- *Corresponding Author:
- L. J. Liu
Department of Pharmaceutical Engineering, Heilongjiang University, Key Laboratory of Chemical Engineering Process and Technology for High-efficiency Conversion, College of Heilongjiang Province, Harbin, 150080, China
E-mail: liulijuan1972@163.com
| Date of Submission | 14 March 2015 |
| Date of Revision | 16 January 2016 |
| Date of Acceptance | 26 February 2015 |
| Indian J Pharm Sci 2016;78(1):143‑150 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The rhizome of Menispermum dauricumDC known as a traditional Chinese medicine, with high content of alkaloids, has been found to possess antitumor activity. In this research, an attempt to correlate fingerprinting with bioactivity was made for quality control of M. dauricum. Firstly, the cytotoxicity of extracts from ten batches of samples against human breast MCF-7 cancer cells was estimated by [3-(4, 5-dimethylthiazole-2-yl)-2, 5-diphenyltetrazoliumbromide] assay. Then, cytotoxic activity-integrated fingerprints were established by high performance liquid chromatography. Eight peaks were selected as the common peaks to evaluate the similarities of samples and hierarchical clustering analysis was used to identify and classify different samples into groups. Assays for determinations of total alkaloids and dauricine contents enabled cytotoxicity coefficient of each extract. The potential usefulness of employing cytotoxicity coefficient was investigated by a combination of Pearson correlation coefficients and multiple linear regression analysis as being the reliable parameter to evaluate the herbal extracts. The results indicated that the level of dauricine (peak 8 in the fingerprint) correlated closely with cytotoxicity and played a significant role in the cytotoxicity of Bei Dou-Gen and could be related to its antitumor properties. It is proposed that the cytotoxicity coefficient value with a cytotoxic activity-integrated fingerprint of key biomarkers (dauricine) may be useful indicators to adopt for the quality control of M. dauricum. The analysis of cytotoxic-activity-integrated fingerprint could correlate fingerprinting with bioactivities and would provide a reasonable strategy for quality control of complex mixture of herbal medicines.
Keywords
Menispermum dauricum, cytotoxic-activity-integrated fingerprint, high performance liquid chromatography, cytotoxicity, quality control
Nowadays traditional Chinese medicines (TCMs) have been widely used for health promotion by millions of people around the world and more and more research groups have focused on them because of its high effectiveness and low toxicity [1]. However, due to the complexity of the chemical compositions of TCMs, the quantity and quality of the safety and efficacy data on herbal medicine are still far from sufficient to meet the criteria need to support their use worldwide [2,3]. Therefore, it is important and urgent to elucidate the biologically active components and sufficiently evaluate the quality based on the pharmacological activities or efficacy. As a result, fingerprint analysis has been introduced and accepted by the World Health Organization (WHO) as a strategy for assessing the quality of herbal medicines [4,5]. The fingerprint analysis, with the characters of entirety and fuzziness by evaluating the quality consistency and stability of herbal products, can be used as a tool to control the quality of TCMs systematically and comprehensively, especially for some unknown components in herbal drugs [1,6-9]. High performance liquid chromatography (HPLC) as an important analytical method has been widely used in fingerprint development to discriminate the different origins of medicinal herbs and conduct the quality control of TCMs [10-18]. However, most of the existing fingerprints characterize the chemical profile of naturally occurring constituents, which could not represent the active components of herbal medicines. So it seems insufficient for the quality control and more and more reports have focused on the activity-integrated fingerprint which contains the active constituents, and evaluated them with suitable integrated systems [19-23].
Menispermum dauricum DC (Menispermaceae), an important medicinal plant with high content of alkaloids in its rhizome, mainly distributed in Heilongjinag, Shanxi, Anhui, Jiangsu, Gansu and Zhejiang provinces in China. The rhizome of this plant, known as Bei Dou-Gen (BDG), has been officially listed in the Chinese Pharmacopoeia as an analgesic and antipyretic to treat sore throats, colitis, dysentery and rheumatic arthritis [24]. Previous studies have shown the presence of alkaloids belonging to various classes such as bisbenzylisoquinoline, aporphine, proporphine, protoberberine and oxoisoaporphine [25,26]. And also, the alkaloids present in BDG possess various bioactivities including antiinflammatory, antitumor and antiarrhythmic effects [27,28]. Beidougen capsules and tablets prepared from the extracts of BDG were listed in Chinese Pharmacopoeia 2010 and determinations of total alkaloids and dauricine contents were used for their quality control whereas the crude herb of BDG has not been evaluated by any quantifiable method [29,30].
Given the importance of the alkaloids of BDG on its activity properties, the alkaloids content and associated bioactivity were used as parameters for quality control testing. Based on multiple linear regression analysis, the potential of cytotoxicity in parallel with fingerprint as quality evaluation measure of BDG was examined.
