- Corresponding Author:
- Vidhya V. Lyer
School of Bio Sciences and Technology, VIT University, Vellore−632 014, India, E-mail: vidhyaiyer.v@vit.ac.in
| Date of Submission | 05 February 2014 |
| Date of Revision | 21 September 2014 |
| Date of Acceptance | 25 September 2014 |
| Indian J Pharm Sci 2014;76(6):548-552 |
Abstract
The results of our previous investigations on extracts of selected marine algae showed that Caulerpa peltata and Padina gymnospora had more promising antiproliferative and antioxidant activities than Gelidiella acerosa and Sargassum wightii. Based on these results, the more active chloroform extract of C. peltata and ethyl acetate extract of P. gymnospora were further analyzed for their constituents by using gas chromatography in tandem with mass spectrometry. The GC-MS analysis (GC % peak area given in parentheses) showed that fucosterol (12.45%) and L-(+)-ascorbic acid 2,6-dihexadecanoate (8.13%) were the major compounds present in P. gymnospora ethyl acetate extract. On the other hand, C. peltata chloroform extract had 1-heptacosanol (10.52%), hexacosanol acetate (9.28%), tetradecyl ester of chloroacetic acid (7.22%), Z,Z-6,28-heptatriactontadien-2-one (6.77%) and 10,13-dimethyl-methyl ester of tetradecanoic acid (5.34%) as major compounds. Also described in the report are the beta-carotene bleaching inhibitory and total reducing activities of the chloroform and ethyl acetate extracts of C. peltata and P. gymnospora, respectively, relative to the other three extracts (aqueous, methanol, chloroform or ethyl acetate) of the two algae.
Keywords
GC-MS, antiproliferative, marine algae, Caulerpa peltata, Padina gymnospora
In recent times, marine algae have been gaining importance as sources of pharmacologically active constituents possessing antioxidant, antiproliferative, antimutagenic, antidiabetic, anticoagulant, antibacterial and antitumor activities [1,2]. Exploration for bioactive compounds led to the screening of selected marine algae from the Tamil Nadu coast for antiproliferative and antioxidant activities [3]. The results of that study indicated that the chloroform (C) extract of Caulerpa peltata J.V. Lamouroux (green alga belonging to Chlorophyta) and ethyl acetate (E) extract of Padina gymnospora (Kützing) Sonder (brown alga belonging to Heterokontophyta) showed significant antiproliferative (in four different human cancer cell lines) and antioxidant (DPPH radical-scavenging and ferrous ion-chelating) activities. GC-MS is an easy and rapid method for analyzing the different constituents present in an unknown sample. This method has also been employed in the identification of compounds in crude extracts of plants and other natural sources. Based on the results of both the previous [3] and the current investigations, the C extract of C. peltata and E extract of P. gymnospora were subjected to GC-MS analysis. Lipid peroxidation is mediated by reactive oxygen species (ROS) causing cellular damage, eventually leading to carcinogenesis. β-Carotene bleaching (BCB) inhibition involves a coupled auto-oxidation of linoleic acid-β-carotene to form peroxyl radicals and bleaching of the yellow colour of β-carotene. Also, the overall reducing/antioxidant capacity of the extracts was determined with the total reducing activity (TRA) assay. Hence, the BCB inhibition and TRA assays were performed for the C and E extracts of C. peltata and P. gymnospora, respectively, along with other three extracts.
Caulerpa peltata and Padina gymnospora were collected between Mar-Jul 2011, from the Mandapam coast of Tamil Nadu, and were authenticated (Central Marine Fisheries Research Institute, Mandapam). As reported earlier by Murugan and Iyer, 2013, washed, shade-dried and powdered algae were separately extracted with four different solvents such as methanol, chloroform, ethyl acetate and distilled water to give M, C, E and A extracts, which were further used for the assays [3].
