- Corresponding Author:
- T. Phaechamud
Department of Pharmaceutical Technology
E-mail: thawatchaienator@gmail.com
| Date of Submission | 5 January 2012 |
| Date of Revision | 13 November 2012 |
| Date of Acceptance | 15 November 2012 |
| Indian J Pharm Sci., 2012, 74 (6): 498-504 |
Abstract
The purpose of this study is to investigate the effects of N-methyl-2-pyrrolidone on the thermosensitive properties of aqueous ethylene oxide-propylene oxide block copolymer (Lutrol® F127)] system. Due to the aqueous solubility enhancement and biocompatibility, N-methyl-2-pyrrolidone is an interesting solubilizer for the poorly water soluble drugs to be incorporated in the Lutrol® F127 system. Effect of N-methyl-2-pyrrolidone on physicochemical properties of Lutrol® F127 system was investigated using appearance, pH, gelation, gel melting temperature and rheology. The antimicrobial activity of the thermosensitive N-methyl-2-pyrrolidone gel was also tested. Lower N-methyl-2-pyrrolidone amount (≤:30%w/w) could shift the sol-gel transition to a lower temperature but the gel-sol transition was shifted to a higher temperature. Higher N-methyl-2-pyrrolidone (≥40%w/w) could shift both sol-gel and gel-sol transitions of the system to a lower temperature. The amount of N-methyl-2-pyrrolidone >60% w/w could reverse the phase of the Lutrol® F127 system to non-newtonian flow at 4° and Newtonian flow at high temperature. Aqueous Lutrol® F127 system containing N-methyl-2-pyrrolidone exhibited antimicrobial activities against Staphylococcus aureus, Escherichia coli and Candida albicans with the N-methyl-2-pyrrolidone in a dose-dependent manner.
Keywords
Antimicrobial, ethylene oxide-propylene oxide block copolymer, gelation, N-Methyl-2-pyrrolidone, thermosensitive gel
N-Methyl-2-pyrrolidone (NMP) is the lactam of 4-methylaminobutyric acid. It is colorless with high boiling point, low viscosity, low toxicity and good biocompatibility [1]. It is a water miscible organic solvent used as a good solvent for sparingly soluble drugs in water. The solubility parameter of NMP is similar to that of ethanol and dimethyl sulfoxide (DMSO) [2]. NMP increased transdermal absorption of some drugs such as phenolsulfonphthalein, ibuprofen and flurbiprofen [3,4] and also estradiol [5]. Atrigel- is the commercial injectable system based on poly (DL-lactide) in NMP used for the periodontal therapy [6,7].
Lutrol- F127 (LF127) (Pluronic F127 or poloxamer 407) consists of ethylene oxide (EO) and propylene oxide (PO) blocks arranged in a triblock structure PEOx-PPOy-PEOx. This nontoxic and nonionic surfactant exhibits reversible thermal characteristics.
Like other surfactants when dispersed in the liquid, at low concentrations, exist individually as monomolecular micelles. This leads to a decrease in the surface tension, as well as surface free energy. The increasing concentration of the polymer in system results in the formation of multimolecular aggregates [8,9]. Polypropylene oxide (PPO) forms central hydrophobic core wherein methyl groups interact via van der Waals forces with substance undergoing solubilization. However, water solubility is believed to be due to the polyethylene oxide (PEO) block by hydrogen bonding interactions between ether oxygen and water molecules. Micellar behavior of different block copolymers is found to be dependent upon solvent composition and temperature [10-12]. Micelle structure consists of hydrophobic PPO block lying in the micelle core and the PEO block in the corona [13,14]. These copolymer molecules aggregate into micelles when the temperature is increased. This micellization is due to the dehydration of hydrophobic PO blocks, which represents the early step in the gelling process. These micelles are spherical with a dehydrated PPO core with an outer shell of hydrated swollen PEO chains [15]. This micellization was followed by gelation for sufficiently concentrated samples. Gel strength increased with increase in temperature and concentration of Poloxamer 407. This polymer facilitated the solubilization of poorly water soluble molecules such as indomethacin [16], insulin [17] and piroxicam [18].
