- *Corresponding Author:
- Alka Mukne
Department of Pharmacognosy and Phytochemistry, Bombay College of Pharmacy, Kalina, Santacruz (East), Mumbai-400098, India
E-mail: alka.mukne@gmail.com
| Date of Submission | 07 April 2017 |
| Date of Revision | 15 May 2017 |
| Date of Acceptance | 28 May 2017 |
| Indian J Pharm Sci 2017;79(3): 438-450 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Garlic (Allium sativum) is a plant known since ages for its beneficial properties. This study was carried out to develop and validate a simple, specific and robust high performance thin layer chromatography method for analysis of constituents present in a thiosulfinate-derivative rich extract of garlic. High performance thin layer chromatography analyses were carried out using aluminium backed silica gel 60F-254 plates as the stationary phase and toluene:ethyl acetate:chloroform:methanol (8:20:12:60% v/v/v/v) as the mobile phase. The method was validated as per ICH Q2 guidelines. The stability of eight components present in the extract of garlic under stress conditions like increased temperature, varying pH and presence of oxidant was also investigated as per the ICH Q1A (R2) guidelines. It was observed that the stability of all constituents present in the extract was majorly dependent on pH, illumination and temperature. The high performance thin layer chromatography method developed was able to estimate changes in content of all constituents present in the extract of garlic when it was subjected to stress conditions, which proves the stability-indicating nature of the method. Results of the study suggested that thiosulfinate derivative-rich extract of garlic should be stored at –20° in a well-closed container, away from light at a pH range of 4-7 to avoid degradation of components present in the extract.
Keywords
Garlic, HPTLC, validation, stability, thiosulfinate derivatives, Allium
Garlic (Allium sativum, family: Liliaceae) is a plant that is known to be useful in treatment of various ailments like chronic cough, constipation and toothache. In ancient Indian and Chinese medicine, garlic was recommended to aid respiration and digestion and to treat various bacterial and parasitic infections. It has been reported in literature that garlic has many experimentally and clinically proven benefits like reduction of risk of cancer, prevention of cardiovascular diseases and hepatoprotective activity [1].
Ajoene and vinyldithiins, along with minor quantities of allyl sulphides are formed as transformation products of allicin when garlic is heated or treated with organic solvents [2]. In 2006, Ledezma et al. reported that ajoene is responsible for most of the biological effects of garlic. Various pharmacological activities of garlic like antithrombotic, antitumor, antifungal and antiparasitic effects have been attributed to ajoene [3]. In 2014, Viswanathan et al. reported the antimycobacterial activity of ajoene rich extract [4]. In 2012, Jacobsen et al. reported that synthetic ajoene was active against Pseudomonas aeruginosa only when kept in ice and it loses its activity in vivo due to instability, whereas, in 2014, Vadekketil et al. proved that ajoene isolated from garlic extract (GE) showed in vitro activity against P. aeruginosa at 37° [5,6]. Vinyldithiins are reported to have potent antiadipogenic and antiinflammatory activities, whereas, allyl sulphides from garlic have been identified as potential chemopreventive agents [7,8].
The International Conference on Harmonization (ICH) Q1A(R2) guideline on “Stability Testing of New Drug Substances and Products” requires that forced degradation studies of compounds should be carried out to evaluate their inherent stability as a part of new drug development process [9]. Evaluation of chemical stability of compounds in extracts is challenging because extracts generally contain many components. It is easier to determine the stability of isolated compounds, but, results from these data do not always accurately reflect the stability of the compound in extract because the stability of a component in the extract is influenced by the presence of other components and various enzymes like esterase and oxidases. For compounds with known pharmacological properties, the permissible limit for variation in content during shelf-life in comparison to the initial assay value is ±5%. Various factors like temperature, moisture, oxidation and pH influence the stability, shelf-life, storage and handling conditions of the compounds [10]. Evaluation of stability of thiosulfinate derivatives in GE is important considering the various pharmacological activities attributed to these compounds. In 2008 Naznin et al. reported the optimal conditions for formation of ajoene and in 2010, the same group reported the stability of ajoene in home-made mayonnaise [11,12]. In 2014, Yoo et al. reported the temperature stabilities of organosulfur compounds present in garlic oil [2]. In 2014, Vadekeetil et al. reported the stability of ajoene in total GE at 4° and 37° in 3 different pH conditions [6]. To the best of our knowledge, complete stability profile of thiosulfinate derivatives in GE or in isolated form has not yet been reported in literature.
Regulatory agencies recommend the use of validated stability-indicating analytical methods for the analysis of compounds with pharmacological activity. Validated HPLC method for analysis of thiosulfinate derivatives in garlic oil has been reported in literature. The method involves the use of C8 column and has a long run time of 45 min [2]. High performance thin layer chromatography (HPTLC) method is a more preferred analysis technique because of its simplicity and highly productive separation. HPTLC method reduces the analysis time drastically and it is possible to analyse many samples at the same time [13]. Various TLC methods for analysis of ajoene have been reported, but the reports provide no information on validation of the developed methods for quantification of ajoene [6,11,14].
In our previous study, eight compounds were isolated from the GE by flash chromatography and the isolates were characterized using proton nuclear magnetic resonance (1H-NMR), liquid chromatography-mass spectrometry (LC-MS) and Fourier-transform infrared spectroscopy (FTIR). The intracellular antitubercular activity of GE in RAW 264.7 mouse macrophage cells was also estimated [15]. The purpose of the present study was to develop and validate a simple, robust HPTLC method for estimation of content of ajoene and other components present in GE. Forced degradation studies of GE were carried out in order to determine the effect of stress conditions on the concentration of all constituents present in the extract and to determine the optimum storage conditions to avoid degradation of constituents in the extract.
Materials and Methods
All chemicals used in this study were of analytical reagent grade and were procured from S. D. Fine Chem Ltd., India. Garlic bulbs were procured from local market and were authenticated at G. N. Khalsa College, Mumbai, India. A voucher specimen of the same (#:pp P1030620) has been deposited at the Herbarium, G. N. Khalsa College, Mumbai, India.
Sample solutions were applied as bands of 5 mm width on aluminium backed TLC silica gel 60 F-254 plates using a Linomat 5 applicator (Camag, Switzerland) equipped with Hamilton 100 μl syringe. A constant application rate of 150 nl/s was used. The application positions were as follows- application start position: 15 mm from the lower edge of the plate, first sample position: 10 mm from the edge of the plate, distance between two tracks: 10 mm, development distance: 85 mm. The mobile phase used for development of plates consisted of toluene:ethyl acetate:chloroform:methanol (8:20:12:60% v/v/v/v). Ascending development was carried out using a 20×10 cm twin trough chamber saturated with the mobile phase (20 ml) for 20 min at 25±2°. Scanning was done at 254 nm using Camag TLC scanner 3, operated by winCATS software (Version 1.2.2, Camag) using deuterium lamp as radiation source. The slit dimension while scanning was 4×0.45 mm and the scanning speed was 20 mm/s.