Based on the higher antiproliferative activity in four different cell lines and antioxidant activities obtained from the previous study, the C extract of C. peltata and E extract of P. gymnospora were subjected to GC-MS analysis. GC-MS analysis of the crude extracts was carried out by using Perkim Elmer-Clarus 680 model with the specific column Elite 5MS ((30 m×0.25 mm ID and 250 µm film thickness, 95% dimethylpolysiloxane) operating in an electron impact mode at 70 eV with helium as a carrier gas at a flow rate of 1 ml/min. The injection volume was 1 µl (split ratio 10:1); temperature was set at 250°. The ion source and transfer temperatures were 230°. The oven temperature was set from 60 to 300° with a 10° increase per min, ending with a 6 min hold at 300° and a total run time of 32 min. Mass spectra were recorded with fragments ranging from 50 to 600 Da. The spectrum obtained after GC-MS analysis was interpreted and compared with the National Institute Standard and Technology (NIST) compounds library [4].
The C extract of C. peltata and E extract of P. gymnospora were examined for BCB inhibition and TRA, and compared with their other three extracts (M, C, E or A). The assays were performed according to the previously reported method [5]. All the chemicals and solvents used for the assays were obtained from SRL, Mumbai.
All the assays were performed at least three times (n=3) and the results have been expressed as mean±standard error of mean (SEM). The assays were compared by using one way analysis of variance (ANOVA), followed by Tukey′s post−hoc test. P<0.05 indicates statistical significance. All statistical analyses were performed using GraphPad Prism 5 software.
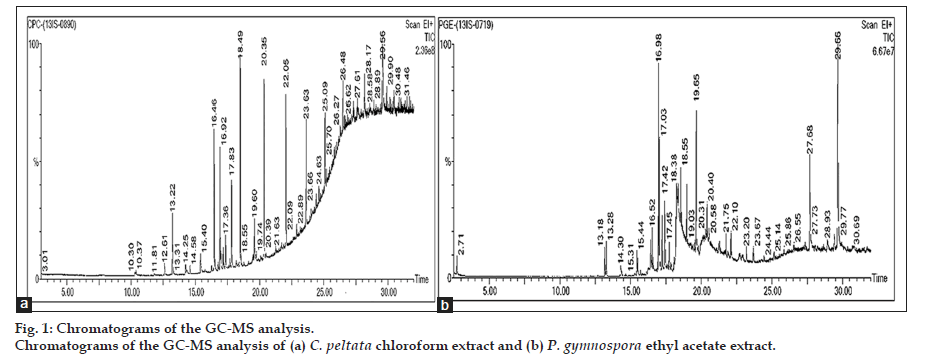
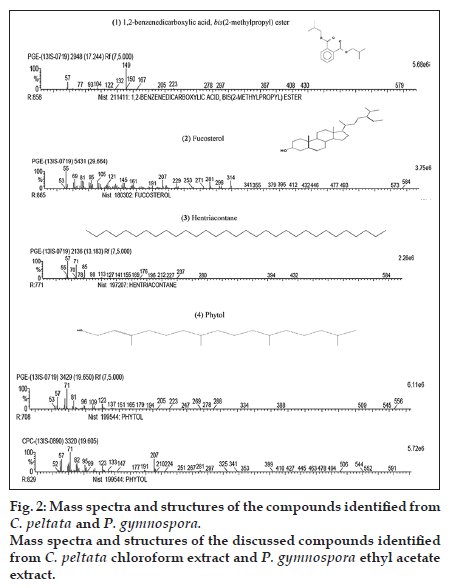
The chromatograms of the GC-MS analysis of C. peltata C extract and P. gymnospora E extract are shown in figs. 1a and b. When the peaks obtained were compared with those in the NIST libraries, eight compounds were identified in the C. peltata C extract and P. gymnospora E extract was found to contain 16 compounds. These compounds were mostly alcohols, esters and long chain hydrocarbons. Fig. 2 displays structures for compounds (1-4) which have been reported earlier in literature in other marine algae and are discussed below. Compounds (1-3) are present in P. gymnospora ethyl acetate extract and (4) is present in both extracts. Compound (1), 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester (fig. 2), was found to be present in P. gymnospora E extract. This compound was also reported in GC-MS analysis of a hexane extract of the antimicrobial red marine alga, Acanthophora spicifera (Vahl) Borgesan [6].
