- *Corresponding Author:
- Salome A. Chime
Department of Pharmaceutical Technology and Industrial Pharmacy, Nsukka-410 001, Nigeria
E-mail: emmymarachi@yahoo.com
| Date of Submission | 14 August 2012 |
| Date of Revision | 06 March 2013 |
| Date of Acceptance | 20 March 2013 |
| Indian J Pharm Sci 2013;75(3):302-309 |
Abstract
The objective of our work was to study the micromeritic properties of lyophilized diclofenac potassium-loaded lipospheres and to evaluate in vivo, the analgesic properties of diclofenac potassium in the lipospheres in addition to other in vitro properties. Solidified reverse micellar solutions were prepared by fusion using 1:1, 2:1, and 1:2% w/w of Phospholipon ® 90H and Softisan ® 154. Diclofenac potassium (1, 3, and 5% w/w) was incorporated into the solidified reverse micellar solutions. Solidified reverse micellar solutions-based lipospheres were formulated by melt homogenization techniques using Ultra-Turrax homogenizer, and thereafter lyophilized to obtain water-free lipospheres. The lipospheres were characterized in terms of particle size and morphology, stability, thermal analysis, drug content, encapsulation efficiency, and loading capacity. The flow properties of the lipospheres were studied using both direct and indirect methods of assessing flow. The analgesic properties of the lipospheres were studied using the hot plate method. Results obtained showed that the yield of diclofenac potassium-loaded lipospheres was high and the particle size ranged from 0.61±0.07 to 2.55±0.04 μm. The lipospheres had high encapsulation efficiency of 95%, which was affected by the amount of drug loaded, while the loading capacity increased with the increase in drug loading. Diclofenac potassium-loaded lipospheres exhibited poor flow. The formulations exhibited good analgesic effect compared with the reference and had 84 to 86% drug release at 13 h. The lipospheres based on solidified reverse micellar solutions could be used for oral delivery of diclofenac potassium.
Keywords
Solidified reverse micellar solution, lipospheres, micromeritics, diclofenac potassium, analgesic
An array of lipid systems such as emulsions, micellar solutions, liposomes, lipid nanoparticles, structured lipid carriers, self-emulsifying lipid formulations, solid dispersions, dry emulsions, solid-liquid compacts, and drug lipid conjugates is available to drug formulators [1]. Among the various lipid systems, lipospheres have been developed to address some issues such as stability and low payload capacity of some lipid systems [1]. Lipospheres are restricted to structures with a lipid core and stabilizing phospholipid layer [2]. These have been utilized in the delivery of antiinflammatory compounds [3,4], local anesthetics, antibiotics, anticancer agents, insect repellents, vaccines, proteins, and peptides [5-8].
The combination of solid inner core with phospholipid exterior confers several advantages on the lipospheres compared with conventional microspheres and microparticles, including high dispersibility in an aqueous medium, and a release rate for the entrapped substance that is controlled by phospholipid coating and carrier. The substance to be delivered need not be soluble in the vehicle since it can be dispersed in the solid carrier [2,9]. Lipospheres also have a lower risk of reaction of substance to be delivered with the vehicle than in emulsion system because the vehicle is a solid material. Moreover, the release rate of substance from the lipospheres can be manipulated by altering either one or both the inner solid vesicle or the outer phospholipid layer.
Solidified reverse micellar solution (SRMS)-based carriers have been investigated, and successfully employed to achieve controlled release of drugs [10-12]. SRMS consisting of phospholipid and solid lipid such as Softisan® 154, a completely hydrogenated palm oil transform into a lamellar mesophase after melting on contact with water. This transformation enables controlled release of solubilized drugs. SRMS also offer a high solubilization rate of different types of drugs [12].
There is a need to present lipospheres formulation in capsule form, as tablets and dried powder for reconstitution, therefore the micromeritic properties of these formulations is of great essence in order to study their flow properties. Therefore, the aim of the work is to study the micromeritic properties of the lyophilized diclofenac potassium-loaded lipospheres, and to evaluate the in vivo analgesic properties of diclofenac potassium in the lipospheres in addition to other in vitro properties such as particle size, morphology, encapsulation efficiency, and loading capacity.
Materials and Methods
The following materials were used as procured from their suppliers without further purification: Hydrochloric acid, sodium hydroxide, monobasic potassium phosphate and Tween® 80, (Merck, Germany), sorbitol (Wharfedale laboratories, England), diclofenac potassium (Healthy Life Pharma, India), Softisan® 154 (Schuppen, Condea Chemie GmbH, Germany), Phospholipon® 90H (Phospholipid GmbH, Köln, Germany), distilled water (Lion water, Nsukka, Nigeria). All other reagents and solvents were of analytical grade and were used as supplied.