Preparation of GE
GE was prepared by the method described by Yoshida et al., 1987 with minor modifications. Briefly, 150 g of crushed garlic was refluxed with hydroalcoholic mixture (1:1, 500 ml) for 2 h at 40°. Ethanol was recovered under vacuum at 30°. The marc that was obtained was filtered using a clean muslin cloth. The filtrate that was obtained was fractionated using ethyl acetate. The ethyl acetate layer was concentrated under vacuum at 30° to obtain a yellowish-brown oily extract. GE was stored at –20° until further use [16].
In our previous study, eight constituents were isolated from GE by flash chromatography followed by further purification by preparative TLC. The isolates were characterized using 1H-NMR, LC-MS and FTIR [15]. The purity of E-ajoene was confirmed by HPLC using the method described by Paek and Ko in 2008 [14]. The isolated E-ajoene was found to be 99.1% pure and was used as standard compound for validating the HPTLC method.
Preparation of standard and extract solutions
About 1 mg of isolated E-ajoene was accurately weighed and dissolved in 10 ml of methanol to obtain 100 μg/ml stock solution of E-ajoene. About 3 mg of GE was dissolved in 1 ml of methanol to obtain 3000 μg/ml stock solution of the extract. These solutions were stored at –20° until further use.
Method validation
Validation of the developed HPTLC method was carried out as per the ICH Q2(R1) guidelines for linearity, specificity, precision, accuracy and robustness for isolated E-ajoene [17]. The method was also validated for linearity, precision and robustness for all the constituents present in GE.
Linearity
Working solutions of E-ajoene were prepared by suitably diluting the stock solution with methanol to obtain 2, 3, 6, 9, 12, 15, 18 and 20 μg/ml of E-ajoene. About 20 μl from each working solution was applied in triplicate to obtain a concentration of 40-400 ng/ band. A stock solution containing 10000 μg/ml of extract was prepared and it was suitably diluted to obtain working solutions containing 750, 1500, 3000, 4500, 6000 and 7500 μg/ml of extract. 20 μl from each working solution was applied in triplicate to obtain a concentration of 15-150 μg/ band.
Calibration curve was constructed by plotting the peak area of E-ajoene/constituents present in GE versus the corresponding concentrations applied. This was repeated six times to get an average standard calibration curve. Scanning of the plates was done at 254 nm. The data obtained was treated by linear regression analysis to determine the linearity of the method.
Specificity
Specificity of the method was evaluated by spotting 20 μl each of E-ajoene (12 μg/ml), extract (3000 μg/ ml), mobile phase and methanol. The plates were then developed and scanned to determine the possibility of interference from any other components of extract or solvents during the quantification process. The peak purity of E-ajoene was assessed by comparing the spectra at three different positions i.e. peak start, middle and end of the bands.
Limit of detection and limit of quantification
Limit of detection (LOD) and limit of quantification (LOQ) were determined on the basis of signal to noise ratio. Various concentrations of stock solution of E-ajoene and methanol were applied on the TLC plate. The peak areas of E-ajoene (S) and methanol (N) were recorded. The concentration of E-ajoene giving S/N ratio of 3:1 and 10:1 were considered as LOD and LOQ, respectively.
Accuracy
Accuracy of the method was determined by recovery studies at three different levels i.e. 50%, 100% and 150%. Known amount of E-ajoene was spiked to pre-analysed sample of extract and the % recovery of E-ajoene was calculated using the equation obtained from calibration curve. The analysis was carried out in triplicate on 3 d.
Repeatability and precision
Repeatability of measurement of peak area was checked by analysing E-ajoene (240 ng/ band) six times without changing the position of the plate. Repeatability was expressed as % relative standard deviation (%RSD). Intra-day precision of the method was determined by spotting bands of freshly prepared solution of E-ajoene (40, 120, 240 and 360 ng/band) and GE (30, 90 and 150 μg/band) in triplicate 3 times a day. Inter-day precision was determined by analysing bands of E-ajoene/GE in triplicate 3 times a day, on 3 different days. The concentrations used in evaluation of precision and robustness of the HPTLC method were decided based on the results obtained for linearity studies. The peak areas for each concentration were noted and difference in the values was expressed as %RSD.
Robustness
Robustness of the method was determined by varying important method parameters like mobile phase composition, mobile phase volume, saturation time, band width, development distance, slit dimension and scanning wavelength. The study was done using two concentrations of E-ajoene (120 ng/band and 360 ng/band). Robustness of the method for analysis of all constituents present in GE was evaluated at two concentrations of extract (30 and 150 μg/band) by varying mobile phase composition, mobile phase volume, saturation time and development distance. The peak area values obtained after making these deliberate changes in the method were recorded. These values were compared with the peak area values obtained using the optimized method and the difference in values was expressed as %RSD.
Spot stability
The time for which the compound remains on the chromatographic plate may influence the stability of its constituents and hence, it is important to evaluate the stability of compounds on the chromatographic plate. To evaluate the spot stability, time between application to development (plates were developed after varying time intervals post application and scanning was done immediately after development) and time between development to scanning (the plates were developed immediately after application and were scanned after varying time intervals) were varied [18]. The peak areas obtained in the study were compared with the peak areas of E-ajoene/other components obtained for the plates that were developed and scanned immediately. The results were expressed as %RSD.
Estimation of content of E-ajoene in GE
To determine the content of E-ajoene in GE, 20 μl of extract (3000 μg/ml) obtained from nine different batches of garlic and E-ajoene (10 μg/ml) were applied on the TLC plate (n=6). Area of the component in the extract having the same Rf value as that of standard E-ajoene was noted and the content of E-ajoene in the extract was calculated using the equation obtained from calibration curve.
Evaluation of stability of constituents present in GE
In this study, seven factors (temperature, pH, hydrolysis, illumination, oxidant, solvents and humidity), which may interfere with the stability of constituents present in GE were studied. Methanolic solution of the extract (3000 μg/ml) was used in this study, unless otherwise mentioned. Each experiment was done in triplicate. The gross weight of each vial was determined before incubating the samples at different conditions. After completion of the incubation period, the gross weight of each vial was adjusted to its initial value by addition of methanol. The samples were filtered through 0.45 μm syringe membrane filter to avoid clogging of the Hamilton syringe. Twenty microlitres of each sample was applied on the TLC plate and the plates were developed and scanned as described in the instrumentation section. The peak areas of each constituent in the treated samples were compared with the corresponding peak area values obtained for the constituent in freshly prepared GE. The % reduction in content of the constituent was calculated and this value was used for determining the stability of the constituent when GE was stored at various conditions.