Some substances such as salts, surfactants, polymers, cosolvents showed marked effects on micellization, clouding and solubilization behavior of LF127 solutions. The addition of sodium chloride could lower the critical micelle concentration (CMC), whereas the addition of urea could increase the CMC. While the addition of sodium dodecyl sulphate caused the formation of polymer-sodium dodecyl sulphate complex or mixed micelle indicating polyelectrolyte nature [19]. Some solvents like ethanol and glycerol triacetate could exhibit amphiphilic behavior and act as cosurfactants by preferably locating at the interface between the PEO-rich and the PPO-rich domains. Diclofenac, ethanol and propylene glycol weaken LF127 gel, whereas sodium chloride, sodium monohydrogen phosphate and glycerine exhibited the opposite effect [20,21]. However, the effect of NMP on the characteristics of LF127 system has not been reported.
LF127 and NMP can be employed for the development of injectable dosage forms owing to their good biocompatibility. Due to the ability of NMP to enhance the aqueous solubility of poorly water soluble drugs, it is a good candidate to be used in LF127 system. The aim of our study was to develop the thermosensitive gels of NMP using LF127 as a gelling agent. The effect of NMP on physicochemical properties of gels was investigated including appearance, pH, gelation and gel melting temperature and rheology. The antimicrobial activity of the system containing NMP was first reported in this study.
Materials and Methods
Lutrol- F127 (LF127) was purchased from Vita Co., Ltd., Bangkok, Thailand. N-methyl-2-pyrrolidone (NMP) (Fluka, New Jersey, USA) was used as received. Sabouraud dextrose agar (SDA), Sabouraud dextrose broth (SDB), tryptic soy agar (TSA) and tryptic soy broth (TSB) (DifcoTM, USA) were used as media for antimicrobial test.
Preparation of Lutrol- F127 gel
The 20% w/w LF127 solutions containing 10, 20, 30, 40, 50, 60 and 80% w/w NMP were prepared using a cold method [8]. Briefly, LF127 was dissolved in distilled water at 4° until a homogeneous solution was obtained and NMP was subsequently added to with the polymer solution.
Evaluation of physical properties of gel
The appearances of the systems such as color and homogeneity were observed visually. The pH values of the systems were measured using a pH meter (Professional Meter PP-15 Sartorius, Goettingen, Germany). All measurements were performed in triplicate.
Gelation and gel melting temperature
The test tube inverting method as described previously [22,23] was employed to roughly determine the phase boundary. Gelation and gel melting temperatures were assessed using a modification of the previously reported technique [22]. Five millilitre aliquot of gel was transferred to a test tube (internal diameter of 1.8 cm) and sealed before immersing in a thermostat water bath at 4°. The temperature was controlled at the heating rate of 0.2°/min, and the transition temperature was monitored at an accuracy of ±1°. The samples were then examined for gelation, which was said to have occurred when the meniscus would no longer move upon tilting through 90° (n=3). The gel melting temperature, the temperature at which a gel started flowing upon tilting through 90°, was recorded (n=3).
Rheological behavior studies
The rheological behavior of prepared systems was investigated by recording their shear stress (F) as a function of shear rate (G) with a Brookfield DV-III Ultra programmable rheometer (Brookfield Engineering Laboratories. Inc., USA). To test the effect of temperature, the measurements were conducted at three different temperatures (4, 28 and 35°) (n=3). These three conditions were selected to represent the temperature in refrigerator, room temperature and temperature of human body, respectively. The continuous check for viscosity change with temperature was rather difficult to perform since there was notably different apparent viscosity of system at different temperature to measure with the same cone of the rheometer. Rheological behavior of the gels is expressed as N value (flowing parameter) of the Martin’s equation [24] given by, Log G=NLog F?Log ?'...(1), where ?' is apparent viscosity. For N value of 1 or close to 1, a rheological behavior is Newtonian and for N value not equal to 1, a rheological behavior is non-Newtonian but are dilatant (if N>1) and pseudoplastics (if N<1), or plastics if yield value was able to be determined [24].
