-1) 3245 (b, OH), 1610 (C=O), 1568, 1471 (C=C-C aromatic ring stretch), 1303 (aromatic C-H stretch), 1244 and 1213 cm-1 (aromatic C-H bend); 1H NMR (DMSO, δ in ppm): 9.85 (1H, s, 3-OH), 7.35-8.62 (12H, m, Ar-H). F3M1, yield: 81%; melting point: 270°, IR (KBr, cm-1) 3504 (b, OH), 1612 (C=O), 1591, 1471 (C=C-C aromatic ring stretch), 1317 (aromatic C-H stretch), 1217 cm-1 (aromatic C-H bend).
F4, yield: 75.5%, melting point: 168-170°, ESI-MS m/z: 285 [M+H]+, IR (KBr, cm-1) 3350 (b, OH), 1616 (C=O), 1568, 1475 (C=C-C aromatic ring stretch), 1344 (aromatic C-H stretch), 1269 and 1209 cm-1 (aromatic C-H bend); 1H NMR (DMSO, δ in ppm): 9.67 (1H, s, 3-OH), 7.4-8.25 (8H, m, Ar-H), 2.59 (3H, s, -S-CH3 of thiomethyl). F4M1, yield: 92%, melting point: 228°, IR (KBr, cm-1) 3352 (b, OH), 1616 (C=O), 1566, 1477 (C=C-C aromatic ring stretch), 1346 (aromatic C-H stretch), 1271 and 1211 cm-1 (aromatic C-H bend); 1H NMR (CDCl3, δ in ppm): 6.9-8.6 (8H, m, Ar-H), 2.3 (3H, s, -S-CH3 of thiomethyl).
F5, yield: 55%, melting point: 190°, ESI-MS m/z: 361 [M+H]+, IR (KBr, cm-1) 3566 (b, OH), 1604 (C=O), 1542, 1460 (C=C-C aromatic ring stretch), 1344 (aromatic C-H stretch), 1259 cm-1 (aromatic C-H bend); 1H NMR (DMSO, δ in ppm): 9.88 (1H, s, 3-OH), 7.09- 7.89 (8H, m, Ar-H), 5.24 (2H, s, -CH2 of benzyloxy benzene ring), 5.19 (1H, s, Ar-OH of the C ring). F5M1, yield: 78%, melting point: 210°, IR (KBr) 3504 (b, OH), 1600 (C=O), 1510, 1460 (C=C-C aromatic ring stretch), 1384 (aromatic C-H stretch), 1261 cm-1 (aromatic C-H bend); 1H NMR (CDCl3, δ in ppm): 9.89 (1H, s, 3-OH), 7.0-8.1 (8H, m, Ar-H), 5.1 (2H, s, -CH2 of benzyloxy benzene ring).
Partition coefficient (log P) is one of the important physical parameters used in the structure-activity relationship studies. It is measured in terms of the log P values, higher the log P value greater will be the lipophilicity and vice versa. The experimental log P values of flavonols F1-F5, were found to be in the range of 1.59, 3.6, 2.47, 2.71 and 2.9 respectively, which is in agreement with the predicted log P values. The corresponding zinc complexes of the synthesized flavonols exhibited values in the range of 2.51, 4.9, 3.8, 3.9, and 4.2, respectively. An increase in its value was observed upon complexation of the ligands.
One of the parameters used in evaluating the antioxidant potential is based on measuring their oxidation potential using current potential relationships at an inert glassy carbon electrode. It is evident that the compound exhibiting antioxidant activity could be easily oxidized. For instance, a powerful reducing agent exhibits a lower positive oxidation potential [21]. Further, there are reports available to substantiate that, any decrease in the phenoxyl radical/phenol redox couple would increase the mammalian cell cytotoxicity [22]. In order to determine the oxidation potential of the test compounds, cyclic voltammetry study for all the test compounds was performed where, the flavonols F1-F5 showed an oxidation potential at 0.633, 0.576, 0.738, 0.641 and 0.928 V, 0.938 V, respectively whereas, their zinc complexes exhibited oxidation potentials at 0.897, 0.718, 0.77, 0.59 and 0.96 V. The complex of F5 did not undergo oxidation in the interval of –0.5 to +1.5 V. However, the zinc complex of F1 showed a reduction potential of −0.374 V. The test compound F4 exhibited two oxidation potentials at 0.59 and 0.96 V and its cytotoxic activity could have been attributed to lowered oxidation potential value of 0.59 V.
When these compounds were tested for their cytotoxicity by MTT assay on MCF-7 cell lines and their safety was evaluated on Vero cell lines, F4, a 2-hydroxy-3- (4-methylsulphanyl-phenyl)-4H-naphthalen-1-one showed cytotoxicity with IC50 at 31.43 μM against MCF-7 cell lines and >200 μM against Vero lines. Test compound F4M1 exhibited IC50 at 34.56 μM and 97.83 μM against MCF-7 and Vero cell lines respectively, when compared to that of the standard quercetin with IC50 at 26.5 μM and >200 μM against MCF-7 and Vero cell lines, respectively as shown in Table 2. These results illustrate that both F4 and its zinc complex are selective cytotoxic on the MCF-7 cell lines and their cytotoxicity is comparable to that of the standard quercetin and were found to be relatively less toxic. Jing Zhou et al. [23] reported that, zinc and copper complexes of quercetin were more effective in suppressing BCG-823, Bel-7402, KB and HL-60 than that of quercetin.