Materials and Methods
HPLC fingerprints were performed on a Hitachi L-2130 series HPLC system including binary solvent delivery pump, manual sampler manager, column compartment and ultraviolet detector, connected to Hitachi L-2000 software. Cell culture was performed in 96-well microplates (Iwaki Glass, Chiba, Japan) and optical density (O.D.) was read on a microplate reader (Spectrafluor, Tecan, Bio-Rad, USA) at 490 nm. Methanol of HPLC grade and other chemicals of analytical grade were from Tianjing Kermel Chemical Factory (Tianjin, China). Water was purified using a Milli-Q water purification system (Milipore, Bedford, MA, USA).
The human breast MCF-7 cancer cells was purchased from the Department of Microbiology and Immunology, Nanjing Medical University (Nanjing, China); The culture medium of Dulbecco’s Modified Eagle Medium (DMEM) was from Gibco (Life Technologies, Grand Island, NY); 3-(4,5-dimethylthiazole-2-yl)-2, 5-diphenyltetrazoliumbromide (MTT) was obtained from Sigma-Aldrich (Deisenhofen, Germany). Ten batches of BDG samples were purchased from different local drug stores (Harbin, China) and were authenticated at Northeast Forestry University, Harbin, China. S10 (Hongri Drug Store, Harbin) was selected as the sample for optimization of the chromatographic conditions and method validation. Authentic reference standard of dauricine was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Preparation of alkaloid extracts
BDG Samples were crushed into powder and sieved through a 20 mesh (0.9 mm) sieve. The powdered sample (100 g) was accurately weighed and immersed in 1000 ml sulfuric acid aqueous solution (pH 2, 55°) for 24 h. The aqueous extracts were filtered and the filtrate was adjusted to pH 9 with ammonia, and then stand for 30 min to precipitate completely. Get rid of the supernatant and take precipitation suction filter. The residue was washed to neutral with deionized water and oven-dried at 55°~60° until the weight remained constant to calculate the extraction yields.
Determination of the total alkaloids in the extracts
Total alkaloids were determined in reference to the method listed in Chinese Pharmacopoeia [29]. In brief, a total of 400 mg of BDG extract and 25 ml of ethyl acetate were mixed and vibrated for 30 min. After filtering, the filtration was evaporated and the residue was dissolve with 10 ml of anhydrous ethanol. Then, 25 ml of sulfuric acid titrant (0.01 mol/l) was added accurately with two drops of methyl red indicator, titrated with sodium hydroxide titrant (0.02 mol/l). Each 1 ml of sulfuric acid titrant (0.01 mol/l) is equivalent to 6.248 mg dauricine (C38H44N2O6).
Determination of dauricine
Determination of dauricine was performed in reference to the method listed in Chinese Pharmacopoeia [29]. In brief, a total of 100 mg of BDG extract and 25 ml of methanol were mixed and sonicated for 30 min. After filtering, the filtration was filtered through a 0.22 μm membrane for HPLC analysis. A standard solution of dauricine was prepared in methanol at a concentration of 0.4 mg/ml. HPLC analysis was performed at the same conditions as fingerprint analysis described above except that the mobile phase acetonitrile:water:triethylamine (45:55:0.5, v/v).
HPLC fingerprints
HPLC condition The chromatographic separation was carried out on a Waters X-Terra MS C18 column (250 mm×4.6 mm, 5 μm), operated at 35°. The analytical condition was set as follows, gradient elution by the mixture of mobile phases A (0.05% triethylamine aqueous solution) and B (methanol) at 0-18 min with the ratio of 80-52% A and 20-48% B; at 18-35 min with the ratio of 52-48% A and 48-52% B; and at 35-40 min with the ratio of 48-35% A and 52-65% B and 40-70 min with the ratio of 35-32% A and 65-68% B. The flow rate was 0.8 ml/min and the detection wavelength was set at 284 nm with the sample injection volume of 20 ml.
Preparation of reference standard solution
The standard solution was prepared by adding an accurately weighed amount of dauricine to a volumetric flask and dissolved with 10 ml methanol to make final concentration 0.4 mg/ml.
Preparation of sample solution
The residue of alkaloid extracts was accurately weighted and dissolved in methanol to obtain the sample solutions at the concentration of 0.8 mg/ml and filtered through 0.22 μm membrane for HPLC analysis.