Preparation of SRMS
Mixtures of Phospholipon® 90H and Softisan® 154 (1:1, 1:2, and 2:1 w/w) were prepared by fusion. In each case the lipids were weighed using analytical balance (Adventurer, Ohaus, China), melted together and stirred at a temperature of 70° using a magnetic stirrer (SR1 UM 52188, Remi Equip., India), until a homogenous, transparent white melt was obtained. The homogenous mixture was stirred at room temperature until solidification to obtain the SRMS [1,13].
Preparation of SRMS-based lipospheres
The diclofenac potassium-loaded lipospheres were prepared using the melt-homogenization technique [14], according to the formula presented in Table 1. The lipospheres were prepared using 1:1, 1:2, and 2:1 (w/w) lipid matrices by hot homogenization using Ultra-Turrax homogenizer (T25 Basic, Digital, Ika, Staufen Germany). In each case 10 g of the lipid matrix was melted at 70° and an appropriate amount of diclofenac potassium was incorporated into the lipidic melt. Sorbitol was dissolved in hot distilled water at the same temperature with the lipidic melt together with Tween® 80. The hot aqueous phase was poured into the lipidic melt and immediately subjected to high shear homogenization with Ultra-Turrax at 5000 rpm for 10 min. An o/w emulsion was finally obtained by phase inversion. The lipospheres obtained after cooling at room temperature were lyophilized using a freeze dryer (Amsco/Finn–Aqua® Lyovac GTZ, Germany), in order to obtain a solidified water-free lipospheres [14]. SLMs containing no drug, which served as negative control, were also similarly formulated.
| Batch | LM ratio | Tween® 80 (% w/w) | LM (% w/w) | Sorbitol (% w/w) | Diclofenac potassium (% w/w) | Distilled water qs (% w/w) |
|---|---|---|---|---|---|---|
| A1 | 1:1 | 1.5 | 10 | 4.0 | 1.0 | 100 |
| A2 | 1:1 | 1.5 | 10 | 4.0 | 3.0 | 100 |
| A3 | 1:1 | 1.5 | 10 | 4.0 | 5.0 | 100 |
| A4 | 1:1 | 1.5 | 10 | 4.0 | 0.0 | 100 |
| B1 | 2:1 | 1.5 | 10 | 4.0 | 1.0 | 100 |
| B2 | 2:1 | 1.5 | 10 | 4.0 | 3.0 | 100 |
| B3 | 2:1 | 1.5 | 10 | 4.0 | 5.0 | 100 |
| B4 | 2:1 | 1.5 | 10 | 4.0 | 0.0 | 100 |
| C1 | 1:2 | 1.5 | 10 | 4.0 | 1.0 | 100 |
| C2 | 1:2 | 1.5 | 10 | 4.0 | 3.0 | 100 |
| C3 | 1:2 | 1.5 | 10 | 4.0 | 5.0 | 100 |
| C4 | 1:2 | 1.5 | 10 | 4.0 | 0.0 | 100 |
LM=Lipid matrix, A1–A4=Contain LM 1:1, B1–B4=Contain LM 2:1, C1–C4=Contain LM 1:2
Table 1: Quantities of materials used for lipospheres formulation.
Percentage yield of lipospheres
After lyophilization, the water-free lipospheres from all the batches were weighed. The yield of the lipospheres (% w/w) was calculated according to the following formula [15]. Percent Yield (%)=(W1/(W2+W3))×100…(1), where, W1 is the weight of lipospheres formulation (g), W2 is the weight of drug added (g), and W3 is the total weight of the lipid, sorbitol and Tween® 80 (g).
Determination of particle size and morphology
About 200 mg of the lipospheres from each batch was placed on a microscope slide and was dispersed in small amount of water. The slide was covered with a cover slip and imaged under a Hund® binocular microscope (Weltzlar, Germany) attached with a Motic image analyzer (Moticam, China), at a magnification of 400×.
Thermal analysis of the SLMs
Differential scanning calorimetry (DSC) was used to determine the thermal characteristics of diclofenac potassium, Softisan® 154, Phospholipon® 90H, SRMS (1:1, 2:1 and 1:2) and the liposphere formulations and also to investigate the state of the drugs in lipospheres. Melting transitions and changes in heat capacity of Phospholipon® 90H, Softisan® 154, diclofenac potassium, SRMS (1:1, 2:1and 1:2), unloaded lipospheres and the drug-loaded lipospheres were determined using differential scanning calorimeter (Netzsch DSC 204 F1, Germany). About 1-10 mg of each specimen was weighed into aluminum pan, hermetically sealed and the thermal behavior determined within the range of 20-500º, at a heating rate of 10 º/min under a 20 ml/min nitrogen flux.
pH studies
The pH of the dispersions of the lipospheres from each batch prior to lyophilization was taken in a time dependent manner: 1 week, 1 month, and 3 months using a pH meter (pH ep® Hanna Instrument, Italy).