| NAME | IC50-VERO (μM) | IC50-MCF (µM) |
|---|---|---|
| Quercetin (std.) | >200 | 26.5 |
| F1 | >200 | >200 |
| F1M1 | >200 | >200 |
| F2 | >200 | >200 |
| F2M1 | >200 | >200 |
| F3 | >200 | >200 |
| F3M1 | >200 | >200 |
| F4 | >200 | 31.43 |
| F4M1 | 97.83 | 34.56 |
| F5 | >200 | >200 |
| F5M1 | >200 | >200 |
Amongst the ten compounds tested for their cytotoxicity, compound F4 exhibited cytotoxicity at 31.43 µM as compared with that of the standard quercetin with its IC50 at 26.5 µM
Table 2: Cytotoxicity of the compounds
A series of flavones and their zinc complexes were synthesized and characterized using spectral data and were found to be in accordance with their proposed structures. Their oxidation potentials were determined by cyclic voltammetric methods. Further, they were evaluated for their cytotoxic efficacy by MTT assay using Vero and MCF-7 cell lines and compound F4 and its zinc complex were found be exhibiting cytotoxicity against MCF-7 cell lines and their IC50 values are comparable with quercetin.
Acknowledgements
Authors thank Manipal University, MODROB and RPS grant under AICTE for providing laboratory facilities.
Financial assistance
Nill.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ravishankar D, Rajora AK, Greco F, Osborn HM. Flavonoids as prospective compounds for anti-cancer therapy. Int J Biochem Cell Biol 2013;45:2821-31.
- Batra P, Sharma AK. Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech 2013;3:439-59.
- Le Marchand L. Cancer preventive effects of flavonoids--a review. Biomed Pharmacother 2002;56:296-301.
- Hirano T, Gotoh M, Oka K. Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL-60 cells. Life Sci 1994;55:1061-9.
- Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr 1999;38:133-42.
- Horsfall JG. Principles of Fungicidal Action. Waltham: Chronica Botanica Co.;1956.
- Arman P, Wain RL. Studies upon the copper fungicides X. The role of leaf exudates in the solution of copper from bordeaux mixture. Ann Appl Biol 1958;46:366-74.
- Fernandez MT, Mira ML, Florencio MH, Jennings KR. Iron and copper chelation by flavonoids: an electrospray mass spectrometry study. J Inorg Biochem 2002;92:105-11.
- Bukhari SB, Memon S, Tahir MM, Bhanger M. Synthesis, characterization and investigation of antioxidant activity of cobalt-quercetin complex. J Mol Struct 2008;892:39-46.
- Gamez P, Koval IA, Reedijk J. Bio-mimicking galactose oxidase and hemocyanin, two dioxygen-processing copper proteins. Dalton Trans 2004;4079-88.
- Rybarczyk-Pirek AJ, Malecka M, Glinka L, Ochocki J. Trans-bis(3-aminoflavone-kappa2N,O)bis(perchlorato-kappaO)copper(II), a new potential antitumour agent. Acta Crystallogr C 2007;63:410-12.
- Jayashree B, Noor F, Yogendra N, Vijay KD. Synthesis of substituted 3-hydroxy flavones for antioxidant and antimicrobial activity. Pharmacol Online 2008;3:586-95.
- Rutkowska E, Pajak K, Jóźwiak K. Lipophilicity--methods of determination and its role in medicinal chemistry. Acta Pol Pharm 2013;70:3-18.
- Hodnick WF, Milosavljević EB, Nelson JH, Pardini RS. Electrochemistry of flavonoids. Relationships between redox potentials, inhibition of mitochondrial respiration, and production of oxygen radicals by flavonoids. Biochem Pharmacol 1988;37:2607-11.
- Aleksandra S, Dragan M, Dejan S, Marija T. Electrochemical Behaviour and Antioxidant and Prooxidant Activity of Natural Phenolics. Molecules 2007;12:2327-40.
- Jesús FA, Mercedes R, Alberto P , Gema A, Sara P, José MR. Comparison of the Simple Cyclic Voltammetry (CV) and DPPH Assays for the Determination of Antioxidant Capacity of Active Principles. Molecules 2012;17:5126-5138.
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 1986;89:271-277.
- Mertens-Talcott SU, Percival SS. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett 2005;218:141-51.
- Mertens-Talcott SU, Talcott ST, Percival SS. Low concentrations of quercetin and ellagic acid synergistically influence proliferation, cytotoxicity and apoptosis in MOLT-4 human leukemia cells. J Nutr 2003;133:2669-74.
- Srivastava S, Somasagara RR, Hegde M, Nishana M, Tadi SK, Srivastava M, et al. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci Rep 2016;6:24049.
- Sun-Waterhouse D, Smith BG, O’Connor CJ, Melton LD. Effect of raw and cooked onion dietary fibre on the antioxidant activity of ascorbic acid and quercetin. Food Chem 2008;111580-5.
- Marozienė A, Nemeikaitė-Čėnienė A, Vidžiūnaitė R, Čėnas N. Correlation between mammalian cell cytotoxicity of flavonoids and the redox potential of phenoxyl radical/phenol couple. Acta Biochim Pol 2012;59:299-305.
- Zhou J, Wang L, Wang J, Tang N. Antioxidative and anti-tumour activities of solid quercetin metal(II) complexes. Transit Metal Chem 2001;26:57-63.