Data analysis
Similarity calculation of the HPLC fingerprints was performed by a professional software named Similarity Evaluation System (SES) for chromatographic fingerprint of Traditional Chinese Medicine (Version 2004A), which was recommended by the State Food and Drug Administration (SFDA) of China. The correlation coefficients of entire chromatographic profiles of samples were calculated and the simulative mean chromatogram was generated as a representative standard fingerprint by SES. The similarities of different chromatographic patterns between the samples tested were compared with the representative standard chromatogram. Hierarchical clustering analysis (HCA), a multivariate analysis technique that provides similarities representation of complex data between groups, was performed using Euclidean distance method. The cluster centers were calculated with the average of all chromatograms belonging to the same cluster. In this study, the HCA of samples S1-S10 was performed using SPSS statistics software (SPSS for Windows 17.0, SPSS Corporation, USA). The nearest neighbor and cosine, which is a pattern similarity measure, were selected as measurement for analysis.
Cytotoxic experiment
Sample preparation for cytotoxic experiment was the same as that for HPLC analysis, except for the last step that the residue was dissolved in 10 ml of medium instead of methanol before cytotoxic assay.
Experimental procedure
The human breast MCF-7 cancer cells were grown in a humidified atmosphere of 5% CO2 in air and fed with the culture medium of DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin solution. The cytotoxicity was measured using a modified MTT assay [31]. Briefly, the cells (5×104) were seeded in each well containing 100 ml of the growth medium in a 96-well plates and routinely cultured for 24 h, and then treated with BDG sample solutions in serial concentrations (0.04, 0.2, 1.0 and 5.0 mg/ml) for an additional 48 h. After incubation, the medium was aspirated and 100 μl of fresh medium plus 10 μl of 5 mg/ml MTT solution were added to the cells followed by incubation at 37° for 4 h. After incubation, the medium containing MTT was removed, and 100 μl of dimethyl sulphoxide (DMSO) was added to each well to dissolve the formazan and the absorbance was read at 490 nm (630 nm as a reference) in a microplate reader. Cytotoxicty is expressed as the concentration of samples inhibiting cell growth by 50% (IC50).
Multiple linear regression analysis
Multiple linear regression analysis (MLR), which is the most commonly used modeling methods in quantitative structure-property relationships (QSPR), is convenient to produce a linear model describing a quantitative property (dependent variable) by means of independent variables [32-35]. We used MLR analysis to search for the relationship between cytotoxicity (dependent variable) and fingerprint (independent variables) of BDG extracts. A forward stepwise multiple linear regression procedure using SPSS version 17.0 for Windows was applied. The optimal number of predictors and the best regression equations were selected on the basis of the following statistical parameters: Square of the multiple correlation coefficient (R), Fisher test value (F) and the significance of individual descriptors (p).
Results and Discussion
To obtain accurate, valid and optimal chromatographic conditions, different HPLC parameters were examined and compared, including various columns (Diamonsil C18 150 mm×4.6 mm, 5 μm; Kromasil C18 250 mm×4.6 mm, 5 μm; Waters X-Terra MS C18 250 mm×4.6 mm, 5 μm), column temperatures (25°, 30°, 35° or 40°), detection wavelength (254 nm, 262 nm, 280 nm and 284 nm (data not shown)). Based on the maximum absorption of the marker compounds in the UV spectra, the detection wavelength was set at 284 nm. Different mobiles phases were also tried, such as methanol:water and acetonitrile:water with different modifiers including triethylamine and phosphoric acid. Finally, the optimized HPLC condition was established by comparing the peak resolution, baseline, elution time and the number of characteristic peaks in each chromatogram after repeated testing.
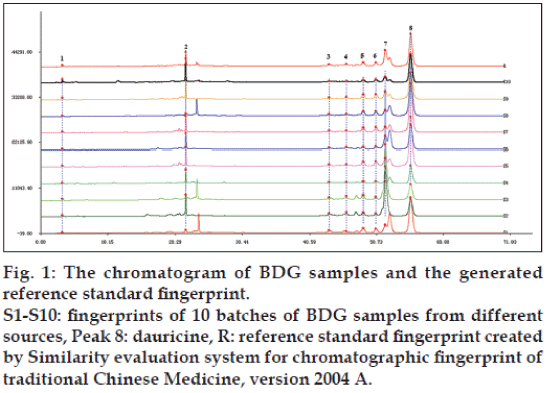
The establishment of fingerprint was operated by the software SES after the selection of “common peaks” in chromatograms. Ten batches of BDG samples from various sources (S1-S10) served as the sample set and the reference standard fingerprint of BDG was generated as shown in fig. 1. The fingerprint showed eight characteristic peaks and peak 8 (dauricine) was chosen as the reference peak to calculate the relative peak area (RPA, Table 1). The area of peak 7 was calculated by merging two poor-resolved peaks at 52.09 and 52.30 min in the reference standard fingerprint. The large RSD values of RPA for peaks 4 (123.1%) and 7 (126.4%) indicated that ten batches of samples from different sources differ greatly in relative contents of these two components, whereas peak 6 differs slightly (13.87%). Similarity analysis was performed based on the standard fingerprint and the similarity indexes were calculated by mean fusion vector method. As listed in Table 2, the similarity values of all samples were more than 0.80 except S2 and S3. The large RPA values for peaks 4 (0.059 and 0.138, respectively) and 7 (2.923 and 3.536, respectively) in these two samples (Table 1) could explain the reason for low similarities with other samples, suggesting that S2 and S3 differ greatly from the others in relative contents of these two components.