Antimicrobial studies
Antimicrobial activity of the samples was tested using an agar cup diffusion method. The standard microbes used in this study were Staphylococcus aureus (ATCC 6538P), Escherichia coli (ATCC 10536) and Candida albicans (ATCC 17110). Ampicillin disc (10 µg) was used as a standard reference. The culture media for antibacterial and antifungal assay were TSA and SDA, respectively. The actively growing broth culture of microbes was prepared and the turbidity was adjusted to contain approximately 108 cells/ml. Then, the swab was spread onto the agar plate in three directions to cover whole plate. The sterilized cylinder cups were then placed carefully on the surface of the agar. The samples were filled into the cup and incubated at 37° for 24 h. Antimicrobial activities were determined by measuring diameters (cm) of clear zones of growth inhibition. The tests were carried in triplicate.
Results and Discussion
The 20% w/w LF127 gel base was transparent liquid at 4° whereas the transparent semisolid gel was formed at 27 and 37°. The 20%w/w LF127 systems containing <40% w/w NMP were transparent liquid at 4° and the preparations containing >50% NMP exhibited as cloudy gel. On the other hand, at 27° and 37°, the prepared systems containing <40% w/w NMP exhibited as transparent gel and the systems containing more than 50% w/w NMP exhibited as transparent solution. These results indicated that the appearance of LF127 systems was affected by the amount of NMP. The phase behavior and microstructure of poloxamer block copolymers was also affected by various organic solvents such as glycerol, propylene glycol and ethanol [25]. It was shown that although polar and completely miscible with water, the PEO-resembling and the PPO-resembling solvents such as NMP could affect the curvature of the self-assembled structure in opposite directions. The PEO-resembling solvents are located in the polar PEO-rich domains far from the interface and increase the curvature. The PPO-resembling solvents are preferably located in the relatively apolar PPO rich domains and at the interface affecting the curvature constant.
The pH of 20% LF127 gel without NMP was 5.20±0.01. The pH values of gel preparations comprising NMP were in the range of 5.16+0.03 to 5.27±0.02. The pH of 1% w/v NMP solution was 5.64±0.04. These results indicated that the NMP amount slightly changed pH value of gel preparations because the pH value of NMP was near to that of the gel base. As desired for any topical and other preparations, the pH of gel was close to the skin pH which is approximately 5.32 [26].
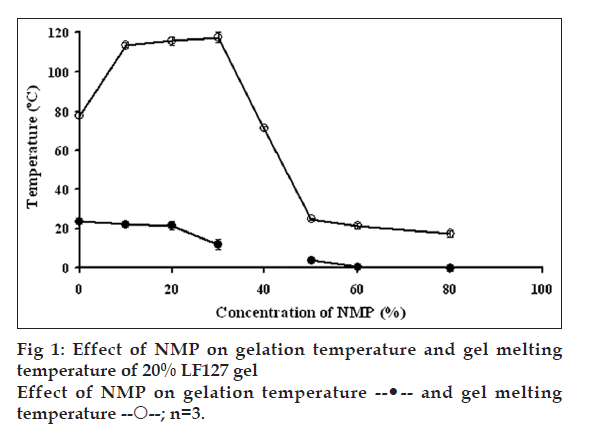
Effect of NMP on sol-gel and gel-sol transitions is shown in fig. 1. Gelation temperature is a temperature that system was changed from sol to gel state, whereas gel melting temperature is a temperature that system was changed to sol state. Sol-gel transition temperature of 20% w/w LF127 gel without NMP was 23.7±0.6°C. The addition of NMP influenced the sol-gel and gel-sol transitions of gels. LF127 system with lower NMP (=30% w/w) could shift the sol-gel transition to a lower temperature but the gel-sol transition was shifted to a higher temperature. LF127 system with higher NMP (=40% w/w) could shift both sol-gel and gel-sol transitions to a lower temperature. Thus, the gelation range of systems containing <30% w/w NMP was broader than that of the systems containing >50% w/w NMP. The sol-gel transition temperature of gel comprising 40% w/w NMP was not found because this system formed gel even at low temperature at 0°. These results suggested NMP decreased the gelation temperature of the system. The prior studies reported that the gelation of thermosensitive gel was affected by various factors, such as temperature and concentrations of polymer, active ingredients/ excipients and electrolytes [27-29]. The gel formation related to micellar packing and volume fraction. The type of structures obtained in the presence of selective solvent appeared to be a function of the volume fraction of the polar/nonpolar components [25]. The effects could be viewed in terms of a reduction of water activity leading to an increase in the active concentration of polymer in systems. The decreased gelation temperature was owing to the interaction between the hydrophobic portions of the polymer molecules, which could disrupt the micellar structure and increase the entanglement of micelles [30]. Most of flavors increased the viscosity of the poloxamer aqueous solution and decreased the gelling point of poloxamer 407 aqueous solution in proportion to amount added. The flavors might bind to the hydrophilic end chains of poloxamer, promoting dehydration resulting in a decrease in the gelation temperature of polymer solution [28]. The dominant hydrophobic portion of NMP caused the decrement of gelation temperature. This is in agreement with previous findings on the effects of hydrophobic molecules on the gel formation of PEO-PPO-PEO block copolymer systems. For example, t-butylbenzene could reduce the gelation temperature of LF127 [31] while benzoic acid and p-hydroxybenzoate esters can cause a decrease in the gelation temperature of the block copolymer. Thus, the more hydrophobic the solute the greater the decrease in gelation temperature [32]. There was a decreased gelation point of LF127 system with increasing amount of lidocaine and prilocaine [33]. This effect was owing to the presence of hydrophobic component inducing micellization resulting in the micellar growth. The ambiguous sol-gel transition temperature of gel comprising 40% w/w NMP might be due to the equilibrium of suitable amount NMP for dehydration of the LF127 molecule, therefore the gel was formed at low temperature.