Encapsulation efficiency
Beer’s plot for diclofenac potassium was obtained at a concentration range of 0.1 to 1.0 mg% in distilled water at a predetermined wavelength of 297 nm. Quantities of the lipospheres equivalent to 0.1 g of diclofenac potassium were weighed out and placed in a 100 ml volumetric flask. The flask was made up to volume with appropriate solvent and heated at 70° with intermittent shaking until the lipospheres completely melted. The dispersion was cooled to room temperature, filtered through a filter paper (Whatman No. 1) and analyzed using spectrophotometer (Jenway 6305 spectrophotometer, Barloworld Scientific Ltd., UK). The drug concentrations were calculated with reference to Beer’s plot. The quantities of the drugs theoretically contained in the lipospheres were compared with the quantity actually obtained from the drug content studies. This was calculated using the equation below: Encapsulation efficiency (EE %)=(ADC/TDC)×100…(2), where ADC is the actual drug content and TDC is the theoretical drug content [1].
Drug loading capacity
Loading capacity (LC) expresses the ratio between the entrapped active pharmaceutical ingredient (API) and the total weight of the lipids [1]. LC was determined using the relationship: LC=(amount of API encapsulated/weight of the lipid)×100…(3).
Bulk and tapped densities
A 10 g quantity of each sample of the lipospheres was weighed out and placed in a 25 ml graduated cylinder. The cylinder was tilted before the sample was poured inside; the volume occupied by the sample was noted as the bulk volume. The bulk density [16,17] was obtained by dividing the mass of the sample weighed out by the bulk volume, as shown by bulk density=mass of powder (M)/bulk volume of powder (VB)…(4).
The cylinder was tapped on a wooden platform by dropping the cylinder from a height of one inch at 2 s interval until there was no further apparent change in volume. The volume occupied by the sample was then recorded as the tapped volume. The tapped density was calculated using the formula: tapped density=mass of powder(M)/tapped volume of powder (VT)...(5).
Flow rate and angle of repose
A funnel was properly clamped on a retort stand. The funnel orifice diameter, base diameter, and efflux tube length were appropriately measured. A 10 g quantity of the sample was weighed out and gradually placed into the funnel with the funnel orifice closed with a shutter. The time taken for the entire sample in the funnel to flow through the orifice was noted. The flow rate was obtained by dividing the mass of the sample by the time of flow in seconds.
The static angle of repose was determined using the fixed base cone method [18-20]. About 10 g of the sample was transferred into an open-ended cylinder placed on a static base cone on a horizontal surface. The cylinder was gradually withdrawn vertically and the sample formed a cone-shaped heap. The height of the sample was determined using a cathetometer; the radius was obtained by dividing the fixed diameter by two. Angle of repose (θ) for each sample was obtained using the equation, θ=tan−1 (height/radius)…(6).
Compressibility index and Hausner’s quotient
Carr’s compressibility indices [18] (%) of the lyophilized lipospheres were obtained using the formula, Carr’s index (%)=((tapped density-bulk density)/tapped density)×100…(7) and Hausner’ ratio was obtained using the formula, Hausner ratio=(tapped density/bulk density)…(8) [18].
Analgesic properties
Analgesic activity was tested in rats using the hot plate method described by Nkomo et al. [19]. All animal experimental protocols were carried out in accordance with guidelines of the Animal Ethics Committee of the Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka. Adult Wistar rats of either sex (120-205 g) were divided into six experimental groups of five rats per group. Diclofenac potassium-loaded lipospheres equivalent to 5 mg/kg of diclofenac potassium were weighed out, dispersed in 0.5 ml of water, and administered orally to the rats using a 1 ml syringe. The control groups received normal saline 5 ml/kg while the reference group received 5 mg/kg of diclofenac potassium pure sample.
Rats were placed on a hot plate maintained at 55±1° and the reaction latency in (seconds) for licking of hind paw or jumping was recorded. The rats which reacted within 15 s and which did not show large variation when tested on three separate occasions were selected for the studies. Recordings were taken before treatment with the different drugs and 30 min, 1, 2, 3, 4, 5, 6, and 7 h posttreatment. Results were expressed as difference between the baseline reaction latency and the reaction latency at recorded times [21].