Fig. 1: The chromatogram of BDG samples and the generated
reference standard fingerprint.
S1-S10: fingerprints of 10 batches of BDG samples from different
sources, Peak 8: dauricine, R: reference standard fingerprint created
by Similarity evaluation system for chromatographic fingerprint of
traditional Chinese Medicine, version 2004 A.
| Peak number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Retention time 3.287 21.79 | 43.58 46.25 | 48.71 | 50.64 | 52.09 | 55.93 | |||
| S1 | 0.007 | 0.021 | 0.015 | 0.006 | 0.072 | 0.069 | 0.630 | 1 |
| S2 | 0.021 | 0.316 | 0.035 | 0.059 | 0.110 | 0.079 | 2.923 | 1 |
| S3 | 0.018 | 0.060 | 0.028 | 0.138 | 0.132 | 0.089 | 3.536 | 1 |
| S4 | 0.011 | 0.166 | 0.012 | 0.006 | 0.049 | 0.064 | 0.368 | 1 |
| S5 | 0.005 | 0.083 | 0.010 | 0.016 | 0.060 | 0.066 | 0.245 | 1 |
| S6 | 0.009 | 0.154 | 0.017 | 0.021 | 0.045 | 0.059 | 1.080 | 1 |
| S7 | 0.007 | 0.076 | 0.009 | 0.017 | 0.052 | 0.062 | 0.208 | 1 |
| S8 | 0.003 | 0.019 | 0.011 | 0.016 | 0.053 | 0.061 | 0.283 | 1 |
| S9 | 0.010 | 0.094 | 0.014 | 0.030 | 0.057 | 0.073 | 0.217 | 1 |
| S10 | 0.027 | 0.186 | 0.016 | 0.017 | 0.058 | 0.063 | 0.228 | 1 |
| RPA | 0.010 | 0.097 | 0.014 | 0.025 | 0.063 | 0.067 | 0.642 | 1 |
| RSD (%) | 66.79 | 76.95 | 50.42 | 123.1 | 41.96 | 13.87 | 126.4 | 0 |
The retention time of common peaks in reference standard fingerprint and RPA values with respect to peak 8 in 10 batches of BDG samples. RPA: Relative peak area, RSD: relative standard deviation, BDG: Bei Dou Gen
Table 1: The relative peak area values of common peaks in fingerprint.
| Sample number | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | RF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 1 | 0.772 | 0.736 | 0.963 | 0.941 | 0.956 | 0.93 | 0.951 | 0.932 | 0.927 | 0.993 |
| S2 | 0.772 | 1 | 0.991 | 0.636 | 0.544 | 0.914 | 0.514 | 0.567 | 0.522 | 0.533 | 0.785 |
| S3 | 0.736 | 0.991 | 1 | 0.581 | 0.493 | 0.884 | 0.462 | 0.527 | 0.470 | 0.473 | 0.747 |
| S4 | 0.963 | 0.636 | 0.581 | 1 | 0.989 | 0.890 | 0.985 | 0.984 | 0.987 | 0.988 | 0.973 |
| S5 | 0.941 | 0.544 | 0.493 | 0.989 | 1 | 0.836 | 0.999 | 0.995 | 0.999 | 0.992 | 0.946 |
| S6 | 0.956 | 0.914 | 0.884 | 0.890 | 0.836 | 1 | 0.816 | 0.848 | 0.821 | 0.825 | 0.967 |
| S7 | 0.930 | 0.514 | 0.462 | 0.985 | 0.999 | 0.816 | 1 | 0.993 | 0.999 | 0.992 | 0.934 |
| S8 | 0.951 | 0.567 | 0.527 | 0.984 | 0.995 | 0.848 | 0.993 | 1 | 0.992 | 0.980 | 0.954 |
| S9 | 0.932 | 0.522 | 0.470 | 0.987 | 0.999 | 0.821 | 0.999 | 0.992 | 1 | 0.993 | 0.937 |
| S10 | 0.927 | 0.533 | 0.473 | 0.988 | 0.992 | 0.825 | 0.992 | 0.980 | 0.993 | 1 | 0.935 |
| RF | 0.993 | 0.785 | 0.747 | 0.973 | 0.946 | 0.967 | 0.934 | 0.954 | 0.937 | 0.935 | 1 |
The similarities of 10 batches of BDG samples from different sources, RF: reference fingerprint, BDG: Bei Dou‑Gen
Table 2: Results of similarities.