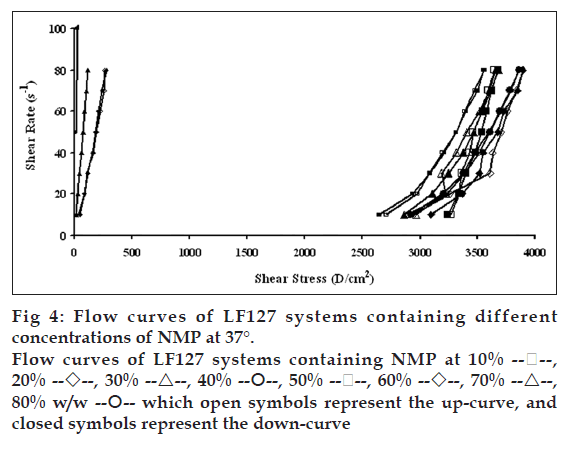
Flow curves of the systems comprising 20% w/w LF127 and different concentrations of NMP at different temperatures, 4, 27 and 37°, are shown in figs. 2, 3 and 4, respectively. The incorporated NMP caused an apparent change in the flow curve of the LF127 system. The flow curve shifted from low to high shear stress along the temperature increased when the concentration of NMP was less than 20%. The unique flow curve with initially increased and subsequently rapidly decreased shear stresses was evident for the systems containing 50 and 60% NMP at 4° because their initial gel strength was destroyed after shear rate was enhanced. As the concentration of NMP was higher than 50%, the flow curve shifted from high to low shear stress when the temperature increased. The change of temperature slightly affected the flow of systems containing 30-40% w/w NMP. The flow curve of system was the non-Newtonian behavior that the up curve did not coincide with the down curve indicating the presence of thixotropy, with a hysteresis loop. But the area of the hysteresis loop of all prepared systems did not increase as the NMP concentration increased. This result suggested that NMP could not enhance the thixotropic properties of the gel. However, the flow curve moved to a higher shear stress value, indicating the higher gel compactness [30].
The flow parameters of LF127 systems containing different concentrations of NMP at 4, 27 and 37° are shown in Table 1. The N values of the L systems containing 0-20% NMP increased with increasing temperature. The N values of 0-20% NMP systems at 4° were close to 1 indicating a Newtonian flow characteristic, while these values at higher temperature were>1 indicating a non-Newtonian flow characteristic [24]. The N values of the prepared gels containing 30-40% NMP were>1, which were not affected by the temperature. The increased NMP concentration from 50 to 80% w/w decreased the N values when the temperature increased. The N values of the prepared gels containing 50-80% NMP at 4° were more than one, indicating the non-Newtonian flow. Moreover, the N values of 50-80% NMP gels at 37° close to 1 indicating the Newtonian flow characteristic. The viscosity coefficients (h) of systems containing 0-20% NMP increased whereas that of 30-40% NMP slightly increased with temperature. On the other hand, the viscosity coefficient of systems containing 50-80% w/w NMP decreased when the temperature increased corresponding with above obtained flow curves.