Effect of temperature
Thermal stability of constituents present in GE was studied by incubating the extract solution stored in amber colored glass vials wrapped with aluminium foil at –20, 4, 25, 40, 80 and 100° for 1, 2, 3, 6 and 24 h. The samples stored at –20, 4 and 25° were additionally analysed on day 7 and day 14. Thermal stability of the constituents in GE was also studied by storing 9 mg of the extract in amber colored glass vials at –20, 4 and 25° for 3 months. After completion of incubation period, the extract was dissolved in 3 ml methanol and the samples were analysed [19].
Acidic and alkaline hydrolysis
Acidic hydrolysis of GE was carried out by refluxing a mixture of 10 ml solution of GE in methanol (6000 μg/ ml) with 10 ml 1N/5N hydrochloric acid separately for 2 h at 60° in the dark. For alkaline hydrolysis, the same procedure was repeated using 1N/5N sodium hydroxide [13].
Effect of pH
The effect of pH on chemical stability of constituents present in GE was studied at a pH range of 1-11 at room temperature. The pH adjustment was done using 1 N sodium hydroxide or hydrochloric acid solution. The solutions (5 ml) were stored in amber colored glass vials wrapped with aluminium foil and were kept in the dark at 25±2°. The samples were analysed after 4 and 8 h of incubation [19].
Effect of illumination
For carrying out photostability studies, 5 ml of the solution was placed in the dark, UV light of 254 nm and natural light at 25±2° for 1, 3, 6 and 24 h. The samples were analysed after completion of the incubation period [19]. The order of reaction for degradation of E-ajoene at pH 1, pH 11 (at which maximum degradation was observed), natural light and UV light was determined by graphical method. Zero order, first order and second order graphs were plotted and the plot with best linearity was taken as order of reaction [13].
Effect of oxidant
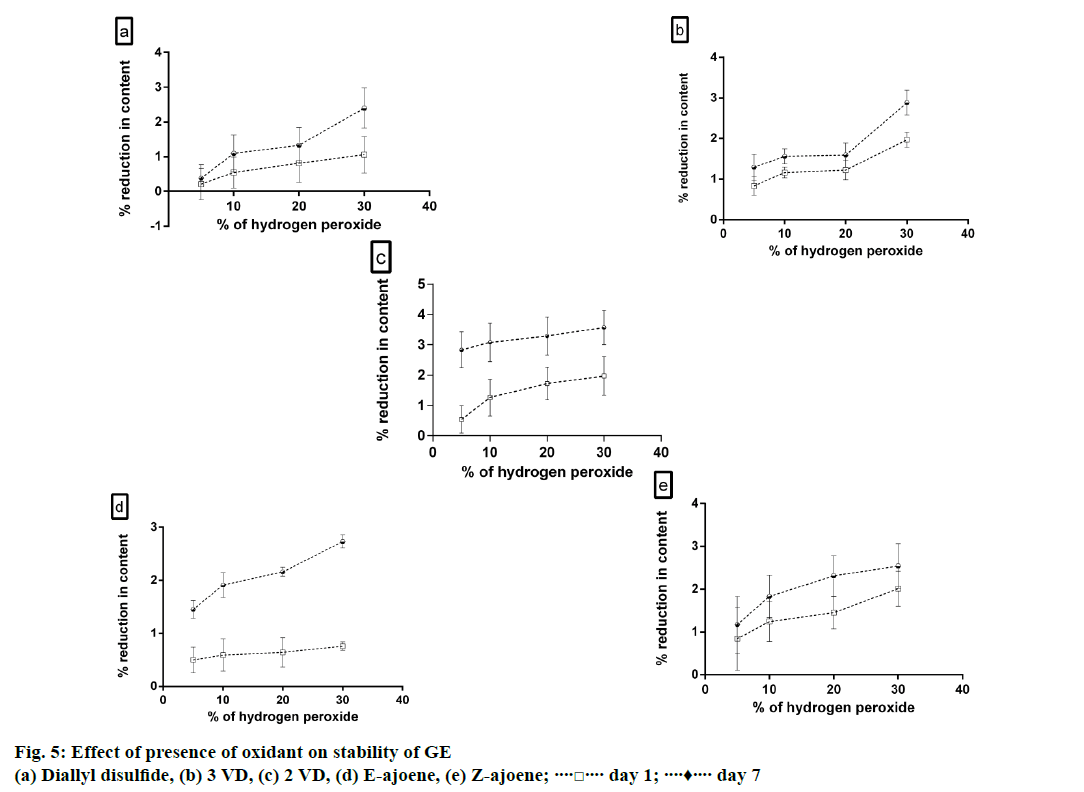
To study the effect of oxidation, hydrogen peroxide of varying concentration (5, 10, 20 and 30%) was used. Test solutions were prepared by mixing stock solutions of extract with varying concentrations of hydrogen peroxide and the samples were stored in well closed amber coloured glass vials. All the samples were stored in the dark at 25° and analysis was done on day 1 and day 7 [19].
Effect of solvents
The effects of various solvents on the stability of constituents present in GE was determined by dissolving 15 mg of extract in 5 ml of various solvents like methanol, ethyl acetate, chloroform, toluene, DMSO and pH 7.4/3.5 phosphate buffer. The samples were packed in tightly closed amber colored glass vials and were stored in the dark at 25±2°. Analysis was done after 6 h, 24 h and 7 d [19].
Effect of humidity
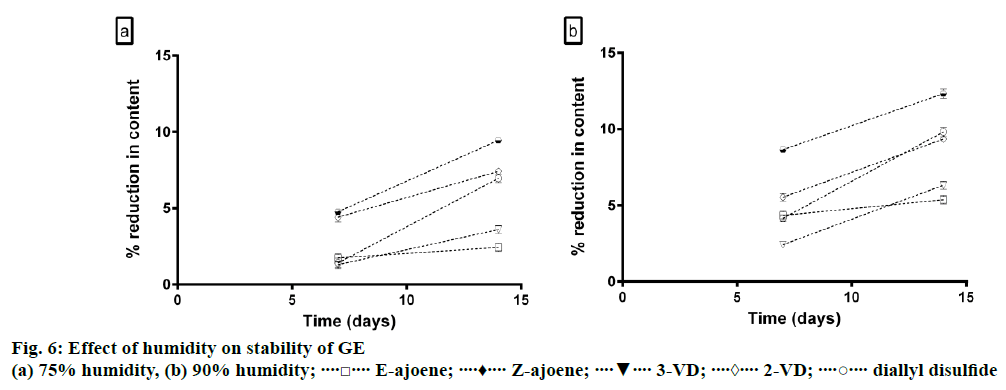
The effect of humidity on the content of constituents present in GE was studied at 75% and 90% relative humidity. Supersaturated solutions of NaCl and KNO3 were used to set up constant humidity conditions of 75 and 90%, respectively in desiccators. About 15 mg of GE was transferred into open amber colored vials and the vials were placed in the desiccators for 7 and 14 d at 25±2°. The treated extracts were then dissolved in 5 ml methanol and HPTLC analysis was carried out [19].