The repeatability of the method was examined by injection of six different samples prepared by the same sample preparation procedure. The interday and intraday precisions were determined by repeated analysis for six times within a day or on five separate days. For the stability test, retention time and peak areas of 8 common peaks in BDG were analyzed every 4 h within 24 h, and the sample solution was found to be rather stable within 24 h. The relative standard deviation (RSD) of retention time and areas of 8 common peaks was used to estimate the repeatability, precision and stability. The results for analysis were shown in Table 3. RSD values for peak areas and retention time were all <3.0%, which could meet the need of fingerprint analysis.
| Number | Precision | Repeatability | Stability | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Interday | Intraday | ||||||||
| RT | PA | RT | PA | RT | PA | RT | PA | ||
| 1 | 0.016 | 0.836 | 0.038 | 0.983 | 1.378 | 1.632 | 0.040 | 0.031 | |
| 2 | 0.546 | 0.589 | 0.519 | 0.816 | 0.845 | 2.075 | 0.548 | 0.917 | |
| 3 | 0.220 | 0.842 | 0.240 | 0.632 | 1.622 | 1.607 | 0.246 | 0.809 | |
| 4 | 0.214 | 0.550 | 0.706 | 0.753 | 1.080 | 1.232 | 0.319 | 0.882 | |
| 5 | 0.232 | 0.352 | 0.347 | 0.894 | 1.481 | 1.923 | 0.344 | 0.870 | |
| 6 | 0.526 | 0.381 | 0.661 | 1.169 | 1.013 | 1.049 | 0.653 | 0.746 | |
| 7 | 0.139 | 0.692 | 0.417 | 0.816 | 1.005 | 1.264 | 0.316 | 0.693 | |
| 8 | 0.591 | 0.751 | 0.220 | 1.517 | 1.632 | 2.317 | 0.661 | 0.734 | |
Precision, repeatability and stability data of eight common peaks. (RSD %, n=6), RT: retention time, PA: peak area, RSD: relative standard deviation
Table 3: Summary of validation of fingerprints
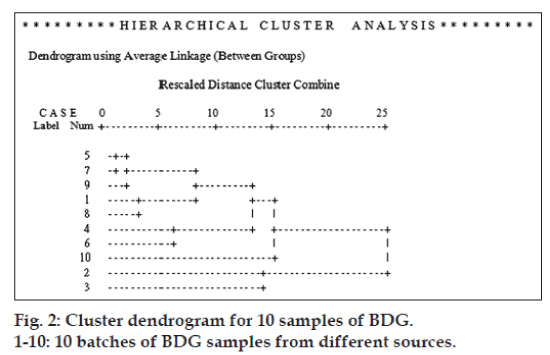
HCA was analyzed after peak areas were normalized by calculation of each peak area in reference to the average areas of 8 common peaks in the reference standard fingerprint. As shown in fig. 2, the samples could be classified into three clusters: (1) S5, S7, S9, S1, S8, S4, S6, S10; (2) S2; (3) S3. S2 and S3 fall into two different clusters despite of their short Euclidean distance. Other eight samples fall into one cluster and S10 differs a bit from other seven samples. Comparing the distance of cluster (1) with (2) and (3), cluster (2) and (3) are more similar to each other than to (1), which indicated that S2 and S3 differ greatly from other samples. The results were consistent with similarities analysis by SES.
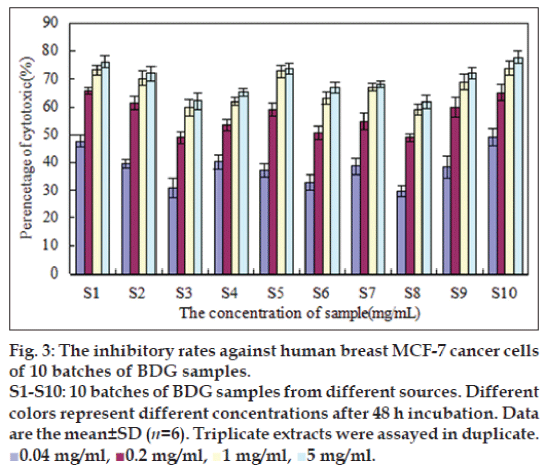
Human breast cancer cells MCF-7 were treated with BDG at different doses and the inhibitory rates were detected by colorimetric MTT assay. Fig. 3 showed the histogram for 10 batches of BDG extracts in different concentrations after 48 h incubation. The shape of these inhibition–dose curves was similar and the results indicated that BDG possessed antiproliferative activity in a dose-dependent manner when the concentration ranged from 0.04 to 1.00 mg/ml. IC50 values for ten batches of BDG samples were listed in Table 4.