The GC-MS profile of the E extract of the brown alga, P. gymnospora, showed the presence of (2) fucosterol (fig. 2) with the highest peak area of 12.45%. Fucosterol has been reported to be a major sterol in brown algae, which is consistent with the results of the present study [7]. Literature reports are available on several biological activities on fucosterol, including antioxidant, cytotoxic, antidiabetic and acetylcholinesterase (ACE) inhibitory activities. A fucosterol isolated from the hexane fraction of the brown marine alga, Sargassum angustifolium C. Agardh was found to be cytotoxic against T47D (breast ductal carcinoma) and HT29 (colon carcinoma) cell lines [8]. Similarly, antioxidant and antidiabetic activities of fucosterol isolated from another brown marine alga, Pelvetia siliquosa Tseng and Chang, have been reported [9,10]. In 1995, Al Easa et al., reported the presence of fucosterol in P. gymnospora collected from Qatar coast [11]. Therefore, it could be hypothesized that the antioxidant and antiproliferative activities of the E extract of P. gymnospora (collected from the Tamil Nadu coast), observed from the earlier study by Murugan and Iyer, 2013, could be due to the presence of this major component, fucosterol.
A hydrocarbon compound, (3) hentriacontane (fig. 2), which was found in P. gymnospora E extract in this study, has already been reported to be a constituent of Phaeophyceae (brown algae) [12]. In an earlier study, the straight chain hydrocarbon, hentriacontane, was found to be present in the Japanese fermented soy food−‘‘Natto’’ when analyzed by gas chromatography, which was responsible for antitumor activity [13].
The compound (4) phytol was found in both C. peltata C extract and P. gymnospora E extract (fig. 2). Phytol, a diterpene alcohol, is a constituent of chlorophyll and is used as a precursor in manufacturing vitamins E and K. It was reported that phytol metabolites activate transcription factors and nuclear receptors [14]. In 2013, a study conducted by Silva et al., phytol was found to inhibit inflammatory response by reducing cytokine production and oxidative stress [15]. Xiao et al. described the extraction of phytol from a nonaqueous two-phase solvent system of two edible marine algae, namely, Undaria pinnatifida (Harvey) Suringar and Sargassum fusiforme (Harvey) Setchell [16].
Tables 1 and 2 show the list of compounds present in C. peltata C extract and P. gymnospora E extract, respectively, apart from the four compounds discussed above and were found to be present in natural sources (fungi and higher plants) other than marine algae. Some of these compounds are reported to have biological activities including antiinflammatory, antioxidant and antibacterial.
| RT(min) | Compound | Mol. formula |
Mol. weight |
Peak area (%) |
Sources | Activity |
|---|---|---|---|---|---|---|
| 13.22 | 3,5−bis (1,1−dimethylethyl)−phenol | C14H22O | 206 | 4.27 | Monochaetia kansesis [17] | Antiinflammatory [18] |
| 15.41 | cis−2,4−dimethylthiane, S, S−dioxide | C7H14O2S | 162 | 2.54 | Ixora coccinea | Antioxidant* [19] |
| 16.47 | Chloroacetic acid, tetradecyl ester | C16H31O2Cl | 290 | 7.22 | Ixora coccinea | Antioxidant* [19] |
| 16.92 | Z,Z−6,28−heptatriactontadien−2−one | C37H70O | 530 | 6.77 | Mukia maderaspatana | Vasodilatory [20] |
| 17.83 | Tetradecanoic acid 10,13−dimethyl−, methyl ester | C17H34O2 | 270 | 5.34 | Actinodaphne madraspatana [21] |
Not found |
| 18.49 | 1−heptacosanol | C27H56O | 396 | 10.52 | Eupatorium odoratum Buddleja crispa |
Antibacterial*, Antioxidant* [22] Nematicidal* [23] |
