- Corresponding Author:
- A. A. Lohade
Department of Pharmaceutics, Bombay College of Pharmacy, Kalina, Mumbai - 400 098, India
E-mail: atullohade@yahoo.co.in
| Date of Acceptance | 23 October, 2007 |
| Indian J Pharm Sci, 2007, 69 (5): 707-709 |
Abstract
Fluticasone propionate (FP) is a lipophilic drug used to control inflammation in patients with asthma, allergic rhinitis and chronic obstructive pulmonary disease (COPD)[1]. The poor aqueous solubility (<1 µg/ml) and dissolution of FP may be an absorption rate limiting step in pulmonary drug delivery and the insoluble particles are removed by the mucociliary clearance in the upper airways and by macrophages in the alveoli[2]. Cyclodextrin complexes might be of a great value in pulmonary delivery by increasing the solubility and dissolution rate of the complexed drug and thereby lead to decreased clearance, increased absorption and faster onset of the action[3]. The objective of the present study was to investigate complexation of FP with 2-hydroxypropyl-β-cyclodextrin (HPβCD) and albumin for improved delivery to lung.
Material and Methods
Equilibrium solubility of FP (Sun Pharmaceutical Industries Ltd, India) was determined in aqueous HPβCD (Gangawal Chemicals, India) solution at 370±20 for 7 d, and solubility constant was calculated from phase-solubility diagram. HPβCD inclusion complex of FP was prepared by the spray drying and freeze drying techniques in the molar ratio 1:1. These complexes were dissolved in bovine serum albumin (S. D. Fine Chemicals Ltd., India) solution, and subjected to spray and freeze drying methods. Microparticles were characterized for various physicochemical properties such as particle size distribution, FTIR, DSC and X-ray diffraction, SEM studies and drug content. In vitro drug release studies were carried out using USP Type IV apparatus in simulated lung buffer (PBS pH 7.4), maintained at 370±0.50, and fine particle fraction (FPF) determined by twin stage impinger (TSI).
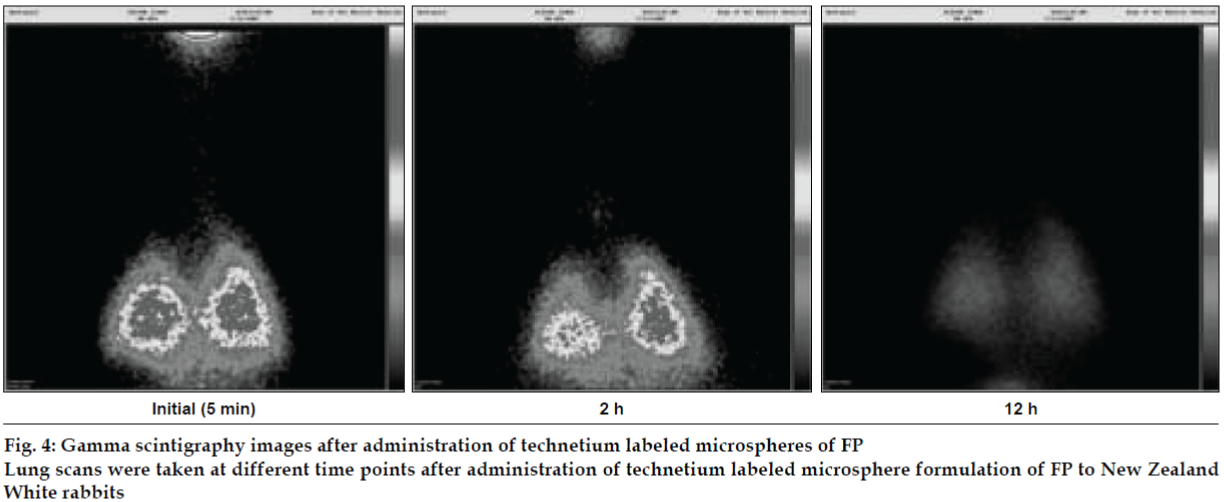
Microspheres were radio labeled with technetium (99mTc) using SnCl2 as reducing agent. In vivo lung deposition studies were carried out using New Zealand rabbits. Scintigraphic images of rabbit lungs were taken at periodic intervals viz., 2, 4, 8 and 12 h using a gamma camera (Millennium MPS Gamma Camera). In vivo efficacy study was carried out in LPS induced bronchoalveolar inflammation in guinea pigs.