Fig. 3: The inhibitory rates against human breast MCF-7 cancer cells
of 10 batches of BDG samples.
S1-S10: 10 batches of BDG samples from different sources. Different
colors represent different concentrations after 48 h incubation. Data
are the mean±SD (n=6). Triplicate extracts were assayed in duplicate.
■0.04 mg/ml, ■0.2 mg/ml, ■1 mg/ml, ■5 mg/ml.
| Samplenumber | Sample source | Total alkaloids (%) | Dauricine(%) | IC50(mg/mL) | ACC values |
|---|---|---|---|---|---|
| S1 | Hongteng | 22.15 | 18.93 | 0.031 | 0.140 |
| S2 | Shi Yitang | 32.28 | 13.37 | 0.385 | 1.193 |
| S3 | Tengfei | 21.14 | 14.85 | 0.431 | 2.038 |
| S4 | Tong Rentang | 33.38 | 17.75 | 0.162 | 0.485 |
| S5 | JiRentang | 27.92 | 21.40 | 0.109 | 0.390 |
| S6 | Xinyuan | 35.95 | 18.69 | 0.281 | 0.782 |
| S7 | Zhan qian | 35.96 | 20.49 | 0.141 | 0.392 |
| S8 | Baofeng | 35.87 | 19.41 | 0.105 | 0.293 |
| S9 | Renmintongtai | 36.11 | 19.91 | 0.473 | 1.310 |
| S10 | Hongri | 34.86 | 21.92 | 0.029 | 0.083 |
The results of the contents of dauricine and total alkaloids, IC50 values and ACC values of 10 batches of BDG samples from different drug stores in Harbin, ACC: activity cytotoxicity coefficient, BDG: Bei Dou‑Gen
Table 4: Summary of determination of the cytotoxicity coefficient.
SES and HCA were used to evaluate the similarities and sort samples into groups based on common peaks in the fingerprint. However, those uncommon peaks especially unique peaks in some samples could not be selected so as to be evaluated. So the potential parameters associated with bioactivity and characteristic components were tested. The contents of dauricine and total alkaloids, and IC50 values of ten batches of samples were determined and the results were listed in Table 4. The cytotoxicity coefficient (ACC) was defined as the ratio of IC50 value to total alkaloids content. The values of ACC of samples were consistent to values of IC50 which represents the cytotoxic activity and lower values correspond to higher cytotoxicity. There was a moderate negative correlation between dauricine and values of IC50 (r=-0.618) whereas there was no linear correlation between total alkaloids content and cytotoxicity (Table 5). A stronger correlation (r=-0.693) was observed for ACC and dauricine content, suggesting that dauricine concentration determined by HPLC was useful indicators of the ACC value in this herb.
| Dauricine (%) | Total alkaloid (%) | |
|---|---|---|
| IC50 | −0.618 NS | −0.06 NS |
| ACC | −0.693* | − |
Pearson correlation coefficients among the contents of dauricine, total alkaloids and cytotoxicity. Pearson correlation significance levels ‑ NS: Not significant, *Significant at P<0.05, ACC: activity cytotoxicity coefficient
Table 5: Summary of multiple linear regression projection
Model was built with data matrix X consisting of the 10 fingerprints, and response vector Y investigated by a combination of the key parameters namely IC50 and ACC. In all cases using ACC as dependent variable, Eqn was obtained by forward stepwise multiple regression techniques following the multi-linear forms: Y=0.518+0.728X4-0.536X8 (P<0.01, R2=0.895). A multiple correlation coefficient (R) of 0.946, coefficient of determination (R2) of 0.895, F value of 29.846, and significant value of 0.000 indicated that the regression equation was valid. VIF values of two variables (X4 and X8) were both 1.003 in the collinearity statistics, suggesting that none of the independent variables were significantly correlated to each other and there was no multicollinearity in the model construction. The regression standardized residual (fig. 4) and normal P-P plot of regression standardized residual (fig. 5) showed that the regression model is appropriate.
Closer examination of the fitting parameters in the equation above, the cytotoxic activities had a close correlation with peaks 4 and 8 in the HPLC fingerprint. Positive regression coefficient of peak 4 indicated that larger peak area of this component corresponded to lower cytotoxicity. Combining with the results of larger RPA values of peak 4 for S2 and S3 listed in Table 1, and higher ACC values for the two samples in Table 5, it could be concluded that peak 4 was a disadvantage of cytotoxicity for BDG.
