- *Corresponding Author:
- R. Dabur
Departments of Biochemistry, University College, Kurukshetra University, Kurukshetra-136 119
E-mail: rajeshdabur@yahoo.com
| Date of Submission | 13 March 2013 |
| Date of Revision | 11 October 2013 |
| Date of Acceptance | 16 October 2013 |
| Indian J Pharm Sci 2013;75(6):688-699 |
Abstract
In the pathogenesis of invasive pulmonary aspergillosis both fungal and host factors play roles. Though cytokines and phagocyte, as host factors, have been shown to participate in defence against Aspergillus species yet the role of cysteine proteases, that is cathepsins, a lysosomal enzymes of phagocytes, remains unknown in fungal infection. Studies are available which shows that cytokines regulate the cysteine proteases processed immune molecules for their further action but their relationship with each other under fungal infection is not clear. Therefore, in this study, we demonstrate the substantial role of cathepsins and cytokines in aspergillosis. In the present murine model of invasive pulmonary aspergillosis, on seventh day of Aspergillus fumigatus infection, both kidney and liver showed significant (P<0.05) fungal burdens, which was also confirmed by histological analyses. The activity profiles of four cathepsins in the kidney and liver tissue were analysed and correlated with blood cytokines level in the presence and absence of antifungal compounds (amphotericin B, a standard drug and 2-(3,4-dimethyl-2,5-dihydro-1H-pyrrole-2-yl)-1-methylethyl pentanoate, isolated in our laboratory from natural source) treatment. The data illustrate that the reduction in fungal load in both organs probably results in a decreased local inflammatory response, as measured by decreased levels of interleukin-4 and interleukin-10 and increased level of interferon gamma in the antifungal compounds treated mice. Interestingly, this altered level of cytokines relates well with the activity level of cathepsins, that is decreased in interleukines (interleukinL-4/interleukin-10) and cathepsins (cathepsin B, cathepsin C and cathepsin L); and increase in interferon gamma and cathepsin H levels in the mice treated with antifungal compounds were observed. These observations support not only the negative (cathepsin B, cathepsin C and cathepsin L) and positive (cathepsin H) role of cathepsins in aspergillosis but also prove the role of cytokines in remodelling of immune response. Overall, the study reveals a correlation between cathepsins and cytokines and their regulatory role in fungal mediated infection.
Keywords
Aspergillus fumigatus, cathepsins, cytokines, antifungal compounds, liver, kidney
Invasive pulmonary aspergillosis (IPA) is responsible for high morbidity and mortality of human life especially in immune-compromised patients such as individuals with renal/liver disease or transplant recipients, hepatocellular carcinoma and severe hepatitis [1-3]. If the infection does not get treated promptly, which initially affects lung tissue, move in the deeper tissues (such as heart, kidney and liver) and could lead to tissue damage and sepsis. Though antifungal drug market is in boom and an increased number of antifungal options significantly decreased the complications in patients with serious Aspergillus infections even than success rate to cure this disease is very low [4,5]. Various reasons are included behind this disappointment such as intrinsic drug resistance, wrong diagnosis and various host-related factors.
Diverse publications explain the importance of phagocytes to guard against fungi [6-9]. Reports are also available where it is proved that Aspergillus species cause infections in patients who have defective phagocytic function [8]. In other words, phagocytes, as one of the host factors, participate in body defence against Aspergillus species. Furthermore, it is also evident that cathepsins such as cathepsin B and L are highly active lysosomal enzymes and play very important role in phagocytosis [10]. Cathepsins are cysteine proteases, act as endopeptidases and are mainly involved in intracellular protein degradation and involved in large number of diseases [11,12]. They are optimally active in the slightly acidic milieu found in lysosomes. Additionally, cysteine proteases in lysosomes play another important role in the functional differentiation of MHC class II-restricted CD4+ T cells [13]. This differentiated immune molecule is then available for antigen presentation under the cytokines control especially interferon gamma (IFN-γ), which is known to potentiate the antifungal activity of macrophages [14] while other cytokines such as interleukin (IL)-4/IL-10 helps in proliferating fungal infection. These cytokines therefore act as another host factors, which have either beneficial or deleterious effect in fungal infection [15].
In recent years, various approaches have been made to eradicate the aspergillosis including the identification of novel antifungal compounds from natural resources. Our group also identified and isolated 2-(3,4-dimethyl-2,5-dihydro-1H-pyrrole- 2-yl)-1-methylethyl pentanoate (DHP), a novel antifungal compound, from Datura metel, which is active at both in vivo and in vitro conditions [16-20]. In the present study, effect of treatment of a standard drug (amphotericin B) and DHP on the profiles of cathepsins and cytokines were conducted and evaluated, and efforts were made to understand the complex interplay between host-related factors and fungal infection. Our research group recently published [21] a report on cathepsins and cytokines expression in the lung organ of IPA mice model and in the present study we are extending their role in deep seated organs. Therefore the overall rationale for this paper is to elaborate the previous work and to explore their expression at kidney and liver organs as well under fungal infection pathological conditions.
Materials and Methods
The 4-methoxy-β-naphthylamide substrates like Z-Phe-Arg-4mβNA, Z-Arg-Arg-4mβNA, Gly–Arg- 4mβNA and Leu-4mβNA were purchased from Bachem Feinchemikalein AG, Bubendorf, Switzerland. Amphotercin B (AmpB) was supplied by Sigma Chemical Co., St. Louis, USA. Cysteine-HCl was supplied by Loba chemical, India. A natural antifungal compound DHP was isolated and established its antifungal properties in both in vitro and in vivo level in the laboratory as described previously [18].
Pathogen cultivation and inoculum preparation
Standard strain (ITCC 4517) was obtained from Indian Agriculture Research Institute, Delhi, India. Organism was grown on Sabouraud dextrose agar (Merck) plated at 37° for 4 days and processed further in same way for inoculum preparations as described by Mittal et al. [21].
Preparation of whole culture filtrate
To study the direct effect of fungal secretary proteins on host factors, Aspergillus fumigatus was cultured in a Sabouraud dextrose agar broth. The medium was dispensed into 250 ml flasks and sterilised at 115o/10 psi for 15 min. The flasks were inoculated with conidia of A. fumigatus and incubated at 37o in a biological oxygen demand incubator for 7 days [17]. Culture filtrates from the culture were collected on seventh day. Filtrates were centrifuged (5000 g, 10 min) and subjected to lyophilisation. The concentrated culture filtrates were dialysed against 10 mM sodium acetate buffer, pH 6.2, with three changes of buffer over a period of 24 h. Dialysed sample of culture filtrate were lyophilised and stored at −80° for further use as fungal secretary protein antigen.