In vitro release studies
Beer’s plot was obtained for diclofenac potassium in simulated intestinal fluid (SIF, pH 7.5) at a concentration range of 0.1 to 1.0 mg% at a predetermined wavelength of 303 nm. The USP paddle method was adopted in this study. The dissolution medium consisted of 900 ml of freshly prepared medium (SIF, pH 7.5) maintained at 37±1°. The polycarbonate dialysis membrane (MWCO 6000-8000, Spectrum Labs, Breda, The Netherlands) selected was pretreated by soaking in the dissolution medium for 24 h prior to use. A quantity of SLM equivalent to 100 mg of diclofenac potassium was weighed and placed in a polycarbonate dialysis membrane containing 2 ml of the dissolution medium, securely tied with a thermoresistant thread and placed in the appropriate chamber of the release apparatus. The paddle was rotated at 100 rpm, and at predetermined time intervals, 5 ml portions of the dissolution medium was withdrawn, appropriately diluted, and analyzed for drug content in a spectrophotometer. Sink condition was maintained by replacing it with 5 ml of fresh medium immediately after each withdrawal.
Statistical analysis
Statistical analysis was done using SPSS version 14.0 (SPSS Inc. Chicago, IL.USA). All values were expressed as mean±SD. Data were analyzed by one-way ANOVA. Differences between means were assessed by a two-tailed Student’s t-test. P<0.05 was considered statistically significant.
Results and Discussion
The result of percentage yield showed that batches A1-A4 SLMs formulated with SRMS 1:1 (Phospholipon® 90H:Softisan® 154) and containing 1, 3, 5, and 0% w/w of diclofenac potassium had 85.9, 85.5, 81, and 69% yield, respectively. Also batches B1-B4 SLMs, formulated with SRMS 2:1 had percentage yield of 81.5, 99.9, 99.5, and 65.0%, respectively. Similarly, batches C1-C4 SLMs, formulated with SRMS 1:2 had percentage yield values of 91, 90, 90, and 69%, respectively. The percentage of the lipospheres obtained from the formulations presented indicated that all the diclofenac potassium-loaded lipospheres exhibited higher percentage yield than the unloaded lipospheres (batches A4, B4, and C4). This is a strong indication that the formulation technique adopted was reliable.
The DSC profiles of Phospholipon® 90H, Softisan® 154, SRMS 1:1, 1:2 and 2:1 and lipospheres loaded with 1% of diclofenac potassium and lipospheres containing no API are shown in Table 2. From the results, the DSC curve of diclofenac potassium pure sample showed a sharp melting peak at 311.4°. This sharp transition showed that diclofenac potassium used was pure and crystalline and was comparable to the melting point reported for diclofenac in BP [18].
| Material | Melting peak (oC) |
|---|---|
| Softisan® 154 | 61.4 |
| Phospholipon 90H | 124.0 |
| Diclofenac potassium | 311.4 |
| SRMS 1:1 | 65.5 |
| SRMS 1:2 | 62.3 |
| SRMS 2:1 | 64.4 |
| A1 | 300.0 |
| B1 | 300.0 |
| C1 | 300.0 |
SRMS: Solidified reverse micellar solution, A1, B1 and C1: SLMs formulated with SRMS 1:1, 2:1 and 1:2 containing 1 % diclofenac potassium.
Table 2: DSC melting peaks of SRMS-based diclofenac-loaded SLMS.
DSC thermogram of Softisan® 154 showed a melting peak at temperature 61.4°. Phospholipon® 90H showed a melting transition at temperature of 124°. The thermogram shows that Phospholipon® 90H consist entirely of stable form because of the sharp and narrow nature of the transition. Thermograms of the drug-free lipid matrices (SRMS 1:1, 1:2, and 2:1) showed sharp endothermic peaks at 65.5, 62.3, and 64.4°, respectively. The DSC results of the lipid matrices showed that the structuring of Softisan® 154 with Phospholipon® 90H generally produced matrices with low enthalpies, without grossly affecting their melting peaks. Reduction in enthalpy generally suggests less crystallinity of lipid matrices [21,22]. Diclofenac potassium-loaded lipospheres prepared with lipid matrix 1:1, 1:2, and 2:1 and containing 1% diclofenac potassium showed transition at 300°. Diclofenac potassium-loaded lipospheres prepared with lipid matrix 1:1, 1:2 and 2:1 and containing 1% diclofenac potassium showed several peaks corresponding to the components. The broad transition showed the presence of diclofenac potassium but with reduced crystallinity. Lower enthalpy suggests less crystallinity and the possibility of retention of an entrapped drug over time [9,21,22].