| 20.34 | Hexacosanol, acetate | C28H56O2 | 424 | 9.28 | Solanecio mannii | Antimicrobial [24] |
*Activity is related to the source or its extract and not the compound; RT: retention time
Table 1: Compounds identified by GC−MS in C. peltata chloroform extract and their natural occurrence
| RT (min) | Compound | Mol. formula |
Mol. weight |
Peak area (%) |
Sources | Activity |
|---|---|---|---|---|---|---|
| 13.29 | 2,6−bis (1,1−dimethylethyl) phenol | C14H22O | 206 | 1.14 | Monochaetia kansesis[17] | Antiinflammatory[18] |
| 15.45 | 3−n−hexylthiolane, S, S−dioxide | C10H20O2S | 204 | 0.79 | Hibiscus rosa−sinensis | Antioxidant*[25] |
| 16.53 | Chloroacetic acid, tetradecyl ester | C16H31O2Cl | 290 | 1.03 | Ixora coccinea | Antioxidant*[19] |
| 16.98 | Z,Z−6, 28−heptatriactontadien−2−one | C37H70O | 530 | 5.10 | Mukia maderaspatana | Vasodilatory[20] |
| 17.04 | 3−methyl−2−(2−oxopropyl) furan | C8H10O2 | 138 | 3.21 | Mallotus tetracoccus | Antipyretic*, Anti−inflammatory*[26] Hepatoprotective*[27] |
| 18.27 | L−(+)−ascorbic acid 2,6−dihexadecanoate | C38H68O8 | 652 | 8.13 | Alstomia boonei Nelumbo nucifera |
Antioxidant[28] |
| 18.55 | Hexacosanol, acetate | C28H56O2 | 424 | 4.43 | Solanecio mannii | Antimicrobial[24] |
| 18.97 | 5,8,11,14− eicosatetraenoic acid, methyl ester, (all Z)− | C21H34O2 | 318 | 2.68 | Michelia champaca | Antimicrobial*, Antioxidant*, Anticancer*[29] |
| 21.75 | Tetrahydro−6−nonyl−2H pyran−2−one, | C14H26O2 | 226 | 0.69 | Parkia Speciosa | Antidiabetic*, Antibacterail*, Mitogenic*[30] |
| 22.10 | Dotriacontyl pentafluoropropionate | C35H65O2F5 | 612 | 0.61 | Schizophylum commune | Cytotoxic*[31] |
| 27.68 | 3−methoxy− (3.beta)− cholest−5−ene | C28H48O | 400 | 4.77 | Orthosiphon tomentosus[32] Kalanchoe pinnata[33] |
Not found |
| 29.42 | 9,10−dihydro−9,10−diethyl anthracene | C18H20 | 236 | 1.06 | Cassia sp. | Antioxidant[34] |
*Activity is related to the source or its extract and not the compound; RT: retention time
Table 2: Compounds identified by GC−MS in P. gymnospora ethyl acetate extract and their natural occurrence
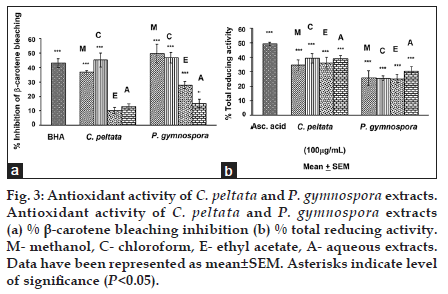
Results for the BCB inhibition and TRA assays have been shown for all four extracts of both C. peltata and P. gymnospora (data for G. acerosa and S. wightii have not been shown). In both BCB inhibition and TRA assays, the C extract of C. peltata showed higher activity (P<0.05) than the other three extracts (E, M and A). Among the extracts of P. gymnospora, the M extract showed the highest activity (P<0.05), followed by C and E extracts for the BCB inhibition assay, whereas the A extract showed the highest activity (P<0.05) for TRA. The M, C, E extracts of P. gymnospora showed almost similar TRA values (fig. 3a-b). The overall results suggest that these compounds (discussed above and in Table 1) present in the C extract of C. peltata might have been responsible for the observed BCB inhibition and TRA activities, whereas the higher TRA of the A extract of P. gymnospora could be attributed to the presence of higher amounts of reducing sugars and polysaccharides. However, although the BCB inhibition of the M extract of P. gymnospora was higher than that of its other extracts, the E extract was subjected to GC-MS analysis based on its greater activity in the previous study [3].
Fig. 3: Antioxidant activity of C. peltata and P. gymnospora extracts. Antioxidant activity of C. peltata and P. gymnospora extracts (a) % β-carotene bleaching inhibition (b) % total reducing activity. M- methanol, C- chloroform, E- ethyl acetate, A- aqueous extracts. Data have been represented as mean±SEM. Asterisks indicate level of significance (P<0.05).