Grouping and treatment
Ethical clearance for the use of animals was obtained from institutional ethics committee. Six week old BALB/c mice of either sex were divided into five groups with six to eight animals in each group. The groups were designated as I-V as shown in Table 1. The mice of group I received only phosphate buffered saline (PBS) and acted as control group. Three groups (Group II, IV and V) of immune-compromised mice received ~ 2×107 conidia by nasal instillation of conidial suspension [18,22]. Groups IV and V were treated with six doses of antifungal compounds, that is DHP and AmpB of 250 and 3.0 mg/kg/day, respectively, from the next day after challenge with A. fumigatus conidia. Group III was treated (50 mg/kg body weight) intravenously with the whole culture filtrate (mixture of fungal secretary proteins only). This mice group is used only to see the direct effect of fungal secretary proteins on the cathepsins and cytokines profiles. In the present report, two antifungal compounds were used; DHP (purified in our laboratory) and AmpB (standard drug) to study the comparative effect between these compounds on fungal infection. Animals were euthanised on the seventh day and kidney, liver and blood samples were collected under aseptic conditions for biochemical and histological studies. Prior to nasal instillation of fungal spores, three groups of BALB/c mice (Group II, IV and V) were administered with cortisone acetate (100 mg/kg/day) subcutaneously for three consecutive days, followed by a single injection per day throughout the postinfection period. Control mice (Group I) were inoculated with an equivalent volume of saline.
| Groups* | Treatment | Symbol used |
|---|---|---|
| in figures | ||
| I | Control (Normal animal) | Normal |
| II | Animals infected with A. fumigatus | Infected |
| III | Fungal secretary proteins for | Antigens |
| direct infection (whole culture | ||
| filtrate) | ||
| IV | Aspergillosis model treated with | Treated (DHP) |
| purified anti‑fungal compound, | ||
| DHP | ||
| V | Aspergillosis model treated with | Treated (AmpB) |
| standard drug, Amphotericin B |
*n=4, four mice per group, DHP= ???, AMPB= ???
Table 1: Groups Of Animals And Their Treatment
Quantification of colony forming units
The colony forming units (CFUs), indicating fungal loads, in various organs of animals were determined as described by Dabur et al. Briefly, the organs of the mice (n=4) were removed aseptically and homogenised in sterile PBS (pH 7.2). Dilutions (10 fold) of organ homogenates were placed on Sabouraud dextrose agar containing 0.05% Triton X-100, and the CFU were counted after incubation at 37° for 48 h.
Histopathology
Aseptically isolated tissues were processed immediately for histological examination by fixing them in 10% neutral buffered formalin and sectioned in a microtome cryostat as explained by Johnson et al. [23]. For the assessment of tissue morphology, 10 μm thick transverse sections of tissues were stained with haematoxylin and eosin (H and E) and examined under a microscope (Eclipse Ti; Nikon) with a digital camera (Nikon Digital Sight DS-Fi1). Image levels were equally adjusted using Nikon NIS Elements BR 3.00 image software.
Cytokine profile
Serum levels of IL-4 and IFN-γ were determined by performing enzyme linked immune sorbent assay (ELISA) in immunoplates (Nunc, Maxisorb). The kit for the estimation of IgE, IL-4, IL-10 and IFN-γ were purchased from Invitrogen, carlsbad, USA and ELISA was performed as per the manufacturer’s instructions. The OD was read at 490 nm in an automated ELISA reader (Molecular Devices, Spectra Max 190). Standard samples of respective interleukins were run in the test for quantification of IL-4 and IFN-γ in the sera samples.
Assay of acid phosphatase
Acid phosphatase assay was performed according to the method of Shiloko and Tappel [24]. Briefly, lung lysosomal fraction was incubated with 32 mM p-nitrophenyl phosphate (PNPP, disodium salt) in 0.2 M sodium acetate buffer (pH 5.5) for 15 min at 37°. The reaction was stopped with 0.16 M Tris–HCl (pH 8.5) containing 0.06 M K2HPO4. The reaction product was measured at 420 nm. This enzyme was used as a lysosomal marker and assay was performed (data not shown), which not only rule out any chance of impurities due to fungal secretary cysteine proteases but also supports that cathepsins assays were carried out in the lysosomal fractions of the host cells.
Preparation of lysosomal fraction for enzyme assay
Lysosomal fraction was prepared (per gram wet tissue weight) in ice cold 0.1 M sodium acetate buffer pH 5.5 containing 0.2 M NaCl, 1 mM EDTA as described by Kamboj et al. [25].
Assay of cathepsin B, L and H
Buffered activator was prepared fresh by dissolving 7 mg of cysteine-HCl in 14 ml of 0.1 M EDTA-phosphate buffer having pH 6.0 containing 1.33 mM EDTA. It was prepared fresh by mixing equal volumes of solution A (EDTA) and p-chloromercuricbenzoic acid (pH 6.0) and solution B (1 mg Fast Garnet GBC per ml water).
To 150 μl of buffered activator, 100 μl enzyme extract was added and after preincubation for 10 min at 37°, the enzymatic reaction was initiated by adding 10 μl of substrate stock solution. After 15 min, 150 μl of coupling reagent was added and mixed well and was allowed to develop the colour for 10 min. The red colour was extracted with 200 μl of n-butanol and intensity of colour was estimated at 520 nm. Blank experiments were also included where the enzyme samples were added after coupling reagent. To calculate specific activities for cathepsin B and L, enzyme, assays were performed by using only one synthetic chromogenic substrate, that is Z-Phe-Arg-4mβNA (6 mg/ml DMSO) in presence of 4 M urea as explained by Kamboj et al. [25] because at this concentration of urea, CPB gets denatured completely and only cathepsin L (CPL) shows its activity. For CPB assay, we also used one more CPB specific substrate, that is Z-Arg-Arg-4mβNA (8 mg/ml DMSO). For cathepsin H assay, we used Leu-4mβNA (40 mg/ml DMSO) as a substrate for this enzyme. One unit of enzyme activity is expressed as that amount of enzyme, which releases one picomoles of 4mβNA per min [26].
Assay of cathepsin C (dipeptidylpeptidase I)
Substrate Gly-Arg-4mβNA (1 mg/ml DMSO) was used to measure cathepsin C activity [27]. To 100 μl of enzyme sample, 150 μl of 0.1 M sodium succinate buffer containing 25 mM NaCl, 2.66 mM cysteine and 1.33 mM EDTA was added. The reaction mixture was incubated for 10 min at 37° and the enzymatic reaction was started by addition of 10 μl of substrate stock solution. After 15 min, 150 μl of coupling reagent was added and allowed to develop colour for 10 min. The red colour was extracted in 200 μl of n-butanol and intensity was estimated at 520 nm. Blank experiments were also included where the enzyme samples were added after coupling reagent. One unit of enzyme activity is expressed as that amount of enzyme, which releases one picomoles of 4mβNA per min.
Enzyme activity calculations
The activity of cathepsins was calculated in terms of picomoles of 4mβNA released per min per ml enzyme homogenate by using the formula, Activity (picomol/min/ml)=(OD520×1012×0.2-3×0.2)/(E×t). Where, E is the molar extinction coefficient of 4mβNA, which equals 36 600 under the assay conditions; 1012 is used for converting moles into picomoles; t is the time of incubation in minutes; 0.2×10−3 is the volume of n-butanol in litres used for extracting the red colour and 10 is used because only 100 μl of enzyme sample is employed.
Statistical analysis
Results are expressed as mean±SD. The two-tailed Student’s t-test and one-way analysis of variance (ANOVA) with Tukey post-hoc test was used to determine significant differences between groups by the use of GraphPad Prism (GraphPad Software, San Diego, CA). A value of P<0.05 was considered statistically significant unless otherwise specified.