In contrast, the standard regression coefficient of peak 8 (dauricine) was negative, suggesting that the larger its peak area was, the higher cytotoxicity would achieve. That is, dauricine played a significant role in the cytotoxicity of BDG and could be related to its antitumor properties. It was proposed that dauricine coupled with ACC would be useful parameters for evaluate the internal quality of BDG.
In this research, the cytotoxicity of extracts from ten batches of M. dauricum against human breast MCF-7 cancer cells was estimated and the cytotoxic activity-integrated fingerprints were established by HPLC. Eight peaks were selected as the common peaks to evaluate the similarities of samples and hierarchical clustering analysis was used to classify different samples into groups. The potential usefulness of employing ACC, total alkaloids and dauricine contents were investigated by a combination of Pearson correlation coefficients and MLR analysis. We found no linear correlation between total alkaloids and the cytotoxic activity determined by MTT assay though total alkaloids have been considered as principle constituents contributing to the bioactivity of BDG. The results of MLR indicated that the level of dauricine (peak 8 in the fingerprint) correlated closely with cytotoxicity and played a significant role in the cytotoxicity of BDG and could be related to its antitumor properties. It is proposed that the ACC value with a cytotoxic activity-integrated fingerprint of key biomarkers (dauricine) may be useful indicators to adopt for the quality control of M. dauricum. The analysis of cytotoxic activity-integrated fingerprint could correlate fingerprinting with bioactivities and would provide a reasonable strategy for quality control of complex mixture of herbal medicines.
Acknowledgements
Authors woud like to thank Professor Xiuhua Wang of Northeast Forestry University, Harbin, China for plant authentication.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Xu L, Han X, Qi Y, Xu Y, Yin L, Peng J, et al. Multiple compounds determination and fingerprint analysis of Lidanpaishi tablet and keli by high-performance liquid chromatography. Anal Chim Acta 2009;633:136-48.
- Su S, Hua Y, Duan JA, Shang E, Tang Y, Bao X, et al. Hypothesis of active components in volatile oil from a Chinese herb formulation, ‘Shao-Fu-Zhu-Yu decoction’, using GC-MS and chemometrics. J Sep Sci 2008;31:1085-91.
- Gan F, Ye R. New approach on similarity analysis of chromatographic fingerprint of herbal medicine. J Chromatogr A 2006;1104:100-5.
- World Health Organization. Guidelines for the Assessment of Herbal Medicines. Vol. 28. Munich: WHO; 1991. p. 6.
- Ni LJ, Zhang LG, Hou J, Shi WZ, Guo ML. A strategy for evaluating antipyretic efficacy of Chinese herbal medicines based on UV spectra fingerprints. J Ethnopharmacol 2009;124:79-86.
- Jin W, Ge RL, Wei QJ, Bao TY, Shi HM, Tu PF. Development of high-performance liquid chromatographic fingerprint for the quality control of Rheum tanguticum Maxim. Ex Balf. J Chromatogr A 2006;1132:320-4.
- Di X, Chan KK, Leung HW, Huie CW. Fingerprint profiling of acid hydrolyzates of polysaccharides extracted from the fruiting bodies and spores of Lingzhi by high-performance thin-layer chromatography. J Chromatogr A 2003;1018:85-95.
- Yang LW, Wu DH, Tang X, Peng W, Wang XR, Ma Y, et al. Fingerprint quality control of Tianjihuang by high-performance liquid chromatography-photodiode array detection. J Chromatogr A 2005;1070:35-42.
- Xie P, Chen S, Liang YZ, Wang X, Tian R, Upton R. Chromatographic fingerprint analysis – a rational approach for quality assessment of traditional Chinese herbal medicine. J Chromatogr A 2006;1112:171-80.
- Jing J, Chan CO, Xu L, Jin D, Cao X, Mok DK, et al. Development of an in-line HPLC fingerprint ion-trap mass spectrometric method for identification and quality control of Radix Scrophulariae. J PharmBiomed Anal 2011;56:830-5.
- Tang D, Yang D, Tang A, Gao Y, Jiang X, Mou J, et al. Simultaneous chemical fingerprint and quantitative analysis of Ginkgo bilobaextractby HPLC-DAD. Anal Bioanal Chem 2010;396:3087-95.
- Yan SK, Xin WF, Luo GA, Wang YM, Cheng YY. An approach to develop two-dimensional fingerprint for the quality control of Qingkailing injection by high-performance liquid chromatography with diode array detection. J Chromatogr A 2005;1090:90-7.
- Yi LZ, Yuan DL, Liang YZ, Xie PS, Zhao Y. Quality control and discrimination of pericarpium citri reticulatae and pericarpium citri reticulatae viride based on high-performance liquid chromatographic fingerprints and multivariate statistical analysis. Anal Chim Acta 2007;588:207-15.