Statistical analysis
The results obtained for method validation and evaluation of chemical stability were statistically analysed by two-way analysis of variance (ANOVA) at 95% confidence interval using GraphPad Prism 6.0 software. The value was considered to be statistically significant when the P<0.05.
Results and Discussion
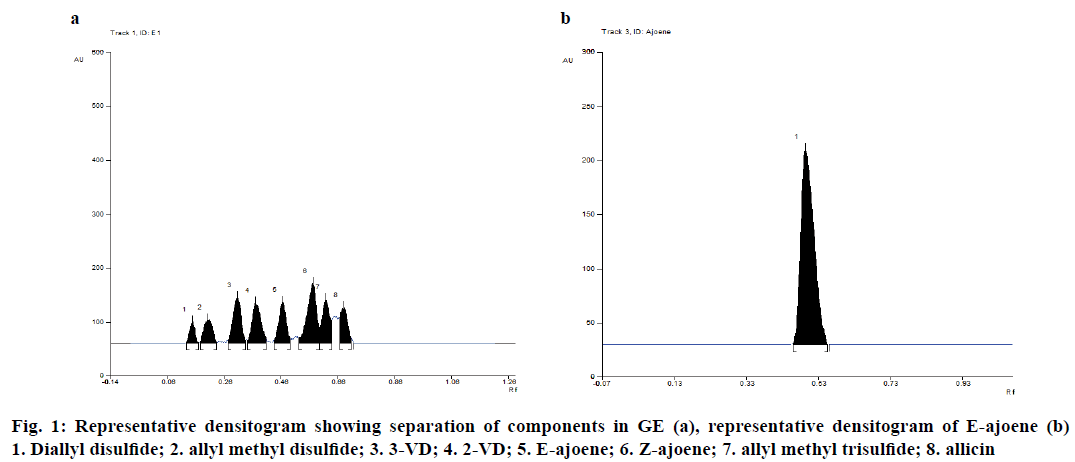
Several trials were carried out to obtain proper separation of the constituents present in GE. Initial trials were carried out using single solvents where efficient separation of components was not obtained. Therefore, combination of solvents was used to achieve separation of components in the extract. Sharp and well-defined peaks were obtained when ethyl acetate, chloroform and methanol were used. Addition of toluene to the mobile phase helped in reducing the tailing and improved the resolution between peaks. Therefore after various trials, toluene:ethyl acetate:chloroform:methanol (8:20:12:60% v/v/v/v) was found to be the optimum mobile phase for obtaining proper separation of components in GE. Chamber saturation time of 20 min was found to be optimum for obtaining a stable baseline and to reduce tailing. Eight constituents were isolated and characterized, as reported in our previous study [15]. The identity of the constituents present in GE along with the corresponding Rf values are summarized in Table 1. A representative densitogram of components present in GE and E-ajoene are shown in Figure 1a and b, respectively.
| Rfvalue ±SD | Name of the constituents |
|---|---|
| 0.15±0.01 | Diallyl disulphide |
| 0.20±0.01 | Allyl methyl disulphide |
| 0.30±0.02 | 3-vinyl-4H-1,2-dithiin (3VD) |
| 0.37±0.01 | 2-vinyl-4H-1,3-dithiin (2VD) |
| 0.49±0.02 | E-ajoene |
| 0.55±0.03 | Z-ajoene |
| 0.62±0.01 | Allyl methyl trisulphide |
| 0.68±0.03 | Allicin |
Table 1: Rf Values and Identity of Constituents present in GE
The linear regression analysis of calibration plots (n=6) showed a linear relationship in the concentration range of 40-400 ng/band of E-ajoene. The linear regression equation that was obtained was Y=8.1279x+95.287 and the correlation coefficient (R2) was 0.9968. Linear regression analysis of peak area values obtained for all other constituents present in GE revealed that linearity of all constituents was observed at a concentration range of 15-150 μg/band of the extract (data not shown).
A method is considered to be specific when no interference from solvent or mobile phase is observed. During the analysis, no interferences from any other component of extract or solvent were observed. The peak purity of E-ajoene was assessed by comparing the spectra at three points (start (s), middle (m) and end (e)). The r (s, m) value was found to be 0.9994 and r (m, e) value was found to be 0.9991. The LOQ value for E-ajoene was as low as 35 ng/band and the LOD value was 12 ng/band. The low LOD and LOQ values, combined with the wide range of linearity suggest that the method can be effectively used for analysing lower concentrations of E-ajoene.
The method was considered to be accurate if the % recovery values obtained were in the range of 90.0- 110.0%. The study was done in triplicate on three different days. Known amounts of E-ajoene were spiked to a sample of extract containing 0.4% w/w of E-ajoene. As evident from Table 2, it was observed that the % recovery values obtained for E-ajoene by the method developed in this study were well within the acceptable range. The difference in % recovery values obtained for each set of study was not statistically significant (P>0.05), indicating that the method was fairly accurate.
| Sample content (ng/band) | Spiked amount (%) | Spiked content (ng/band) | Total content (ng/band) (Theoretical) | Total content (ng/band) (Practical) | % Recovery±SD |
|---|---|---|---|---|---|
| 120 | 50 | 60 | 180 | 177.2 | 98.4±1.43 |
| 120 | 100 | 120 | 240 | 237.6 | 99±0.26 |
| 120 | 150 | 180 | 300 | 301.9 | 100.63±0.56 |
Table 2: Recovery Studies of E-Ajoene at 50, 100 and 150% by Standard Addition Method
The %RSD obtained for repeatability study was within the acceptable limits, which suggests that the method was reproducible. The inter-day and intra-day precision of the method was assessed by comparing the peak area responses for three different concentrations of E-ajoene as well as the constituents of GE on three different days (inter-day) and same day (intra-day). The proposed method was considered to be precise if the %RSD was <2%. Results of the study, summarized in Table 3 indicate that the method is precise for evaluation of E-ajoene and all constituents in GE. Robustness of the developed analytical method for analysis of E-ajoene was evaluated by making deliberate changes in the method. The method was considered to be robust when %RSD<2.