Results and Discussion
There are many reports on the immune system impaired by A. fumigatus but there are hardly any available data, which can explain the involvement of cytokines and cathepsins together in progression of IPA in any organ of the infected body. In 2011, our research laboratory reported a finding on these molecules in the lung tissue of IPA infected mice [21]. As lung is the first organ get affected by aspergillosis and if this disease is not treated promptly at this stage may leads to systemic aspergillosis and going to damage the deeper located organs such as heart, kidney, liver and others, and could lead to tissue sepsis. As our earlier study reports findings on these molecules from lung only, the overall rationale for this paper is to extend the previous work and to look their expression at kidney and liver organs as well.
In the present study, a murine model of IPA was developed in the same manner as described earlier [18]. To answer first two immediate questions, that is any link between cathepsins and IPA and to check propagation of fungal burden in the deeper organs, three groups of mice were prepared. One group was given only buffer solution and treated as control and second and third groups were infected with a specific dose of A. fumigatus conidia so that aspergillosis can propagate beyond the pulmonary system, that is up to liver and kidney. Third group of mice, also infected with A. fumigatus, was treated with a particular dose of DHP (250 mg DHP/kg body weight of mice), an antifungal compound isolated in our laboratory.
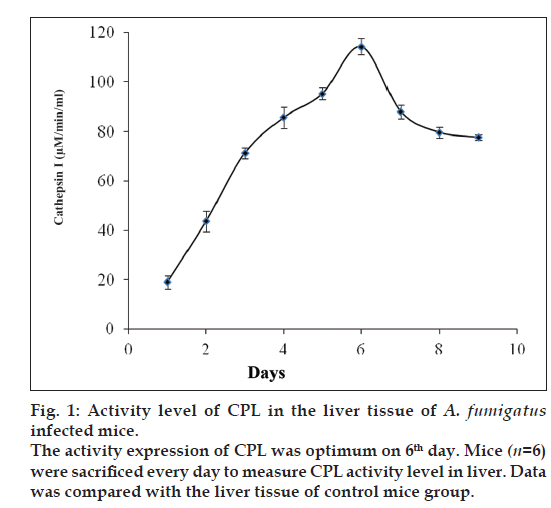
For initial correlation between cathepsins and IPA, A. fumigatus infected and control mice (n=4, each group) were sacrificed every day after infection and kidney and liver tissues were collected to study the activity level of an (CPL) by using a chromogenic substrates (Z-Phe-Arg-4mβNA). CPL was chosen for initial study being the most studied lysosomal enzyme under diverse pathological conditions. Interestingly, as shown in fig. 1, a regular increase in the CPL activity was observed in liver tissue till sixth day and then after it remains almost constant. Parallel observation was also noticed in kidney tissue (data not shown). Almost similar rise in CPL activity was reported previously [21] in the lung tissue of pulmonary aspergillosis mice model. Since CPL activity was observed optimum at sixth day and constant thereafter, therefore seventh day was selected to sacrifice all groups of mice for further analysis.
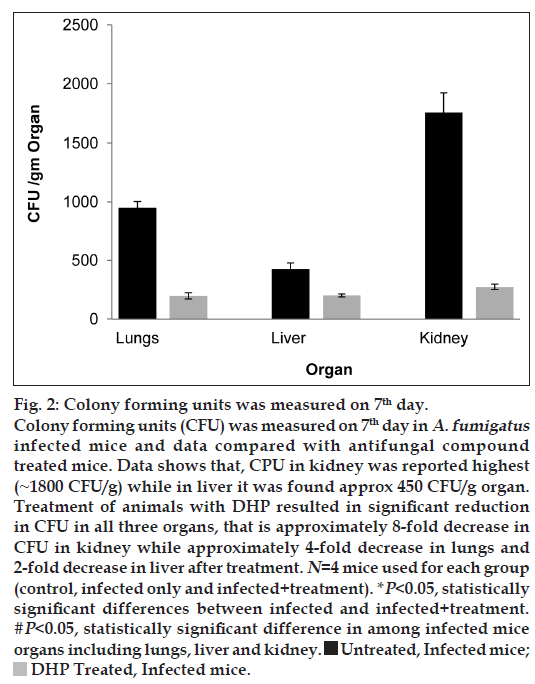
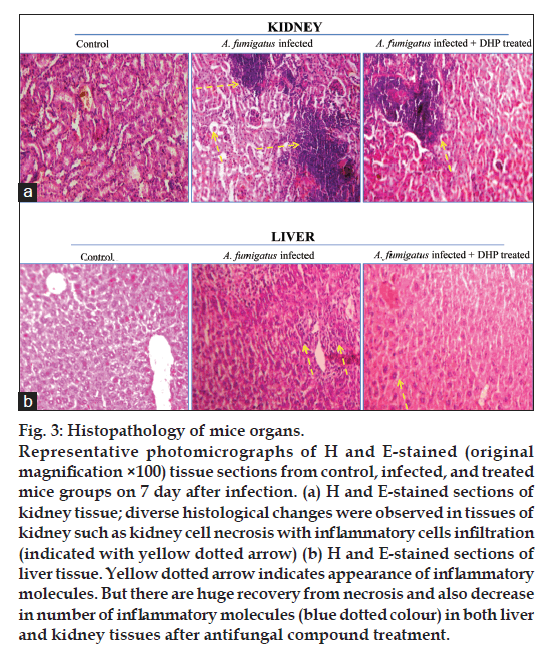
To answer another question, that is the fungal burden propagation in the initial (lungs) and deeper organs (liver and kidney) of the infected mice, CFU was measured only on the seventh day in A. fumigatus infected mice and data compared with antifungal compound treated mice. As shown in fig. 2, data explains not only the significant fungal burden propagation in the deeper organs of the infected mice but also depicts the strong protective effect of DHP on aspergillosis. This fact was further evaluated using histological approach. Fig. 3a and b, shows the large number of inflammatory molecules in both organs (kidney and liver) due to fungal infection. Necrosis was also observed in fungal infected kidney cells. But the level of these inflammatory molecules and cell necrosis gets decreased after DHP treatment. It is very clear that cathepsins has some role in fungal infection and infection propagates well in the deeper organs and IPA converts into systemic aspergillosis with time. In other words, our model is ready for further investigations.
Fig 2: Colony forming units was measured on 7th day.
Colony forming units (CFU) was measured on 7th day in A. fumigatus infected mice and data compared with antifungal compound
treated mice. Data shows that, CPU in kidney was reported highest
(~1800 CFU/g) while in liver it was found approx 450 CFU/g organ.
Treatment of animals with DHP resulted in significant reduction
in CFU in all three organs, that is approximately 8-fold decrease in
CFU in kidney while approximately 4-fold decrease in lungs and
2-fold decrease in liver after treatment. N=4 mice used for each group
(control, infected only and infected+treatment). *P<0.05, statistically
significant differences between infected and infected+treatment. #P<0.05, statistically significant difference in among infected mice
organs including lungs, liver and kidney  Untreated, Infected mice;
Untreated, Infected mice;  DHP Treated, Infected mice.
DHP Treated, Infected mice.
Fig 3: Histopathology of mice organs.