- Xie B, Gong T, Tang M, Mi D, Zhang X, Liu J, et al.An approach based on HPLC-fingerprint and chemometrics to quality consistencyevaluation of liuwei dihuang pills produced by different manufacturers. J Pharm Biomed Anal 2008;48:1261-6.
- Wei H, Sun L, Tai Z, Gao S, Xu W, Chen W. A simple and sensitive HPLC method for the simultaneous determination of eight bioactive components and fingerprint analysis of Schisandra sphenanthera. AnalChim Acta 2010;662:97-104.
- Yang DZ, An YQ, Jiang XL, Tang DQ, Gao YY, Zhao HT, et al.Development of a novel method combining HPLC fingerprint and multi-ingredients quantitative analysis for quality evaluation of traditional Chinese medicine preparation. Talanta 2011;85:885-90.
- Shen DD, Wu QL, Sciarappa WJ, Simon JE. Chromatographic fingerprints and quantitative analysis of isoflavones in tofu-type soybeans. Food Chem 2012;130:1003-9.
- Liu LJ, Jiang WH, Zhang LJ, Li F, Zhang QB. Chemical correlation between shuanghuanglian injection and its three raw herbs by LC fingerprint. J Sep Sci 2011;34:1834-44.
- Lu HM, Liang YZ, Wu XJ, Qiu P. Tentative fingerprint-efficacy study of Houttuynia cordata injection in quality control of traditional Chinese medicine. Chem Pharm Bull (Tokyo) 2006;54:725-30.
- Kong WJ, Zhao YL, Shan LM, Xiao XH, Guo WY. Investigation on the spectrum-effect relationships of EtOAc extract from Radix Isatidis based on HPLC fingerprints and microcalorimetry. J Chromatogr B 2008;871:109-14.
- Chang YX, Yan DM, Chen LL, Ding XP, Qi J, Kang LY, et al. Potency fingerprint of herbal products Danshen injection for their quality evaluation. Chem Pharm Bull (Tokyo) 2009;57:586-90.
- Kong WJ, Zhao YL, Xiao XH, Wang JB, Li HB, Li ZL, et al.Spectrum-effect relationships between ultra performance liquid chromatography fingerprints and anti-bacterial activities of Rhizomacoptidis. Anal Chim Acta 2009;634:279-85.
- Yi LZ, Yuan DL, Liang YZ, Xie PS, Zhao Y. Fingerprinting alterations of secondary metabolites of tangerine peels during growth by HPLC-DAD and chemometric methods. Anal Chim Acta2009;649:43-51.
- Chinese Pharmacopoeia. Vol. 1. Beijing: Chemical Industry Press; 2010. p. 92.
- Yu BW, Chen JY, Wang YP, Cheng KF, Li XY, Qin GW. Alkaloids from Menispermum dauricum. Phytochemistry 2002;61:439-42.
- Zhang X, Ye W, Zhao S, Che CT. Isoquinoline and isoindole alkaloids from Menispermum dauricum. Phytochemistry 2004;65:929-32.
- Lin M, Xia B, Yang M, Gao S, Huo Y, Lou G. Anti-ovarian cancer potential of two acidic polysaccharides from the rhizoma of Menispermum dauricum. Carbohydr Polym 2013;92:2212-7.
- Lin M, Xia B, Yang M, Gao S, Huo Y, Lou G. Characterization and antitumor activities of a polysaccharide from the rhizoma of Menispermum dauricum. Int J Biol Macromol 2013;53:72-6.
- Chinese Pharmacopoeia. Vol. 1. Beijing: Chemical Industry Press; 2010. p. 377.
- Chinese Pharmacopoeia. Vol. 1. Beijing: Chemical Industry Press; 2010. p.641.
- Sargent JM, Taylor CG. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br J Cancer 1989;60:206-10.
- Mandal AS, Roy K. Predictive QSAR modeling of HIV reverse transcriptase inhibitor TIBO derivatives. Eur J Med Chem 2009;44:1509-24.
- Ghasemi J, Saaidpour S, Brown SD. QSPR study for estimation of acidity constants of some aromatic acids derivatives using multiple linear regression (MLR) analysis. J Mol Struct 2007;805:27-32.
- Tistaert C, Dejaegher B, Nguyen Hoai N, Chataigné G, Rivière C, Nguyen Thi Hong V, et al. Potential antioxidant compounds in Mallotus species fingerprints. Part I: Indication, using linear multivariate calibration techniques. Anal Chim Acta 2009;649:24-32.
- Massart DL, Vandeginste BG, Buydens LM, De Jong S, Lewi PJ, Smeyers-Verbeke J. Handbook of Chemometrics and Qualimetrics. Amsterdam: Elsevier; 1997.