| Intra- and inter-day precision data for isolated E-ajoene | ||||||
| Amount spotted (ng/ band) | Intra-day precision | Inter-day precision | ||||
| SD in peak area | %RSD | SD in peak area | %RSD | |||
| 40 | 0.428 | 0.106 | 1.422 | 0.354 | ||
| 120 | 1.158 | 0.183 | 1.659 | 0.153 | ||
| 240 | 1.363 | 0.097 | 1.458 | 0.070 | ||
| 360 | 1.602 | 0.091 | 1.788 | 0.060 | ||
| Intra- and inter-day precision data for all constituents present in GE | ||||||
| Compounds | Intra-day precision (SD in peak area±%RSD) |
Inter-day precision (SD in peak area±%RSD) |
||||
| 30 (µg/ml) | 90 (µg/ml) | 150 (µg/ml) | 30 (µg/ml) | 90 (µg/ml) | 150 (µg/ml) | |
| Diallyl disulphide | 1.65±0.21 | 1.02±0.06 | 1.52±0.06 | 1.42±0.18 | 1.70±0.10 | 1.07±0.04 |
| Allyl methyl disulphide | 0.76±0.13 | 0.85±0.07 | 0.52±0.02 | 1.11±0.20 | 1.25±0.10 | 1.74±0.09 |
| 3VD | 0.75±0.04 | 1.36±0.37 | 1.26±0.01 | 1.56±0.09 | 1.41±0.09 | 1.38±0.01 |
| 2VD | 1.12±0.06 | 1.80±0.05 | 0.75±0.01 | 1.34±0.08 | 1.68±0.05 | 1.57±0.03 |
| E-ajoene | 0.67±0.10 | 1.45±0.08 | 0.44±0.01 | 1.24±0.19 | 1.40±0.08 | 1.65±0.07 |
| Z-ajoene | 0.56±0.08 | 0.96±0.05 | 0.40±0.01 | 1.08±0.16 | 1.33±0.07 | 1.03±0.04 |
| Allyl methyl trisulphide | 0.98±0.24 | 1.29±0.17 | 1.15±0.08 | 1.54±0.37 | 1.31±0.17 | 1.34±0.09 |
Table 3: Intra-Day and Inter-Day Precision Data
As observed in Table 4, it was observed that slight changes in the polarity of the mobile phase did not have any significant effect on the peak areas observed for E-ajoene (P>0.05), however, significant tailing was observed. Changing the mobile phase volume or saturation time caused a reduction in the peak area, though not statistically significant (P>0.05). However, it was observed that these changes caused significant tailing in the peak of E-ajoene. It can therefore be concluded that chamber saturation and mobile phase composition have a significant bearing on the separation of components in GE. As evident from Table 4, when the slit dimension was changed, significant reduction in the peak area values of E-ajoene were observed (P<0.05).
| Parameters | 120 ng/band | 360 ng/band | ||
|---|---|---|---|---|
| %RSD in peak area | Rf±SD | %RSD in peak area | Rf±SD | |
| Mobile phase composition (optimized:toluene:ethyl acetate:chloroform:methanol (8:20:12:60 %v/v/v/v) | ||||
| Toluene:ethyl acetate:chloroform: methanol (8:19:11:59 v/v/v/v) | 0.776 | 0.53±0.016 | 0.416 | 0.52±0.016 |
| Toluene:ethyl acetate:chloroform:methanol (8:21:13:61 v/v/v/v) | 0.398 | 0.48±0.016 | 0.25 | 0.48±0.011 |
| Mobile phase volume (optimised: 20 ml) | ||||
| 18 ml | 1.10 | 0.49±0.007 | 0.39 | 0.5 |
| 22 ml | 1.00 | 0.50 | 0.16 | 0.49±0.01 |
| Chamber saturation time (optimised: 20 min) | ||||
| 18 min | 1.11 | 0.49 | 0.20 | 0.50±0.01 |
| 22 min | 1.70 | 0.50±0.01 | 0.39 | 0.50±0.013 |
| Scanning wavelength (optimised: 254 nm) | ||||
| 250 nm | 0.006 | 0.49±0.01 | 0.09 | 0.49±0.02 |
| 240 nm | 1.89 | 0.5±0.02 | 0.45 | 0.50±0.01 |
| Slit dimension (optimised: 4´0.45 mm) | ||||
| 4 mm×0.30 mm | 1.72 | 0.49 | 0.22 | 0.50±0.02 |
| 5 mm×0.30 mm | 2.28 | 0.49±0.01 | 0.62 | 0.50±0.02 |
| Band width (optimised: 5 mm) | ||||
| 4 mm | 0.48 | 0.49±0.011 | 0.28 | 0.5 |
| 6 mm | 1.43 | 0.50±0.01 | 0.48 | 0.5±0.007 |
| Development distance (optimised: 85 mm) | ||||
| 80 mm | 0.26 | 0.54±0.03 | 0.250 | 0.54±0.04 |
| 90 mm | 0.37 | 0.43±0.047 | 0.47 | 0.44±0.043 |
| Time between application to development | ||||
| 3 h | 0.99 | 0.49±0.02 | 0.55 | 0.49±.0.01 |
| 6 h | 2.48 | 0.50±0.017 | 1.76 | 0.49 |
| 24 h | 12.20 | 0.49±0.014 | 9.14 | 0.49±0.009 |
| Time between development to scanning | ||||
| 3 h | 0.24 | 0.49±0.007 | 0.10 | 0.50±0.02 |
| 6 h | 2.60 | 0.49±0.01 | 1.94 | 0.50 |
| 24 h | 15.71 | 0.49±0.01 | 13.10 | 0.50±0.017 |
Table 4: Data for Robustness of HPTLC method for analysis of E-Ajoene
The absorbance maxima obtained for E-ajoene was 250 nm. However, there was no significant change in peak areas when the plates were scanned at 250 nm and 254 nm (P>0.05). The absorbance maxima for all other constituents present in GE were also found to be close to 254 nm. Therefore, the plates were scanned at 254 nm throughout the study. As evident from Table 4, scanning at 240 nm led to significant decrease in the peak area of E-ajoene (P<0.05).
Reduction in the band width led to an increase in the peak area, but this change was not statistically significant (P>0.05). Increasing the band width led to significant reduction in the peak area (P<0.05). Varying the development distance did not have any significant effect on the peak area of E-ajoene, but as expected, changes in Rf values were observed. The %RSD values for all parameters evaluated in the study for all constituents present in the extract (results not shown) and isolated E-ajoene were <2. The results of the study indicate the robustness of the analytical method, however, band width and slit dimension while scanning were found to have significant effects on the peak areas of the constituents.
In order to evaluate the stability of E-ajoene on the chromatographic plate, the time between application to development and development to scanning were varied. As summarized in Table 4, it was observed that when the plates were developed 3 h post application, there was no significant change in peak area (P>0.05), whereas, the peak area values had significantly reduced when the plates were developed after 6 or 24 h post application (P<0.05). Similar results were observed when the time between developments to scanning was varied. It was concluded from the study that the plates should be developed and scanned within 3 h after application.
To estimate the content of E-ajoene in GE, 20 μl of stock solution of GE obtained from six different batches of garlic was applied in triplicate on a TLC plate along with isolated E-ajoene. A spot at Rf = 0.49±0.02, corresponding to Rf of E-ajoene was seen in the chromatogram of the extract along with other components. The peak area values of E-ajoene in nine batches of extract (prepared using garlic purchased from different vendors) were recorded and the content of E-ajoene in extract was determined using the calibration curve. It was observed that 100 mg of GE contained 0.56±0.33 mg of E-ajoene.