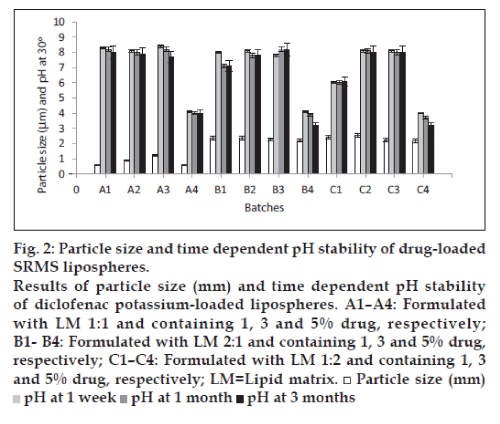
The photomicrographs of lipospheres presented in fig. 1a-c, showed that the shapes of diclofenac potassium-loaded lipospheres formulated were spherical. From the results of particle size presented in fig. 2, it can be seen that diclofenac potassium lipospheres prepared with the lipid matrix, SRMS 1:1 (A1-A3) exhibited the lowest mean particle sizes, while lipospheres prepared with the lipid matrix, SRMS 1:2 and containing 1 and 3% diclofenac potassium, i.e., batches C2 and C3 had the largest mean particle diameter of 2.44±0.07 and 2.55±0.04 µm, respectively. Particle size may be a function of either one or more of the following: Formulation excipients, degree of homogenization, homogenization pressure, rate of particle size growth, and crystalline habit of the particle [1]. Particle size was affected by the amount of drug incorporated into the lipospheres and the ratio/combination of lipid matrix used in the formulation as shown in fig. 2. Increase in amount of drug incorporated into the lipospheres increased the particle size. The ratios of the two lipids used in the formulation of the lipid matrices also affected the size of the lipospheres. The results indicated that the encapsulated drug may be in the inner core of the lipospheres. This may be the possible reason for the increased particle size seen in lipospheres formulated with SRMS 1:2 lipid matrix, containing higher Softisan® 154 ratios. The particle size, however, varied significantly (P<0.05) within the subbatches and across the batches.
From the results of pH studies carried out on of diclofenac potassium-loaded lipospheres presented also in fig. 2, the lipospheres formulated with SRMS 1:1 lipid matrix and containing no drug (A4) had a stable pH of 4.0. The pH of the lipospheres formulated with lipid matrices with ratios 1:2 and 2:1 (phospholipid: Softisan® 154) exhibited significant reduction (P<0.05) in pH of approximately 4.0 to 3.2 in 3 months. For the lipospheres loaded with diclofenac potassium, the pH values ranged from 6.0 to 8.4 in 1 week, 6.0 to 8.2 in 1 month, and 6.1 to 8.2 in 3 months. However, the decrease in pH of the lipospheres indicated that the preparation would need a buffer to keep the pH more stable. The slight decline in the pH values in most liposphere formulations was not attributed to drug degradation since there was also a fall in the pH of the unloaded lipospheres [1]. This phenomenon could be attributed to the liberation of free fatty acids from the lipid matrices on storage.
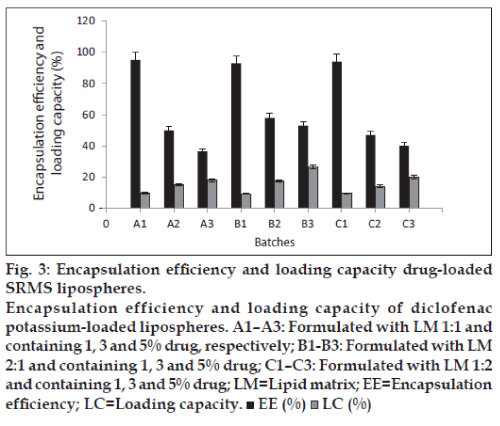
The ability of the lipospheres to accommodate active molecules is an important property and this can be expressed by the entrapment efficiency (EE%) and loading capacity. EE% defines the ratio between the weight of entrapped API and the total weight of API added to the dispersion, while LC expresses the ratio between the entrapped API and the total weight of the lipids [1]. Generally, the EE% and LC were affected by the total amount of API in the formulations as shown in fig. 3. From the results, lipospheres loaded with 1% diclofenac potassium had the highest encapsulation efficiency, followed by lipospheres containing 3% drug loading. The lipid matrix with 5% drug loading had lowest encapsulation efficiency, which may be as a result of saturation of the lipid matrix at high drug loading. LC increased with the increase in drug content, as lipospheres loaded with 5% diclofenac potassium had higher LC than those loaded with 3 and 1% API. Both EE% and LC are dependent on several parameters, such as the lipophilic properties of the API, the screening of the most appropriate lipid composition/ratio and surfactant combination, as well as the production procedures used [1].