The present study was carried out to identify the compounds present in C. peltata C and P. gymnospora E extracts by using GC-MS, and to compare the BCB inhibition and TRA of these extracts with the other solvent extracts of the two algae (M, C, E or A). This is the first report of these compounds present in the C and E extracts of C. peltata and P. gymnospora collected from the Tamil Nadu coast.
Acknowledgemnets
The authors are grateful to Mr. Ramalingam (former CMFRI employee, Madapam) for authenticating and to Mr. Raju for collecting the marine algae. The authors thank the Sophisticated Instrumentation Facility (SIF), VIT University, Vellore for GC-MS analysis.
References
- Smit AJ. Medicinal and pharmaceutical uses of seaweed naturalproducts: A review. J App Phycol 2004;16:245-62.
- Folmer F, Jaspars M, Dicato M, Diederich M. Photosynthetic marine organisms as a source of anticancer compounds. Phytochem Rev 2010;9:557-79.
- Murugan K, Iyer VV. Differential growth inhibition of cancer cell lines and antioxidant activity of extracts of red, brown and green marine algae. In Vitro Cell Dev Biol Animal 2013;49:324-34.
- Raja Rajeswari N, Ramalakshmi S, Muthuchelian K. GC-MS analysis of bioactive components from the ethanolic leaf extract of Canthium dicoccum (Gaertn.) Teijsm and Binn. J Chem Pharm Res 2011;3:792-8.
- Murugan K, Iyer VV. Antioxidant and antiproliferative activities of marine algae, Gracilaria edulis and Enteromorpha lingulata, from Chennai coast. Int J Cancer Res 2012;8:15-26.
- Zakaria NA, Ibrahim D, Shaida SF, Supardy NA. Phytochemical composition and antibacterial potential of hexane extract from Malaysian red algae, Acanthophora spicifera (Vahl) Borgesen. World App Sci J 2011;15:496-501.
- Patterson GW. The distribution of sterols in algae. Lipids 1971;6:120-7.
- Khanavi M, Gheidarloo R, Sadati N, Ardekani MR, Nabavi SM, Tavajohi S, et al. Cytotoxicity of fucosterol containing fraction of marine algae against breast and colon carcinoma cell line. Pharmacogn Mag 2012;8:60-4.
- Lee S, Lee YS, Jung SH, Kang SS, Shin KH. Antioxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch Pharm Res 2003;26:719-22.
- Lee YS, Shin KH, Kim BK, Lee S. Antidiabetic activities of fucosterol from Pelvetia siliquosa. Arch Pharm Res 2004;27:1120-2.
- Al Easa HS, Kornprobst JM, Rizk AM. Major sterol composition of some algae from Qatar. Phytochemistry 1995;39:373-4.
- Heilbron I, Phipers RF, Wright HR. The chemistry of the algae. Part 1. The algal sterol Fucosterol. J Chem Soc 1934;1572-6.
- Takahashi C, Kikuchi N, Katou N, Miki T, Yanagida F, Umeda M. Possible antitumor-promoting activity of components in Japanese soybean fermented food, Natto: Effect on gap junctional intercellular communication. Carcinogenesis 1995;16:471-6.
- Kitareewan S, Burka LT, Tomer KB, Parker CE, Deterding LJ, Stevens RD, et al. Phytol metabolites are circulating dietary factors that activate the nuclear receptor RXR. Mol Biol Cell 1996;7:1153-66.
- Silva RO, Sousa FB, Damasceno SR, Carvalho NS, Silva VG, Oliveira FR, et al. Phytol, a diterpene alcohol, inhibits the inflammatoryresponse by reducing cytokine production and oxidative stress. FundClin Pharm 2013;28:455-64.
- Xiao XH, Yuan ZQ, Li GK. Preparation of phytosterols and phytol from edible marine algae by microwave-assisted extraction and high-speed counter-current chromatography. Sep Purif Technol 2013;104:284-9.