Results and Discussion
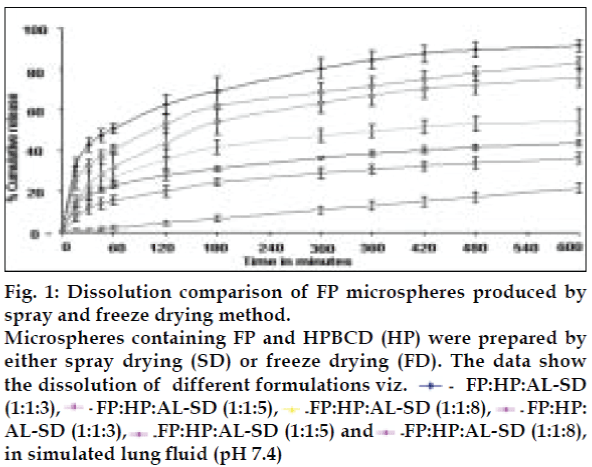
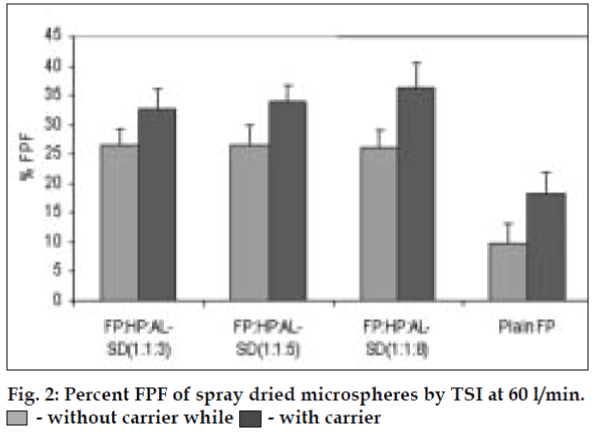
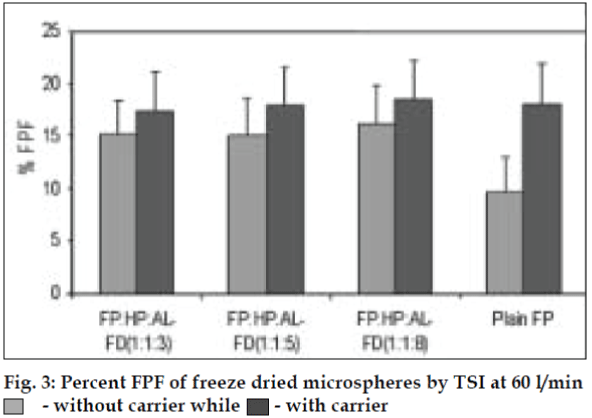
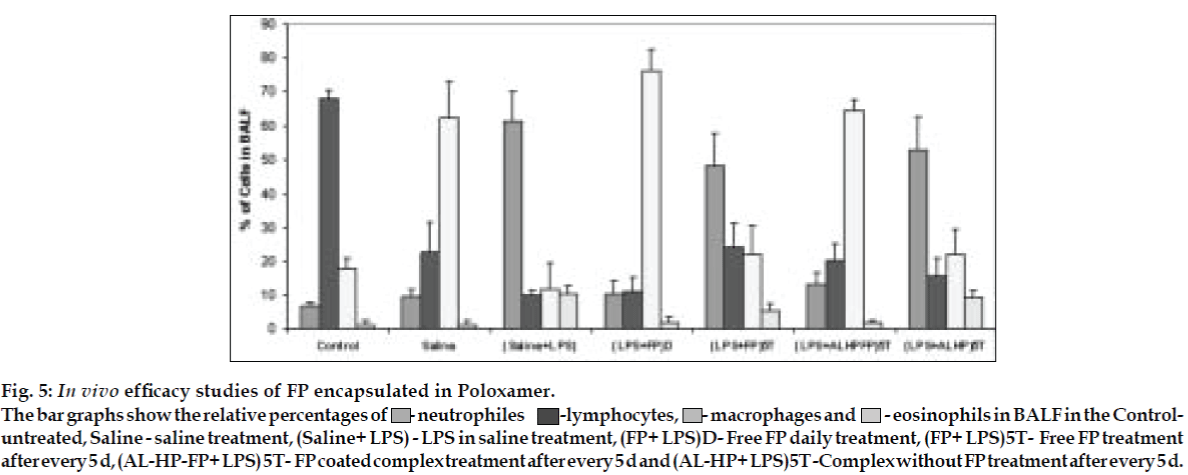
AL type solubility diagram was obtained for FP which could be attributed to formation of 1:1 M inclusion complex with HPβCD (Higuchi and Connors method[4]). Release data from microspheres showed significant improvement of the dissolution rate of FP, and a controlled release was obtained in the presence of higher amount of albumin (fig. 1). Spray-dried products presented higher release profiles than freezedried products. The surface of microspheres prepared by spray drying was spherical and smooth with an average diameter of 4 μm where as freeze-dried microspheres formed irregularly shaped microparticles with average diameter of 15 μm. DSC and XRD studies revealed loss of drug crystallinity. FTIR spectra confirmed the existence of inclusion complex. DPI bends showed a FPF of 35-42% in case of spray dried microparticles, compared to blends prepared with free drug and freeze dried particles (10-18%). (figs. 2 and 3). The lung deposition was calculated as ratio of activity in the lungs to activity emitted from device, and found to be only 8-10% of the total administered dose. The gamma scintigraphic images showed the presence of activity in lungs for up to 12 h (fig. 4), whereas free 99mTc cleared rapidly over a period of 6 min. More activity was deposited in peripheral region then central region of lungs indicating fine particles produced by microspheres. Furthermore, a decrease in the counts in the central region was observed over the time period resulting in a decrease in central to peripheral ratio (Table 1). In the in vivo efficacy studies, formulation delivered every 5th day of therapy with FP encapsulated in microspheres was as effective as daily FP therapy in decreasing lung inflammation and showed a marked lowering of total neutrophil counts (fig. 5).