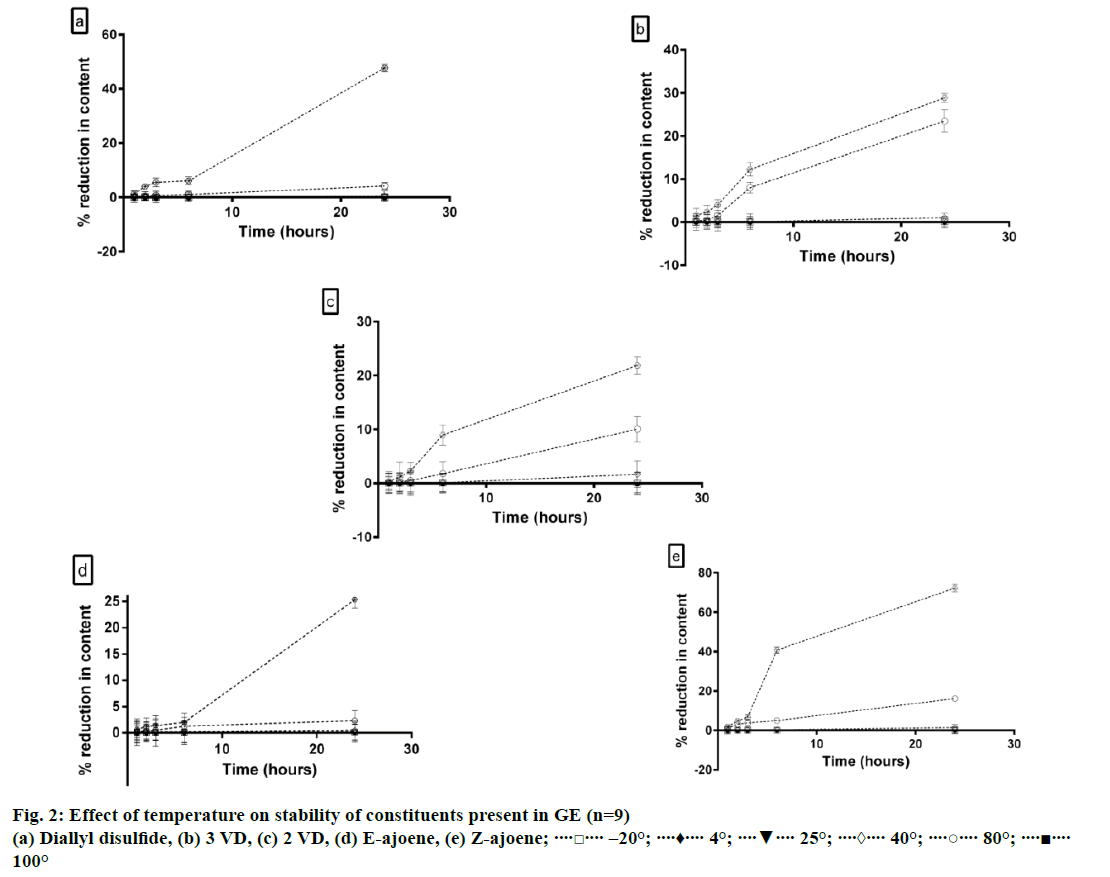
The effects of temperature, illumination, humidity, oxidant, pH and solvent on the stability of the constituents present in GE were evaluated in order to determine the optimum storage conditions for the extract. The results for evaluation of stability of five major constituents present in GE viz. diallyl disulphide, 3-VD, 2-VD, E-ajoene and Z-ajoene are summarized below. Allyl methyl disulphide, allyl methyl trisulphide and allicin, which were the minor constituents present in GE were highly unstable when GE was subjected to stress conditions. Therefore, the peak areas of these compounds after treatment of GE were not quantifiable.
It was observed that all the constituents, except allyl methyl disulphide, allicin and allyl methyl trisulphide were stable up to 40°. The content of allyl methyl disulphide, allicin and allyl methyl trisulphide reduced significantly after 6 h of heating at 40°. Significant reduction in content of all other constituents was observed only after 6 h of incubation at 80° (P<0.05). E-ajoene was significantly more stable than Z-ajoene at all the temperatures evaluated (P<0.05). Results of the study are summarized in Figure 2.
When a methanol solution of the extract was kept at 25, 4 and –20° for extended period of time, it was observed that all the constituents remained extremely stable at –20° up to 14 d. Some degradation was observed at 4°, though not statistically significant (P>0.05). Significant reduction in concentration of the thiosulfinate derivatives was observed when the extract was stored at 25° (P<0.05) for 14 d.
When the yellowish-brown oily extract was stored in dark at 25, 4 and –20° for 3 mon, it was observed that at 4° and –20°, the variations in content of each constituent present in the extract in comparison to the initial values were not statistically significant (P>0.05). However, almost 40% degradation was observed when the extract was stored at 25° for 3 mon (P<0.05).
Our results are in agreement with the findings reported by Yoo et al. in 2014 that the instability of allicinderivatives increased with increase in temperature [2]. The results of the study revealed that though thiosulfinate derivatives were stable at 25 to 40° for a short period of time, it is advisable to keep the extract at –20 or 4° for long-term storage.
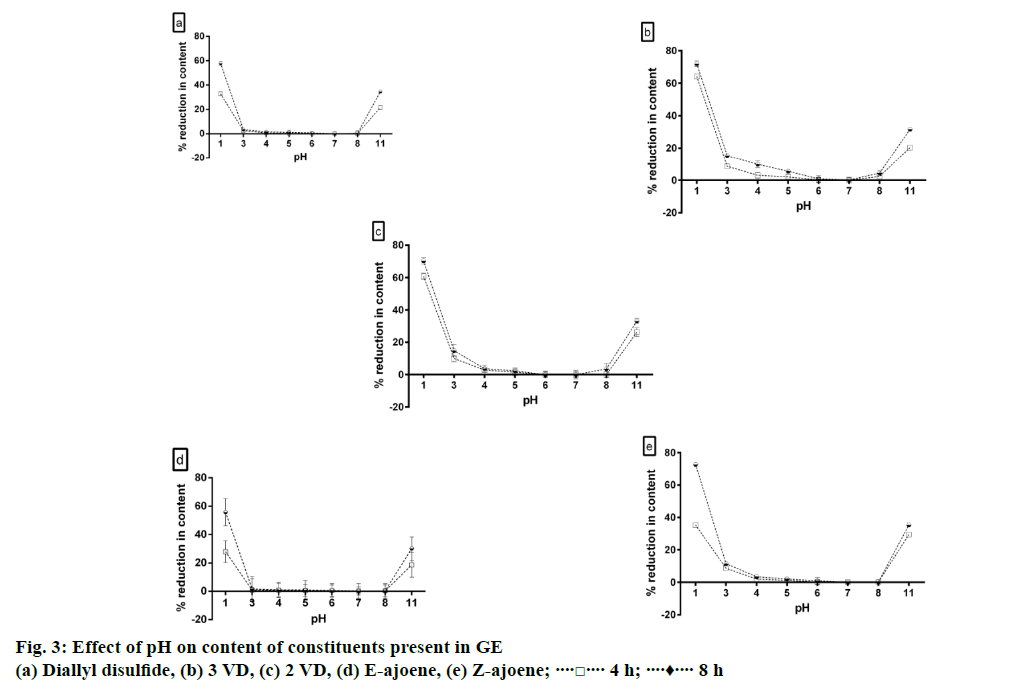
It was observed that the constituents degraded significantly in extremely acidic (pH 1) or basic pH (pH 11). Allyl methyl disulphide, allicin and allyl methyl trisulphide degraded rapidly at all the pH values used in the study. As demonstrated in Figure 3, all constituents, except allyl methyl disulphide, allicin and allyl methyl trisulphide, present in GE were most stable in the pH range of 4-7.