The lyophilized diclofenac potassium-loaded lipospheres were further characterized in terms of their flowability. This is because of the possibility of presenting the formulations in capsule or tablet form, or as granules for reconstitution. The results of micromeritic properties presented in Table 3 showed that the micromeritic properties of diclofenac lipospheres could not be assessed by the direct method of flow under gravity. However, the indirect methods of assessing powder fluidity were employed. From the result presented in Table 3, the samples had decreased bulk density range of 0.090±0.006 to 0.160±0.005 g/ml and tapped density range of 0.120±0.017 to 0.300±0.010 g/ml. Powder fluidity is important in capsule filling because variability in flow rate will automatically cause variability in capsule filled weight and active ingredient variation. A decrease in bulk density may be associated with a reduction in particle size and a loose packed powder bed, which is unlikely to flow because of inherent cohesiveness of the fine particles. Conversely, an increase in bulk density causes a decrease in porosity, and there is an increase in interparticulate contact. From the result presented in Table 3, the samples had decreased bulk density, showing poor flowability of the samples. Angle of repose was used as indirect methods of assessing flowability of powder because of their relationship with inter particle cohesion. The result of angle of repose showed that the lipospheres had poor flowability. Lipospheres formulated with SRMS 1:1 (A1-A4) exhibited angle of repose that ranged from 32.4±0.5 to 38.1±0.4° which indicated that the lipospheres exhibited poor, but passable flow. Other batches of diclofenac potassium-loaded SLMs showed angle of repose above 40°. Drug incorporation affected the flow properties because of changes in size and size distribution, particle density, structure, and geometry of the lipospheres due to drug encapsulation. Hausner’s ratio of less than or equal to 1.25 indicate good flow, while values greater than 1.25 indicate poor flow [14]. Therefore, batches A1, A2 and C3 lipospheres, loaded with 1, 3 and 5% diclofenac potassium and batch B4 lipospheres formulated with SRMS 2:1 without any loaded drug had good flow. Carr’s index in the range of 5-16 indicates good flow, 18-21 shows fair flow, while values above 38 shows very poor flow [16]. Therefore, batches A2 and A3 formulated with SRMS 1:1 and loaded with 1 and 5% diclofenac potassium exhibited good flow. The flow rate of diclofenac potassium-loaded lipospheres based on SRMS was affected by the ratio/composition of the lipid matrix used in the formulation. Lipospheres formulated with lipid matrix having equal ratios of phospholipid and Softisan®154 or having higher ratios of phospholipid (SRMS 1:1 and 2:1) significantly exhibited fair flow more than lipospheres containing higher ratio of Softisan®154 (1:2) as shown in Table 3. However, some factors can affect the flow rate of particles. They include particle size, particle size distribution, particle shape, particle density, particle cohesion, moisture content, and relative humidity of the environment among others [16]. The flow rate of the diclofenac potassium lipospheres based on SRMS can be improved by reducing the ratio of triglyceride in the formulation, addition of glidants which will reduce the interparticulate friction, e.g., Cab-O-Sil and talc and proper storage of lipospheres in a moisture resistant container.
| LM/Drug ratio | Batch | ℓB (g/ml)* | ℓT (g/ml)* | A.R. (°)* | H.R. | C.I. (%) |
|---|---|---|---|---|---|---|
| 1:1 (1) | A1 | 0.160±0.005 | 0.240±0.006 | 38.1±0.4 | 1.50 | 33.3 |
| 1:1 (3) | A2 | 0.090±0.006 | 0.140±0.016 | 32.4±0.5 | 1.56 | 35.7 |
| 1:1 (5) | A3 | 0.100±0.008 | 0.120±0.017 | 33.0±0.6 | 1.20 | 16.7 |
| 1:1 (0) | A4 | 0.110±0.005 | 0.120±0.016 | 36.8±0.4 | 1.09 | 8.3 |
| 2:1 (1) | B1 | 0.120±0.005 | 0.150±0.005 | 46.8±0.1 | 1.25 | 20.0 |
| 2:1 (3) | B2 | 0.140±0.005 | 0.220±0.010 | 45.0±0.6 | 1.57 | 36.4 |
| 2:1 (5) | B3 | 0.100±0.006 | 0.200±0.010 | 48.0±0.1 | 2.00 | 50.0 |
| 2:1 (0) | B4 | 0.100±0.001 | 0.170±0.070 | 43.1±0.5 | 1.70 | 41.2 |
| 1:2 (1) | C1 | 0.210±0.006 | 0.300±0.010 | 46.3±0.4 | 1.43 | 30.0 |
| 1:2 (3) | C2 | 0.130±0.001 | 0.210±0.060 | 42.5±0.5 | 1.62 | 38.1 |
| 1:2 (5) | C3 | 0.120±0.006 | 0.180±0.010 | 43.1±0.5 | 1.50 | 33.3 |
| 1:2 (0) | C4 | 0.130±0.005 | 0.160±0.010 | 44.7±0.4 | 1.23 | 18.8 |
Values shown are mean±SD (*n=3), A, B, and C=diclofenac potassium-loaded SLMs, ℓB and ℓT=bulk and tapped densities, AR=angle of repose, HR=Hausner’s ratio, CI=Carr’s compressibility index , LM= lipid matrix.