Representative photomicrographs of H and E-stained (original
magnification ×100) tissue sections from control, infected, and treated
mice groups on 7 day after infection. (a) H and E-stained sections of
kidney tissue; diverse histological changes were observed in tissues of
kidney such as kidney cell necrosis with inflammatory cells infiltration
(indicated with yellow dotted arrow) (b) H and E-stained sections of
liver tissue. Yellow dotted arrow indicates appearance of inflammatory
molecules. But there are huge recovery from necrosis and also decrease
in number of inflammatory molecules (blue dotted colour) in both liver
and kidney tissues after antifungal compound treatment.
To elaborate this correlation between cathepsins and fungal infection with more evidences, five groups of mice were made (Table 1) and studied the various cathepsins (CPB, CPL, CPC, CPH) activity profiles in the kidney and liver organs of the animals under investigations. Same groups of animals were also used for inflammation study by histological approach. As shown in Table 2, the scoring was performed on seventh day after A. fumigatus infection in three organs (lungs, kidney and liver) of all the animal groups used in our study as per Table 1. The symptoms in animals were graded on a scale from 0 to 4, where 0–no inflammation, 1–minimal, 2–mild, 3–moderate and 4–marked. The scores in group I animals were 0 while it observed 3 to 4 in all organs of the infected group (Group II). The histological examination of organs in the animals of Group V (250 mg/kg) showed no inflammation while there was minimal to mild inflammation in other experimental groups (Table 2). Data clearly depicts that A. fumigatus mediated inflammation has spread out even in the deeper organs and this condition get reversed by the treatment of antifungal compounds (DHP, AmpB). In this table, lung tissue data was incorporated only for relative study. Moreover, this histological data itself acts as a direct evidence to use present murine model for studying any role of cathepsins, cytokines as host factors in fungal mediated disease progression.
| Mice group no | Lung | Liver | Kidney |
|---|---|---|---|
| I | 0 | 0 | 0 |
| II | 4 | 3 | 3 |
| III | 3 | 3 | 2 |
| IV | 2 | 1 | 1 |
| V | 1 | 0 | 0 |
*n=4, four mice in each group. Grading: 0=None, 1=Minimal, 2=Mild, 3=Moderate, 4=Marked
Table 2: Scoring In Animals Based On The Histopathological Observations On The 7th Day Of Infection
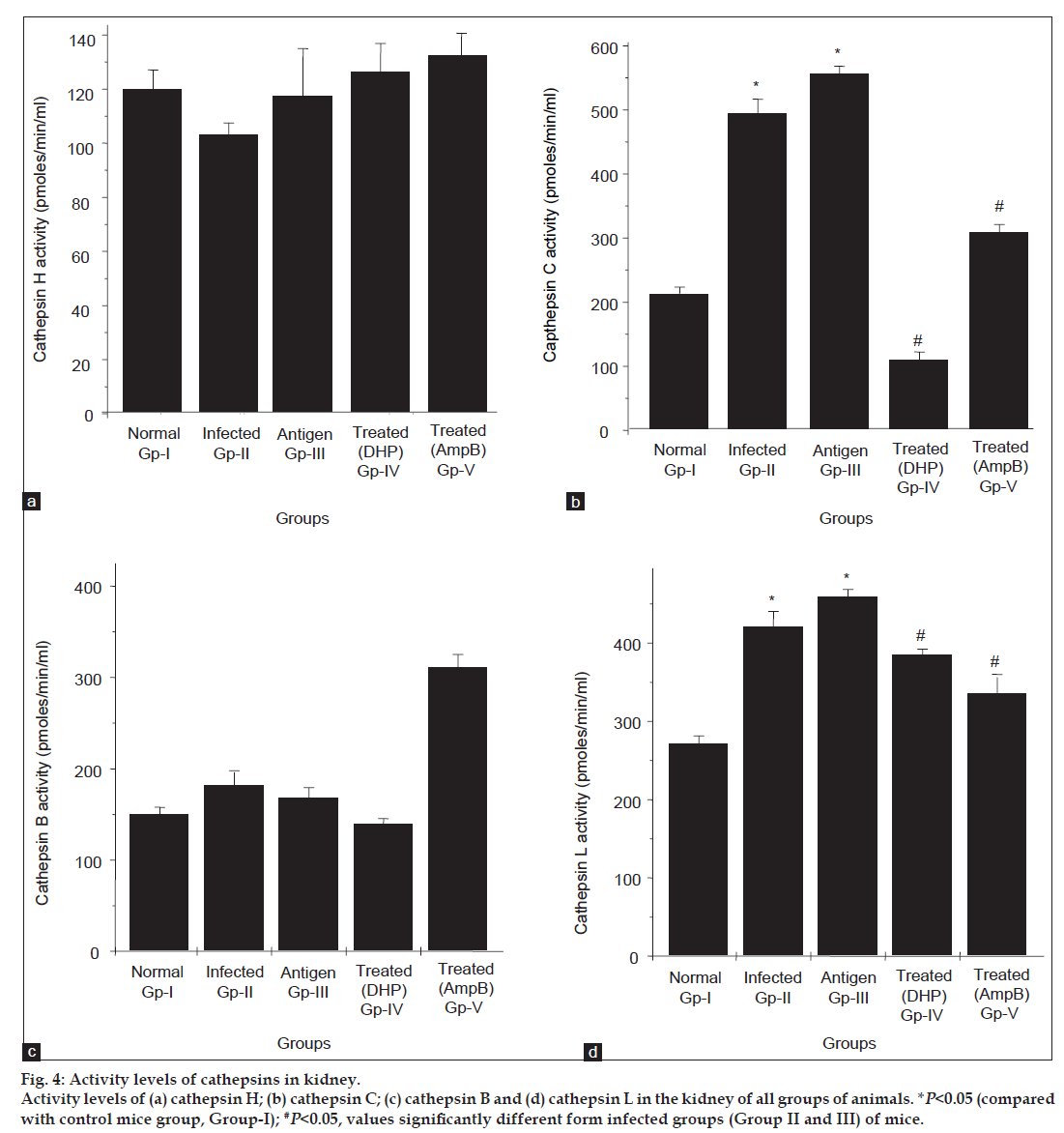
To explore any regulatory role of host-factor (specifically, cathepsins) in fungal mediated disease progression, we examined the activity profile of these enzymes (CPB, CPL, CPC, CPH) in all five groups, that is under infected and treated conditions. As seen in fig. 4b, A. fumigatus infection (Group II and III) resulted in a significant increase in CPC activity level, with a further ~3.0 fold increase above control. However, the activity of this enzyme was found low in groups (Group IV and V) treated with antifungal compounds. Likewise the activity level of another cathepsin, that is CPL was also observed high (~1.5 fold) in infected groups of mice if compared with control and this activity get decreased after antifungal treatment (fig. 4d). Downregulation in the activity of CPC (~100% inhibition by DHP and 60% inhibition by AmpB) and CPL (~20-30% inhibition by both compounds) after treatment indicates the physiological importance of these enzymes in progression of aspergillosis in kidney organ of the mice. Evidences are available which explained that cathepsin L activates cathepsin C, which further activates other cathepsins and enzymes [28]. Our data also supports this evidence, an elevation in CPL activity (fig. 4d, Group II and III) possibly activates CPC and once CPC gets activated, it may undergo autocatalysis, which further resulted more increase in the CPC activity (fig. 4b, Group II and III). Furthermore, literature reports that cathepsin C is one of the major processing enzymes known so far. It activates a number of serine granule enzymes, granzymes A and B, cathepsin G, neutrophil elastrase and chymase [29]. Likewise in antifungal treated groups a sharp decrease in CPC activity compared with CPL, further elaborate the possibility that after diminishing the effect of antifungal compounds CPL would reactivate the CPC activity. Additionally, elevated level of cathepsins is harmful to the cell/tissues as it may lead to cell death and help fungus in its progression. In contrast to these cathepsins, no significant change in the activity profile of CPB and CPH was observed in all groups of mice under investigations (fig. 4a and c).