It was also observed that there was a quick fluctuation in pH on addition of acid or base to GE. It has been reported that sulfoxide groups present in organosulphur components of garlic undergo thio-Claisen condensation reaction, which led to the formation of S+ and O- groups simultaneously [20]. The presence of positive and negative charge simultaneously may be the possible reason for quick fluctuation in pH on addition of acid or base.
The reduction in concentration of E-ajoene due to changes in pH apparently follows second order reaction. The Eqn., 1/[At]=1/[A0]+kt, where, At was the concentration of E-ajoene in GE at time t and A0 was the concentration of E-ajoene in GE at time t0, allowed the calculation of the rate constant (k), which was equal to the slope of the linear regression curve obtained graphically i.e. 0.0254 for pH 1 and 0.0088 for pH 11. Half-life of E-ajoene in GE at pH 1 and 11 were calculated using the Eqn., t1/2=1/k[A0]. Half-life period of E-ajoene in GE at pH 1 and 11 were 6.320 h and 18.24 h, respectively. This data indicates that E-ajoene is significantly more unstable at pH 1 as compared to pH 11 (P<0.05).
Acidic and alkaline hydrolysis of GE led to significant reduction in content of all constituents (P<0.05). Percent reduction in the content of all constituents in GE after 2 h of acid hydrolysis was significantly greater than % reduction after alkaline hydrolysis (P<0.05). More than 80% reduction in peak areas of all the constituents was observed after acid and alkaline hydrolysis.
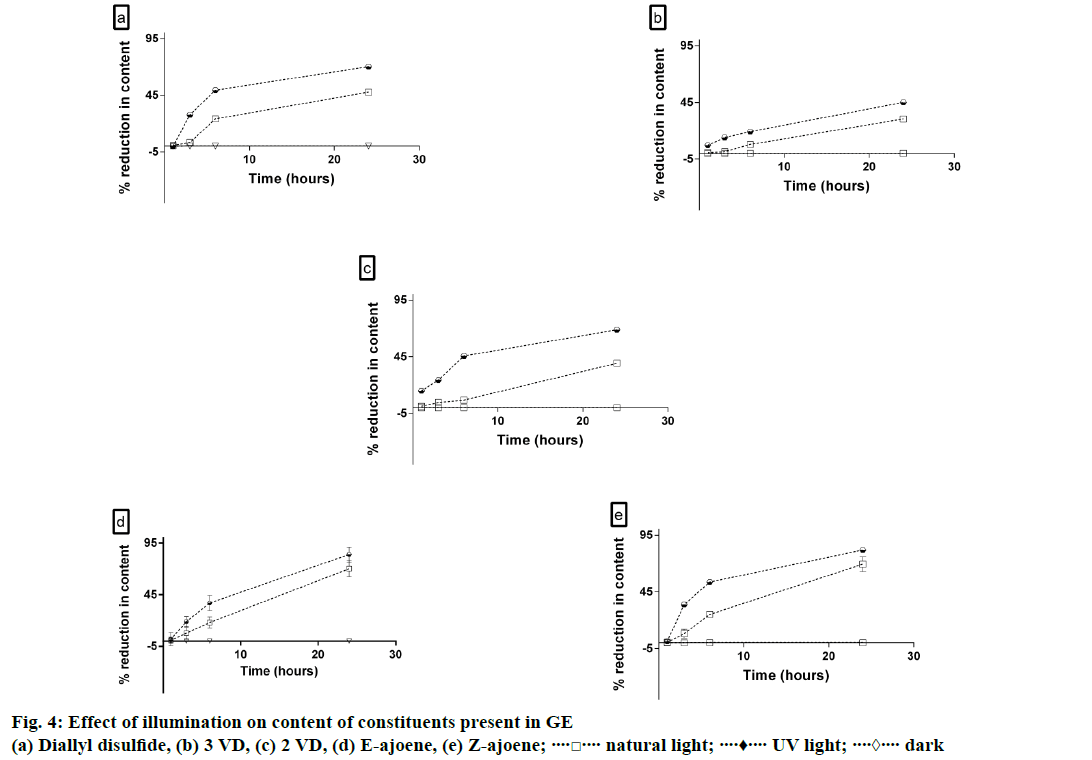
Peaks of allyl methyl disulphide, allicin and allyl methyl sulphide were below the detectable limits after just 1 h of exposure to UV light. All other constituents remained stable when stored in dark, but, they started degrading significantly within 3 h of exposure to natural light. Results of the study are summarized in Figure 4. In 2010, Ao et al. hypothesized that light degradation of a compound was promoted by absorbed energy from the photons [19]. Since the energy of ultraviolet light was much higher than the energy of natural light, constituents in GE degraded more in presence of UV light.
The order of reaction of degradation of E-ajoene in presence of light was determined graphically. It was observed that degradation of E-ajoene in presence of UV light followed first order reaction kinetics as the best correlation coefficient was obtained for the plot of logn (remaining concentration) versus time. The degradation of E-ajoene in natural light followed zero order kinetics.
The reaction kinetics for degradation of E-ajoene in GE in presence of natural light was determined using the Eqn., [At]=–kt+[A0], where At was the concentration of E-ajoene in GE at time t and A0 was the concentration of E-ajoene in GE at time t0. The value of k obtained after using this equation was same as the slope of line obtained graphically i.e. –0.1847. The half-life of E-ajoene in presence of natural light was calculated using the Eqn., t1/2=[A0]/2k. The half-life of E-ajoene when exposed to natural light was 16.2 h.
For degradation of E-ajoene in presence of UV light, the Eqn., logn [At]=logn[A0]–kt, where, At=concentration of E-ajoene in GE at time t, A0=concentration of E-ajoene in GE at time t=0) was used to determine the value of the rate constant (k), which was same as the slope of the linear regression curve obtained graphically i.e. –0.0344.