Table 3: Flow properties of the lipospheres.
The result of analgesic/antinociceptive properties of diclofenac potassium lipospheres shown in Table 4 showed that the lipospheres increased the antinociceptive time from 3-4 s at 30 min to 18-20 s at 7 h. The diclofenac potassium-loaded lipospheres had higher analgesic effect significantly different from the reference drug (P<0.05). At 7 h there was an increase in antinociceptive time of the liposphere formulations, unlike the reference drug which had a decrease in the pain reaction time from 18.96 s at 6 h to 15.26 s at 7 h as shown in Table 4. The reference drug exhibited maximum analgesic effect at 6 h while the diclofenac potassium-loaded lipospheres could not reach maximum analgesic effect at 7 h. This result showed that the liposphere formulations of diclofenac potassium had very good sustained release effect.
| Group | 30 min | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | 7 h |
|---|---|---|---|---|---|---|---|---|
| A1 | 3.20 ±1.08 | 6.30 ± 0.58* | 8.30 ± 1.24* | 10.00 ± 2.54* | 12.30 ± 1.80* | 15.00 ± 1.32* | 16.50 ± 1.20* | 20.40 ± 2.95* |
| B1 | 3.10 ±0.46 | 5.20 ± 1.52* | 8.20 ± 0.43* | 9.70 ± 1.07* | 12.90 ± 2.91* | 15.70 ± 2.25* | 17.70 ± 1.52* | 19.00 ± 1.16* |
| C1 | 3.50 ±0.66 | 7.10 ± 2.04* | 11.30 ± 1.84* | 12.30 ± 1.52* | 13.70 ± 1.62* | 16.20 ± 1.24* | 17.40 ± 0.83* | 18.80 ± 1.06* |
| D | 4.30 ± 0.97 | 7.00 ± 1.57* | 9.20 ± 1.75* | 11.60 ± 2.60* | 14.90 ± 2.60* | 15.70 ± 2.18* | 18.90 ± 1.59* | 15.20 ± 2.03* |
| E | 2.70 ± 0.66 | 2.70 ± 0.91 | 3.60 ± 0.48 | 3.10 ± 0.49 | 3.30± 0.50 | 4.00 ± 0.93 | 3.70 ± 0.74 | 3.70 ± 0.88 |
*Significant at P<0.05 compared to control; Values shown are mean±SD (n=5), A1, B1, and C1=Diclofenac potassium-loaded lipospheres, D=Pure diclofenac potassium and E=Control
Table 4: Analgesic/antinociceptive properties of diclofenac potassium-loaded lipospheres.
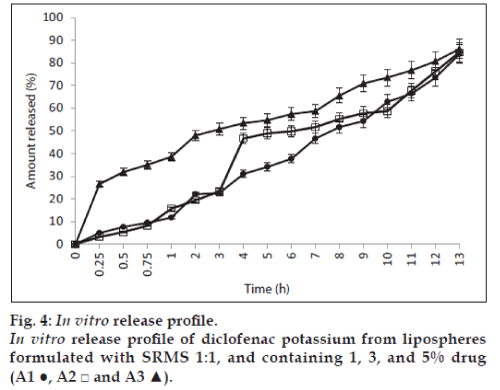
The results of the in vitro release of the lipospheres studied using lipospheres formulated with lipid matrix ratio 1:1 is shown in fig. 4 and show that batches A1, A2 and A3 containing 1, 3 and 5% of diclofenac potassium exhibited 11.7, 15.1, and 38.6% release of diclofenac potassium at 60 min (T60). The lipospheres, however, showed about 84 to 86% drug release at 13 h. The results showed that drug release was affected by the amount of drug loading. Batch A3 containing 5% of diclofenac potassium had higher release of drug at different time intervals and also exhibited an initial burst effect as shown in fig. 4. This may be due to saturation of the lipid matrix with increased drug loading leading to the presence of encapsulated drug in the periphery of the lipospheres. The results showed that the formulations had good sustained release properties.