Fig 4: Activity levels of cathepsins in kidney.
Activity levels of (a) cathepsin H; (b) cathepsin C; (c) cathepsin B and (d) cathepsin L in the kidney of all groups of animals. *P<0.05 (compared
with control mice group, Group-I); #P<0.05, values significantly different form infected groups (Group II and III) of mice.
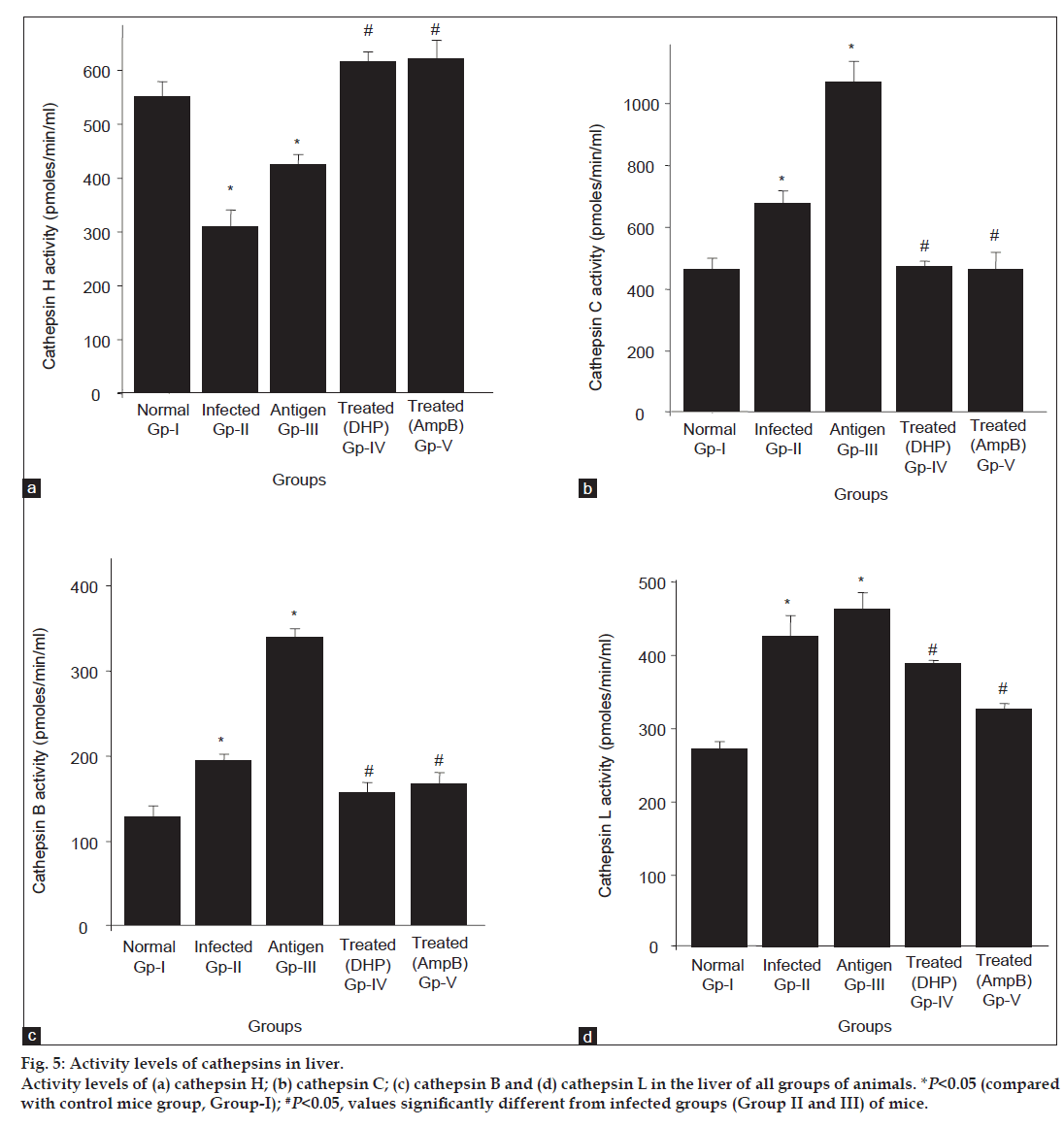
Similar to kidney, increased and decreased in the CPC and CPL activities (fig. 5b and d, respectively) were also observed in the liver tissues under both conditions, that is in the infected (Group II and III) and antifungal treated (Group IV and V) groups. While opposing to kidney, major alterations in the levels of other cathepsins (CPB and CPH) were also observed in the liver in all groups of compared with control. As shown in fig. 5c, CPB activity was also detected high (~1.5- to 3.0-fold) in the infected groups (Gp II and III) compared with control, however, this activity gets reversed after antifungal treatments and come to the level of control group. Contrary to all cathepsins studied under present investigations, CPH activity pattern was found altogether different in both conditions (fig. 5a). The activity of this enzyme was noticed significantly low in the liver tissue of the infected groups of mice (Group II and III) while its level gets up-regulated after the treatment (Group IV and V). In other words, CPH works opposite to CPB, CPC and CPL in both infected and treated groups of mice (fig. 5a). One possible reason could be the inhibitory effect of some metabolites secreted by fungus, which may be responsible for decreasing the CPH activity after infection but this regulatory effect of these metabolites gets reversed after antifungal treatment.
Fig 5: Activity levels of cathepsins in liver.
Activity levels of (a) cathepsin H; (b) cathepsin C; (c) cathepsin B and (d) cathepsin L in the liver of all groups of animals. *P<0.05 (compared
with control mice group, Group-I); #P<0.05, values significantly different from infected groups (Group II and III) of mice.
These findings from kidney and liver tissues provide direct link between cathepsins and fungus where these host lysosomal enzymes are playing either positive or negative role in fungal mediated disease progression. In our previous reports on these cathepsins from lung tissue [21], we also observed increased in the activity level of CPL and CPB after fungal infections while after treatment CPB activity gets down-regulated completely, whereas CPL activity level altered slightly. However, other cathepsins (CPC and CPH) from lung tissues of treated and untreated infected mice groups were not showing any significant alteration in their activity levels. The cathepsins data from lungs is relatively different than the data from kidney and liver organs. These variations in cathepsins levels depict clearly their differential role which seems very much organ specific.