| Time | Left lung | Right lung | ||||||
|---|---|---|---|---|---|---|---|---|
| C (%) | I (%) | P (%) | C/P | C (%) | I (%) | P (%) | C/P | |
| Initial (5 min) | 25.23 | 40.44 | 34.33 | 0.735 | 26.43 | 39.97 | 33.60 | 0.786 |
| 2 h | 23.97 | 39.28 | 36.75 | 0.652 | 24.35 | 37.21 | 38.44 | 0.633 |
| 6 h | 21.93 | 39.05 | 39.02 | 0.562 | 23.12 | 36.96 | 39.92 | 0.579 |
| 12 h | 18.39 | 38.88 | 42.73 | 0.579 | 21.70 | 33.25 | 45.04 | 0.482 |
Table 1: Percent Activity in Central, Intermediate and Peripheral Region of Lungs
Fig. 1: Dissolution comparison of FP microspheres produced by spray and freeze drying method.
Microspheres containing FP and HPBCD (HP) were prepared by either spray drying (SD) or freeze drying (FD). The data show the dissolution of different formulations viz. FP:HP:AL-SD (1:1:3), FP:HP:AL-SD (1:1:5), FP:HP:AL-SD (1:1:8), FP:HP: AL-SD (1:1:3), FP:HP:AL-SD (1:1:5) and FP:HP:AL-SD (1:1:8), in simulated lung ß uid (pH 7.4)
Fig. 5: In vivo efficacy studies of FP encapsulated in Poloxamer.
The bar graphs show the relative percentages of  - neutrophiles -lymphocytes,
- neutrophiles -lymphocytes,  - macrophages and
- macrophages and  - eosinophils in BALF in the Controluntreated, Saline
- eosinophils in BALF in the Controluntreated, Saline  - saline treatment, (Saline+ LPS) - LPS in saline treatment, (FP+ LPS)D- Free FP daily treatment, (FP+ LPS)5T- Free FP treatment after every 5 d, (AL-HP-FP+ LPS) 5T- FP coated complex treatment after every 5 d and (AL-HP+ LPS)5T -Complex without FP treatment after every 5 d.
- saline treatment, (Saline+ LPS) - LPS in saline treatment, (FP+ LPS)D- Free FP daily treatment, (FP+ LPS)5T- Free FP treatment after every 5 d, (AL-HP-FP+ LPS) 5T- FP coated complex treatment after every 5 d and (AL-HP+ LPS)5T -Complex without FP treatment after every 5 d.
Albumin microsphere systems of these FP- HPβCD complexes prepared by spray drying are an effective treatment regimen for asthma, which can provide localized sustained action.
Acknowledgements
The authors wish to thank BRNS-DAE (Sanction no. 2004/35/5/BRNS) for financial assistance, Burculodomo Ingredients for lactose and Sun Pharmaceutical Ltd., India for FP.
References
- Ventresca GP, Mackie AE, Moss JA, McDowell JE, Bye A. Absorption of oral ßuticasone propionate in healthy subjects. Am J Resp Med 1994;149:A214.
- Derendorf H, Hochhaus G, Meibohm B, Möllmann H, Barth JJ. Pharmacokinetics and pharmacodynamics of inhaled corticosteroids. Allergy ClinImmunol 1998;101:S440.
- Kinnarinen T, Jarho P, Järvinen K, Jarvinen T. The in vitro pulmonary deposition of a budesonide / γ-cyclodextrin inclusion complex. J Inclusion Phenomena MacrocyclicChem 2002;44:97–100.
- Higuchi T, Connors KA. Phase solubility analysis. Adv Anal ChemInstrum 1965;4:117-212.