- Yogeswari S, Ramalakshmi S, Neelavathy R, Muthumary J. Identification and comparative studies of different volatile fractions from Monochaetia kansensis by GCMS. Global J Pharmacol 2012;6:65-71.
- Costantino L, Parenti C, Di Bella M, Zanoli P, Baraldi M. Antiinflammatory activity of newly synthesized 2,6-bis-(1,1- dimethylethyl) phenol derivatives. Pharmacol Res 1993;27:349-58.
- Shyam P, Suresh PK. Comparative analysis of three leaf extracts of Ixora coccinea Linn. for their protective and antioxidant potentials and correlation with analytical data. Int J Pharm Bio Sci 2013;4:937-49.
- Mallikadevi T, Paulsamy S, Jamuna S, Karthika K. Analysis for phytoceuticals and bioinformtics approach for the evaluation of therapeutic properties of whole plant methanolic extract of Mukia maderaspatana (L.) M. Roem. (Cucurbitaceae)- A traditionsl medicinal plant in western districts of Tamil Nadu, India. Asian J Pharm Clin Res 2012;5:163-8.
- Saravanan D, Kasisankar V, Asharani IV. GC-MS analysis of phytocomponents in the leaves of Actinodaphne madraspatana Bedd. Int J Res Pharm Sci 2013;4:469-73.
- Venkataraman B, Samuel LA, Pardha Saradhi M, Narashimharao B, Naga Vamsi Krishna A, Sudhakar M et al. Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian J Pharm Clin Res 2012;5:99-106.
- Sultana N, Akhter M, Khan RA, Afza N, Tareen RB, Malik A. Nematicidal natural products from the aerial parts of Buddleja crispa. Nat Prod Res 2010;24:783-8.
- Mbosso EJ, Ngouela S, Nguedia JC, Beng VP, Rohmer M, Tsamo E. In vitro antimicrobial activity of extracts and compounds of some selected medicinal plants from Cameroon. J Ethnopharmacol 2010;128:476-81.
- Bhaskar A, Nithya V, Vidhya VG. Phytochemical screening and in vitro antioxidant activities of the ethanolic extract of Hibiscus rosa-sinensis L. Ann Biol Res 2011;2:653-61.
- Ramalakshmi S, Muthucheian K. Analysis of bioactive constituents from the leaves of Mallotus tetracoccus (Roxb.) Kurz, by gas chromatography-mass spectrometry. Int J Pharm Sci Res 2011;2:1449-54.
- Okwu DE, Ighodaro BU. GC-MS evaluation of bioactive compounds and antibacterial activity of the oil fraction from the leaves of Alstonia boonei De Wild. Der Pharma Chemica 2010;2:261-72.
- Huang B, Ban X, He J, Tong J, Tian J, Wang Y. Comparative analysis of essential oil components and antioxidant activity of extracts of Nelumbonucifera from various areas of China. J Agric Food Chem 2010;58:441-8.
- Lee SW, Wendy W, Julius YFS, Desy FS. Characterization of antimicrobial, antioxidant, anticancer property and chemical composition of Michelia champaca seed and flower extracts. Stamford J Pharm Sci 2011;4:19-24.
- Available from: http://www.globinmed.com. Available from: http://www.globinmed.com/index.php?option=com_content and view=article and id=83456:parkia-speciosa#1 [Last accessed on 2014 Jan 22].
- Aina DA, Oloke JK, Awoyinka OA, Adebayo EA, Akoni OI, Agbolade JO et al. Comparative cytotoxic effect of metabolites from wild and mutant strains of Schizophylum commune grown in submerged liquid medium. Am J Res Comm 2013;1:219-40.
- Shukla RS, Bhaskar VV. Steroidal composition of the root extract of Orthosiphon tomentosus Benth (Lamiaceae) used in Tribal medicine. J Pharm Res 2009;2:1137-38.
- Majaz Q, Nazim S, Shaikh S, Gomase P, Choudhari A. Phytochemical analysis of chloroform extract of roots of Kalanchoe pinnata by HPLC and GCMS. Int J Pharm Sci Res 2011;2:1693-9.
- Dave H, Ledwani L. A review on anthaquinones isolated from Cassia species and their applications. Indian J Nat Prod Resour 2012;3:291-319.