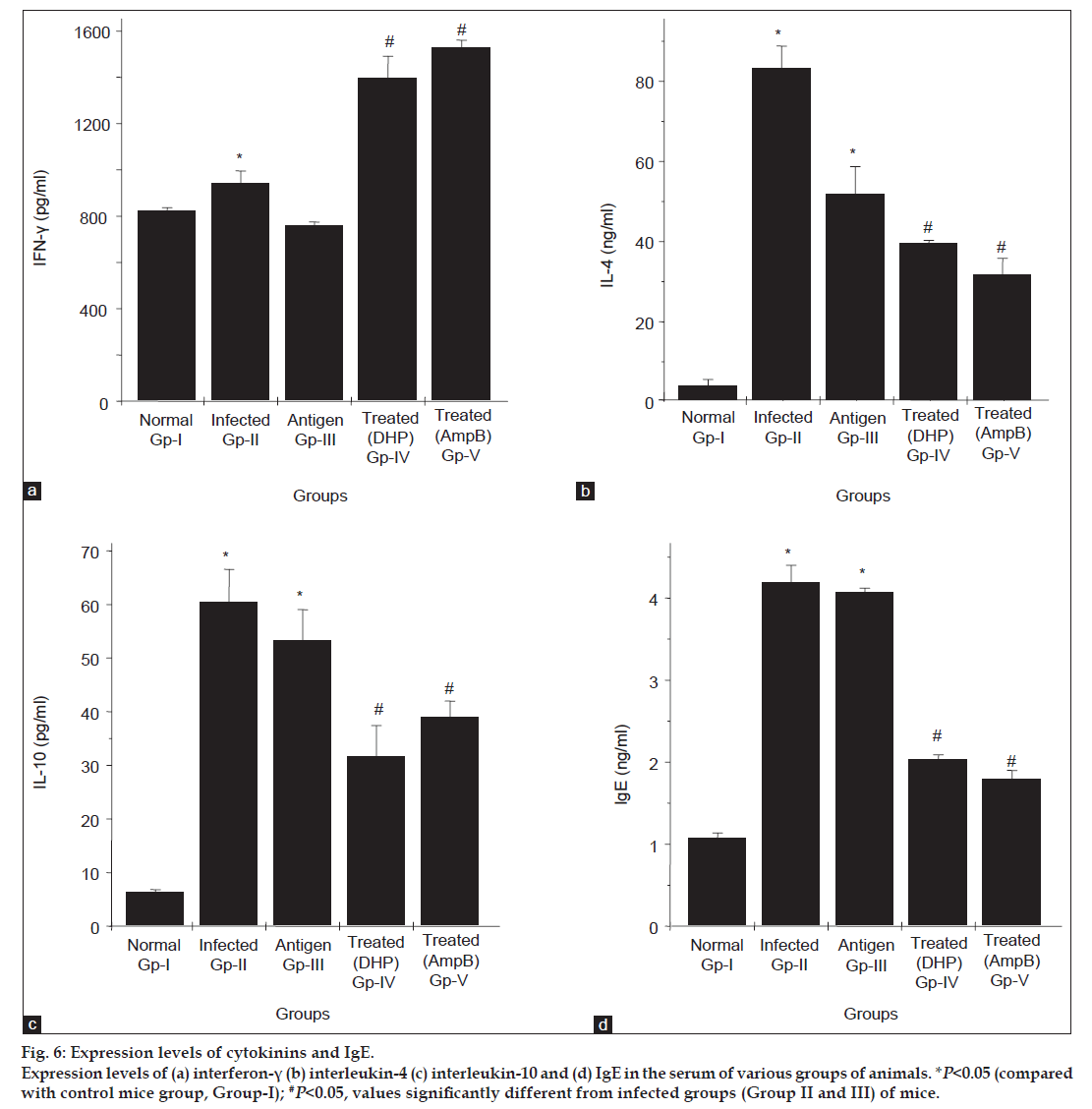
Cytokines specifically IL-4, IL-10 and IFN-γ are well reported to be involved in fungal mediated infections and play the essential role in immune-functional differentiation. To see the effect of A. fumigatus on these cytokines levels in IPA model, profiles of these cytokines in all five groups were analysed. As seen in fig. 6a and c, the serum levels for IL-4/ IL-10 decreased (~2 fold) while level found high for IFN-γ in the animals treated with antifungal compounds (Group IV and V) compared with infected mice groups (Group II and III). As evident through a published report [30] the level of IgE gets regulated by IL-4/IL-10, therefore the study was also carried out for IgE in all experimental groups. As per fig. 6d, the level of this antibody is found similar to the level of IL-4 and IL-10 in all groups of mice, that is its level increased in infected groups and decreased after treatment.
Fig 6: Expression levels of cytokinins and IgE.
Expression levels of (a) interferon-γ (b) interleukin-4 (c) interleukin-10 and (d) IgE in the serum of various groups of animals. *P<0.05 (compared
with control mice group, Group-I); #P<0.05, values significantly different from infected groups (Group II and III) of mice.
Aspergillosis is a challenging disease because of involvement of various confounding factors including cytokines. Some cytokines may have either beneficial or deleterious effect on fungal infection, which is further responsible for alterations in the immune-system of the body. Specifically, Th1 and Th2 pathways directly relate to the severity of infection. It is known that Th1 cells produce IFN-γ, which activates macrophages, the key effectors in invasive aspergillosis, whereas Th2 cells secrete IL-4 and IL-10, which activates B cells and switch antibody synthesis to IgE. In other words, after cytokine production, Th1 seems to be involved in the protection against microorganisms, whereas Th2 is involved in allergy and parasitic infections and the overall outcome may be worse [31] and may further be responsible for pathological changes in the body. In our present experimental model of aspergillosis (Group I-V), we exploited the divergent level of IFN-γ and IL-4/IL-10/ IgE. As per data seen in fig. 6b-d, decreased in the IL- 4, IL-10 and IgE levels and increased in IFN-γ level (fig. 6a) after treatment with antifungal compounds (Group IV and V) indicates not only the modulation of immune response from Th2 type to Th1 type but also supports the protective effect of antifungal compounds against fungal infection.
Interestingly, these alterations in the cytokines level correspond to the cathepsins activities, that is activity profile of CPC/CPL from kidney and CPC/CPL/CPB from liver (figs. 4b, 4d, 5b-d) match splendidly with IL-4/IL-10/IgE serum level under both infected and treated conditions. However, the activity level of other cathepsin, that is CPH (fig. 5a) was observed very much similar to IFN-γ level if we see antifungal treated mice groups (Group IV and V), that is level of IFN-γ and CPH was found significantly high in antifungal treated infected mice groups compared with infected groups only.
These outcomes explained the regulatory role of cathepsins in fungal disease. Our findings advocate that there are possibility of friendly relationship between some specific cathepsins (CPL/CPC, in both organs; CPB, in liver only) and fungus, that is these cathepsins are someway helping A. fumigatus for promoting systemic aspergillosis. Data also depicts that the level of these cathepsins are under the control of IL-4/IL-10. This interpretation is based on an observation given by Gocheva et al. [32] in cancer model. According to this group, IL-4 induces cathepsin protease activity in tumour associated macrophages to promote cancer growth and invasion. Beside the negative role of cathepsins in physiology, our data also supports the positive role of CPH in regulation of systemic aspergillosis, that is CPH (in liver only) seems to have any regulatory role, which does not allow fungus to cause infection and this cathepsin works under the command of IFN-γ. This elucidation supports findings of Lafuse et al. [33] and gave strong evidences that IFN-γ increases cathepsin H mRNA levels in mouse macrophages, while cytokines, IL-2, IL-4, IL-10 have no effect on cathepsin H mRNA levels in mouse peritoneal macrophages.