As summarized in Figure 5, it was observed that the content of all the constituents had significantly reduced after incubation of extract with 30% hydrogen peroxide for 7 d. The stability of extract in presence of oxidant was dependent on the concentration of hydrogen peroxide present in the solution. It was observed that E- ajoene was more stable than Z-ajoene in presence of oxidant. It has been reported in literature that ajoene possesses free radical scavenging activity [21]. We hypothesize that the possible reason for reduction of content of ajoene in GE after incubation with oxidant may be due to the reaction of ajoene with the free radicals generated to form a degradant or a by-product.
The effect of humidity on content of constituents present in GE was studied at 75% and 90% relative humidity. It was observed that the content of all the constituents significantly reduced after 14 d of incubation at both 75% and 90% relative humidity (P<0.05). The results of the study are summarized in Figure 6. Gradual degradation of all the constituents was observed after 7 d of incubation with the solvents. The order of stability of GE in solvents was methanol>ethanol>ethyl acetate>DMSO>acetone> chloroform>toluene>phosphate buffer 7.5>phosphate buffer 3.5.
It was observed that GE was extremely stable in phosphate buffer pH 7.4 and pH 3.5. From the study it was concluded that the constituents present in GE degrades rapidly when acid or base is added directly to the extract, but it remains stable when the pH of the solution is adjusted before addition of extract. A simple, robust and reproducible HPTLC method for estimation of content of ajoene in GE was developed. The method was also validated for estimation of other components present in the extract. The method proposed in this study is stability-indicating as small changes in concentration of all constituents present in GE were detected when the extract was subjected to stress conditions.
The forced degradation studies of GE revealed that stability of constituents in GE is dependent majorly on illumination, pH and temperature. Oxidation and humidity also play an important role in long- term stability of GE. To minimize the degradation of GE, we suggest that the extract should be stored in a dark place in an air tight container. The storage temperature should be –20 or 4°. The pH of the extract should preferably be maintained in the range of 4-7 for long term storage. Moreover, it is advisable that stock solutions should not be stored for more than 3 d in order to avoid degradation of GE.
Acknowledgements
This work was supported by Department of Atomic Energy- Board of Research in Nuclear Sciences for supporting this work (Project sanction no: 35/14/39/2014- BRNS).
Conflict of interest
All authors declare no conflict of interests.
Financial support
Nil.
References
- Bayan L, Koulivand PH, Gorji A. Garlic: A Review of Potential Therapeutic Effects. Avicenna J Phytomed 2014;4:1-4.
- Yoo M, Kim S, Lee S, Shin D. Validated HPLC Method and Temperature Stabilities for Oil-Soluble Organosulfur Compounds in Garlic Macerated Oil. J Chromatogr Sci 2014;52:1165-72.
- Ledezma E, Apitz-Castro R. Ajoene the Main Active Compound of Garlic (Allium sativum): A New Antifungal Agent. Rev Iberoam Micol 2006;23:75-80.
- Viswanathan V, Phadatare AG, Mukne A. Antimycobacterial and Antibacterial Activity of Allium sativumBulbs. Indian J Pharm Sci 2014;76:256-61.
- Jakobsen TH, van Gennip M, Phipps RK, Shanmugham MS, Christensen LD, Alhede M, et al. Ajoene, A Sulfur-Rich Molecule from Garlic, Inhibits Genes Controlled by Quorum Sensing. Antimicrob Agents Chemother 2012;56:2314-25.
- Vadekeetil A, Kaur G, Chhibber S, Harjai K. Applications of Thin-Layer Chromatography in Extraction and Characterisation of Ajoene from Garlic Bulbs. Nat Prod Res 2015;29:768-71.
- Jan CR, Lo HR, Chen CY, Kuo SY. Effect of Allyl Sulfides from Garlic Essential Oil on Intracellular Ca2+ Levels in Renal Tubular Cells. J Nat Prod 2012;75:2101-07.
- Keophiphath M, Priem F, Jacquemond-Collet I, Clément K, Lacasa D. 1, 2-vinyldithiin from Garlic Inhibits Differentiation and Inflammation of Human Preadipocytes. J Nutr 2009;139:2055-60.
- https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2__Guideline.pdf.
- Gafner S, Bergeron C. The Challenges of Chemical Stability Testing of Herbal Extracts in Finished Products Using State-of-the-art Analytical Methodologies. Cur Pharm Anal 2005;1:203-15.
- Naznin MT, Akagawa M, Okukawa K, Maeda T, Morita N. Characterization of E-and Z-Ajoene Obtained from Different Varieties of Garlics. Food Chem 2008;106:1113-19.
- Naznin MT, Maeda T, Morita N. Stability of E-and Z-Ajoene in Home-Made Mayonnaise. Int J Food Prop 2010;13:317-27.
- Akabari AH, Shah DR, Shah SA, Suhagia BN. Kinetic Determinations of Pitavastatin Calcium by Stability Indicating HPTLC Method. J Liq Chromatogr Relat Technol 2015;38:521-31.
- Paek SH, Ko C. Inventors; Samsung Electronics Co., Ltd., assignee. Method of Using Ajoene as Alcohol Dehydrogenase Inhibitor, Composition for Removing Hangover Comprising Ajoene and Method of Preparing the Same. United States patent application US 11/970,261. 2008 Jan 7.
- Nair SS, Gaikwad SS, Kulkarni SP, Mukne AP. Allium sativumConstituents Exhibit Anti-tubercular Activity In vitro and in RAW 264.7 Mouse Macrophage Cells Infected with Mycobacterium tuberculosisH37Rv. Pharmacogn Mag2017;13:S209-S15.
- Yoshida S, Kasuga SH, Hayashi NO, Ushiroguchi TS, Matsuura HI, Nakagawa SH. Antifungal Activity of Ajoene Derived from Garlic. Appl Environ Microbiol 1987;53:615-17.
- https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf.
- Srivastava MM. An overview of HPTLC: A Modern Analytical Technique with Excellent Potential for Automation, Optimization, Hyphenation and Multidimensional Applications. In:Srivastava M, editor. High-Performance Thin-Layer Chromatography (HPTLC). Berlin, Heidelberg: Springer; 2010. p. 3-24.
- Ao M, Shi Y, Cui Y, Guo W, Wang J, Yu L. Factors Influencing Glabridin Stability. Nat Prod Commun 2010;5:1907-12.
- Block E. The Organosulfur Chemistry of the Genus Allium–Implications for the Organic Chemistry of Sulfur. Angew Chem Int Ed Engl 1992;31:1135-78.
- Naznin MT, Maeda T, Morita N. Antioxidant Functions of E-and Z-ajoene Derived from Japanese Garlic. Int J Food Prop 2010;13:821-29.