Acknowledgements
We thank Phospholipid GmbH, Köln, Germany for providing sample of Phospholipon® 90H.
References
- Attama AA, Okafor CE, Builders PF, Okorie O. Formulation and in vitro evaluation of a PEGylated microscopic lipospheres deliverysystem for ceftriaxone sodium. Drug Deliv 2009;16:448-616.
- Rawat M, Saraf S. Liposphere: Emerging carries in delivery of proteins and peptides. Int J Pharm SciNanotechnol 2008;1:207-14.
- Chime SA, Attama AA, Onunkwo GC. Sustained release indomethacin-loaded solid lipid microparticles, based on solidified reverse micellar solution (SRMS): In vitro and in vivo evaluation. J Drug DelivSciTechnol 2012;22:485-92.
- Maha N, Samar M, Nahed DM, El Shamy AA. Lipospheres as carriers for topical delivery of aceclofenac: Preparation, characterization and in vivo evaluation. AAPS PharmSciTech 2008;9:154-62.
- Masters DB, Domb AJ. Lipospheres local anesthetic timed release for perineural site application. Pharm Res 1998;15:1038-45.
- Khopade AJ, Jain NK. Long circulating lipospheres targeted to inflamed tissue. Pharmizie 1997;52:165-6.
- Amselem S, Alving CR, Domb AJ. Lipospheres for vaccine delivery systems. Drugs Pharm Sci 1996;77:149-68.
- Domb AJ, Marlinsky A, Maniar M, Teomim L. Insect repellant formulations of N.N diethyl-m-toluamide (DEET) in a liposphere system: Efficiency and skin uptake. J Am Mosq Control Assoc1995;11:29-34.
- Domb AJ, Bergelson L, Amselem S. Lipospheres for controlled delivery of substances. In: Benita S, editor. Microencapsulation Method and Industrial Applications. NY: Marcel Dekker Inc.; 1996. p. 337-410.
- Friedrich I, Müller-Goymann CC. Characterization of solidified reverse micellar solutions (SRMS) and production development of SRMS-based nanosuspension. Eur J Pharm Biopharm 2003;56:111-9.
- Umeyor EC, Kenechukwu FC, Ogbonna JD, Chime SA, Attama AA. Preparation of novel solid lipid microparticles loaded with gentamicin and its evaluation in vitro and in vivo. J Microencapsul 2012;29:296-307.
- Schneeweis A, Muller-Goymann CC. Controlled release of solid-reversed micellar-solution (SRMS) suppositories containing metoclopramide-HCl. Int J Pharm 2000;196:193-6.
- Friedrich I, Reichl S, Muller-Goymann CC. Drug release and permeation studies of nanosuspensions based on solidified reverse micellar solutions (SRMS). Int J Pharm 2005;305:167-75.
- Jaspart S, Bertholet P, Piel G, Dogne JM, Delattre L, Evrad B. Solid lipid microparticles as sustained release system for pulmonary drug delivery. Eur J Pharm Biopharm 2007;65:47-56.
- Attama AA, Nkemnele MO. In vitro evaluation of drug release from self micro-emulsifying drug delivery systems using a biodegradable homolipid from Capra hircus.Int J Pharm 2005;304-10.
- Aulton ME. Pharmaceutics: The Science of Dosage Form Design. 3rd ed. Edinburgh: Churchill Livingstone; 2007. p. 197-210.
- Ngwuluka NC, Idiakhoa BA, Nep EI, Ogaji I, Okafor SI. Formulation and evaluation of paracetamol tablets manufactured using the dried fruit of Phoenix dactylifera Linn as an excipient. Res Pharm Biotech 2010;2:25-32.
- Yüksel N, Türkmen B, Kurdoğlu AH, Başaran B, Erkin J, Baykara T. Lubricant efficiency of magnesium stearate in direct compressible powder mixtures comprising cellactose® 80 and pyridoxine hydrochloride. FABAD J Pharm Sci 2007;32:173-83.
- Nkomo M, Nkeh-Chungag BN, Kambizi L, Jamot EN, Iputo JE. Antinociceptive and antiinflammatory properties of Gunneraperpensa(Gunneraceae). Afr J Pharm Pharmacol 2010;4:263-9.
- British Pharmacopoeia, vol. 111. The Commission Office, London; 2009. p. 6578-85.
- Attama AA, Muller-Goymann CC. A critical study of novel physically structured lipid matrices composed of a homolipid from caprahircus and theobroma oil. Int J Pharm 2006;322:67-78.
- Attama AA, Muller-Goymann CC. Investigation of surface-modified solid lipid nanocontainers formulated with a heterolipid-templatedhomolipid. Int J Pharm 2007;334:179-89.