Besides this, reports are available where lysosomal cysteine proteases has been shown to be involved in the antigen processing and in differentiation of functional CD4+ T cell subsets [34]. By using various cysteine protease specific inhibitors, the roles of cathepsins are also established in immune system, for example CPB inhibitor CAO74 was reported to change the digestion pattern of lysosomal enzyme and modulate the immune response from Th2 type to Th1 type [35]. Similar modulation can also be predicted through our cathepsins and cytokines data. In the present experimental model after antifungal treatment, we observed significant decrease in the CPB/CPL/CPC/IL-4/IL-10/IgE level while level of CPH/IFN-γ was noticed to be increasing. These observations further prove the role of cathepsins and cytokines in remodelling of immune response.
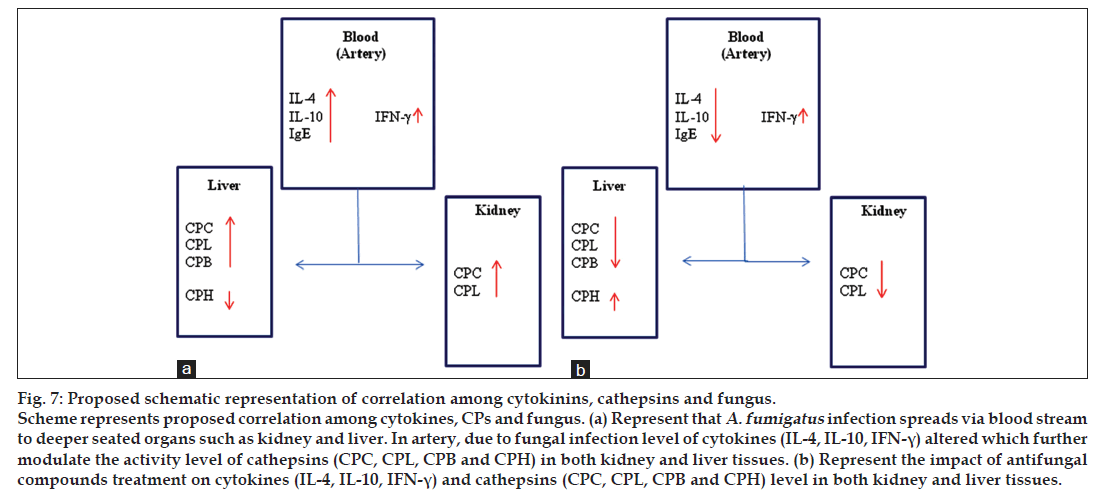
Cathepsins activity/protein levels have been reported diverse in various pathophysiological conditions. Reduction in the activities of CPB and CPL in diabetes, whereas increased activities of CPL in myocardium failure, kidney hypertensive models and various type of cancerous diseases were observed. Similarly, increase in CPB and CPL were reported in muscle of dystrophic Hamsters, but in same muscles no change was found for CPC level [36-38]. Also there is growing evidence that the expression of cathepsin H is increased in disease states including breast carcinoma, melanoma and prostate carcinoma [39-43]. In contrast, decreased cathepsin H expression has also been reported in squamous cell carcinoma of the head and neck [44]. These studies explain that cathepsins do not increase or decrease relative to other cathepsins rather individual cathepsin has its own level/ role, which may differ from other cathepsins in pathological conditions. Fig. 7 illustrates some key host factors, which seems to have any role in fungal mediated disease. Overall, present data uncovers a correlation among cathepsins, cytokines and fungus, which is a novel outcome and could be used further as a drug target to cure secondary effects of fungal diseases. In other words, antagonists for CPL/CPC/ CPB and agonist for CPH along with antifungal drugs may perhaps be used as a combinatorial approach to regulate aspergillosis.
Fig 7: Proposed schematic representation of correlation among cytokinins, cathepsins and fungus.
Scheme represents proposed correlation among cytokines, CPs and fungus. (a) Represent that A. fumigatus infection spreads via blood stream
to deeper seated organs such as kidney and liver. In artery, due to fungal infection level of cytokines (IL-4, IL-10, IFN-γ) altered which further
modulate the activity level of cathepsins (CPC, CPL, CPB and CPH) in both kidney and liver tissues. (b) Represent the impact of antifungal
compounds treatment on cytokines (IL-4, IL-10, IFN-γ) and cathepsins (CPC, CPL, CPB and CPH) level in both kidney and liver tissues.
In recent years, various approaches have been made to eradicate the aspergillosis. Despite significance advancement in antifungal therapies, overall mortality rate is still very high. We started to find new target biomolecules, which may involve directly or indirectly in fungal-mediated disease progression. Our recently published report on lung tissues clearly depicts the involvement of cathepsins and cytokines in pulmonary aspergillosis. The present data from kidney and liver organs of A. fumigatus infected mice further give us a support to develop relationship between these host factors in aspergillosis. Antifungal compound (DHP/ AmpB) treatment results, clearly indicate that these molecules are involved in aspergillosis. Although the specific role of these host factors such as cathepsins in the aspergillosis is still uncertain, yet the use of inhibitors/activators against the specific cysteine protease may enhance the potency of antifungal drugs under such pathological conditions. Moreover, this combinatorial approach may help in improving the quality of life of immuno-compromised persons. More studies are still needed to be carried out on these host factors pertaining to elaborate their relationship in A. fumigatus and also to establish their role in immune system modelling.
References
- Latgé JP. The pathology of Aspergillus fumigatus. Trends Microbiol 2001;9:382-9.
- Maschmeyer G, Haas A, Cornely OA. Invasive aspergillosis: Epidemiology, diagnosis and management in immunocompromised patients. Drugs 2007;67:1567-601.
- Kedziora K, Slominski JM, Gil K, Porzezinska M, Gorzewska A. Invasive aspergillosis of the paranasal sinuses, lung and brain. Pneumonol Alergol Pol 2008;76:400-6.
- Steinbach WJ, Stevens DA. Review of newer antifungal and immunomodulatory strategies for invasive aspergillosis. Clin Infect Dis 2003;37:S157-87.
- Enoch DA, Ludlam HA, Brown NM. Invasive fungal infections; A review of epidemiology and management options. J Med Microbiol 2006;55:809-18.
- Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest1982;69:617-31.
- Robertson MD, Seaton A, Milne LJ, Raeburn JA. Resistance of spores of Aspergillus fumigatus to ingestion by phagocytic cells. Thorax 1987;42:466-72.
- Cohen MS, Isturiz RE, Malech HL, Root RK, Wilfert CM, Gutman L, et al. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defense against fungi. Am J Med 1981;71:59-66.
- Park SJ, Burdick MD, Mehrad B. Neutrophils mediate maturation and efflux of lung dendritic cells in response to Aspergillus fumigatus germ tubes. Infect Immun 2012;80:1759-65.
- Rodriguez-Franco EJ, Cantres-Rosario YM, Plaud-Valentin M, Romeu R, Rodríguez Y, Skolasky R, et al. Dysregulation of macrophage-secreted cathepsin B contributes to HIV-1-linked neuronal apoptosis. PLoS One 2012;7:e36571.
- Sever S, Altintas MM, Nankoe SR, Möller CC, Ko D, Wei C, et al. Proteolytic processing of dynamin by cytoplasmiccathepsin L is a mechanism for proteinuric kidney disease. J Clin Invest 2007;117:2095-104.
- Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest 2010;120:3421-31.
- Zhang T, Maekawa Y, Hanba J, Dainichi T, Nashed BF, Hisaeda H, et al. Lysosomal cathepsin B plays an important role in antigenprocessing, while cathepsin D is involved in degradation of the invariant chain inovalbumin-immunized mice. Immunology 2000;100:13-20.
- Ibrahim-Granet O, Philippe B, Boleti H, Boisvieux-Ulrich E, Grenet D, Stern M, et al. Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect Immun 2003;71:891-903.
- Knutsen AP. Immunopathology and immunogenetics of allergic bronchopulmonary aspergillosis. J Allergy (Cairo) 2011;2011:785983.
- Rajesh, Sharma GL. Studies on antimycotic properties of Datura metel. J Ethnopharmacol 2002;80:193-7.
- Dabur R, Singh H, Ali M, Gupta J, Sharma GL. A novel antifungal pyrrole derivative from Datura metel leaves. Pharmazie 2004;59:568-70.
- Dabur R, Diwedi SK, Yadav V, Mishra V, Singh R, Singh H, et al. Efficacy of 2-(3,4-Dimethyl-2,5-Dihydro-1H-Pyrrole-2-yl)-1-Methylethyl pentanoate in a murine model of invasive aspergillosis. Antimicrob Agents Chemother 2005a;49:4365-7.
- Dabur R, Chhillar AK, Yadav V, Kamal PK, Gupta J, Sharma GL. In vitro activity of 2-(3, 4-dimethyl-2, 5-dihydro-1H-pyrrol-2-yl)-1-methylethyl pentanoate, a novel antifungal dihydro pyrrole derivative. J Med Microbiol 2005b;54:1-4.
- Dabur R, Mandal TK, Sharma GL. Post-antifungal effects of the antifungal compound 2-(3,4-dimethyl-2,5-dihydro-1H-pyrrol-2-yl)-1-methylethyl pentanoate on A.fumigatus. J Med Microbiol 2007;56:815-8.
- Mittal A, Mandal TK, Bindal R, Dabur R . Cathepsin B is a negative regulator in pulmonary aspergillosis. Int J Biosci Biochem Bioinforma 2011;1:125-30.
- Graybill JR, Bocanegra R, Najvar LK, Luther MF, Loebenberg D. SCH56592 treatment of murine invasive aspergillosis. J Antimicrob Chemother 1998;42:539-42.
- Johnson TS, El-Koraie AF, Skill NJ, Baddour NM, El Nahas AM, Njloma M, et al. Tissue transglutaminase and the progression of human renal scarring. J Am Soc Nephrol 2003;14:2052-62.
- Shiloko S, Tappel AL. Acid phosphatase of the lysosomal and soluble fraction of rat liver. Biochem Biophys Acta 1963;73:76-80.
- Kamboj RC, Pal S, Raghav N, Singh H. A selective colorimetric assay for cathepsin L using Z-Phe-Arg-4-methoxy-beta-naphthylamide.Biochimie 1993;75:873-8.
- Kamboj RC, Raghav N, Mittal A, Khurana S, Sadana R, Singh H. Effects of some Antituberculous and AntiLeprotic Drugs on Cathepsins B, H and L. Indian J Clin Biochem 2003;18:39-47.
- Pal S, Raghav N, Kamboj RC, Singh H. Dipeptidyl peptidase I from goat brain: Purification, characterization and its action on Leuenkephalin. Neurochem Int 1993;22:59-68.
- Dahl SW, Halkier T, Lauritzen C, Dolenc I, Pedersen J, Turk V, et al. Human recombinant pro-dipeptidyl peptidase I (cathepsin C) can be activated by cathepsins L and S but not by autocatalytic processing. Biochemistry 2001;40:1671-8.
- Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci USA 1999;96:8627-32.
- Kobayashi N, Nagumo H, Agematsu K. IL-10 enhances B-cell IgE synthesis by promoting differentiation into plasma cells, a process that is inhibited by CD27/CD70 interaction. Clin Exp Immunol 2002;129:446-52.
- Sambatakou H, Pravica V, Hutchinson V, Denning DW. Cytokines profiling of pulmonary aspergillosis. Int J Immunogenet 2006;33:297-302.
- Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. IL-4 induces cathepsin protease activity in tumor-associatedmacrophages to promote cancer growth and invasion. Genes Dev 2010;24:241-55.
- Lafuse WP, Brown D, Castle L, Zwilling BS. IFN-gamma increases cathepsin H mRNA levels in mouse macrophages. J Leukoc Biol 1995;57:663-9.
- Tamara TL, Hawley M, Rock KL, Goldberg AL. Gamma interferon causes a selective induction of the lysosomal proteases, cathepsins B and L, in macrophages. FEBS Lett 1995;363:85-9.
- Maekawa Y, Himeno K, Ishikawa H, Hisaeda H, Sakai T, Dainichi T, et al. Switch of CD4+ T cell differentiation from Th2 to Th1 bytreatment with cathepsin B inhibitor in experimental leishmaniasis. J Immunol 1998;161:2120-7.
- Peres GB, Juliano MA, Simões MJ, Michelacci YM. Lysosomal enzymes are decreased in the kidney of diabetic rats. Biochim Biophys Acta 2012;1832:85-95.
- Huang D, Li Y, Cheng X. Contribution of lysosomal cysteine proteases in cardiac and renal diseases. World J Hypertens 2012;2:29-33.
- Kominami E, Bando Y, Ii K, Hizawa K, Katunuma N. Increases in cathepsins B and L and thiol proteinase inhibitor in muscle of dystrophic hamsters. Their localization in invading phagocytes. J Biochem 1984;96:1841-8.
- Friedrich B, Jung K, Lein M, Türk I, Rudolph B, Hampel G, et al. Cathepsins B, H, L and cysteine protease inhibitors in malignant prostate cell lines, primary cultured prostatic cells and prostatic tissue. Eur J Cancer 1999;35:38-44.
- Gabrijelcic D, Svetic B, Spaic D, Skrk J, Budihna M, Dolenc I, et al. Cathepsins B, H and L in human breast carcinoma. Eur J Clin Chem Clin Biochem 1992;30:69-74.
- del Re EC, Shuja S, Cai J, Murnane MJ. Alterations in cathepsin H activity and protein patterns in human colorectal carcinomas. Br J Cancer 2000;82:1317-26.
- Kos J, Stabuc B, Schweiger A, Krasovec M, Cimerman N, Kopitar-Jerala N, et al. Cathepsins B, H, and L and their inhibitors stefin A and cystatin C in sera of melanoma patients. Clin Cancer Res 1997;3:1815-22.
- Sivaparvathi M, Sawaya R, Gokaslan ZL, Chintala SK, Rao JS. Expression and the role of cathepsin H in human glioma progression and invasion. Cancer Lett 1996;104:121-6.
- Budihna M, Strojan P, Smid L, Skrk J, Vrhovec I, Zupevc A, et al. Prognostic value of cathepsins B, H, L, D and their endogenous inhibitors stefins A and B in head and neck carcinoma. Biol Chem Hoppe Seyler 1996;377:385-90.