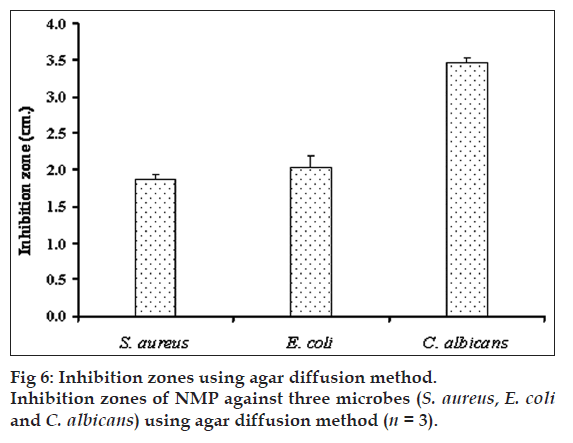
Antimicrobial activity of gels containing different concentrations of NMP against S. aureus, E. coli and C. albicans (fig. 5) were tested by agar diffusion method. Antibacterial activity against S. aureus and E. coli was increased as the amount of NMP was increased from 20-40% w/w while antifungal activity against C. albicans was enhanced by increasing the NMP amount. The gel base did not exhibit the clear zone. The inhibition zones of pure NMP against S. aureus, E. coli and C. albicans were 1.9±0.1, 2.0±0.2 and 3.5±0.1 cm, respectively (fig. 6) hence NMP could inhibit all tested microbes. The solubility parameter of NMP is similar to those of ethanol and DMSO [34]. Therefore, NMP could solubilize the lipid in cell membrane and promote the leakage of microbial cell membranes.
| NMP (% w/w) | 4° | 27° | 37° | |||||
|---|---|---|---|---|---|---|---|---|
| N | Viscosity coefficient | N | Viscosity coefficient | N | Viscosity coefficient | |||
| (mean±SD) (mean±SD, [Dyne/cm [2] ]N s) | (mean±SD) | (mean±SD, [Dyne/cm [2] ]N s) (mean±SD) (mean±SD, [Dyne/cm [2] ]N s) | ||||||
| 0 | 1.01±0.02 | -0.29±0.04 | 6.95±1.43 | 22.89±4.84 | 10.50±2.24 | 35.77±8.05 | ||
| 10 | 0.99±0.01 | -0.20±0.01 | 7.00±0.45 | 22.71±1.48 | 8.67±1.32 | 29.18±4.69 | ||
| 20 | 1.02±0.01 | 0.05±0.02 | 6.89±0.73 | 22.67±2.63 | 8.25±1.30 | 27.59±4.49 | ||
| 30 | 6.09±0.35 | 20.04±1.17 | 6.66±0.97 | 22.00±3.33 | 7.42±1.14 | 24.61±3.88 | ||
| 40 | 6.67±0.84 | 22.14±2.90 | 7.25±0.37 | 24.12±1.35 | 7.45±0.56 | 24.60±1.93 | ||
| 50 | 6.74±1.27 | 22.33±4.63 | 2.92±0.46 | 6.47±1.51 | 1.08±0.05 | 0.70±0.13 | ||
| 60 | 1.84±0.39 | 4.44±1.26 | 1.01±0.03 | 0.49±0.08 | 1.01±0.03 | 0.21±0.06 | ||
| 80 | 3.16±1.05 | 7.57±3.66 | 1.05±0.17 | -0.58±0.26 | 1.04±0.05 | -0.59±0.12 | ||
Table 1: Flow parameters of the aqueous l systems containing different concentrations of Nmp at different temperatures (N=3)
NMP affected the thermosensitive property of the aqueous LF127 system. Lower NMP amount (=30% w/w) could shift the sol-gel transition to a lower temperature but the gel-sol transition was shifted to a higher temperature. Higher NMP amount (=40% w/w) could shift both sol-gel and gel-sol transitions of that system to a lower temperature. The amount of NMP> 60% could reverse the phase of the LF127 system to non-Newtonian flow at 4° and the Newtonian flow at high temperature. Aqueous LF127 system containing NMP exhibited antimicrobial activities against S. aureus, E. coli and C. albicans in a dose-dependent manner.
Acknowledgements
This work was kindly supported by the Research, Development and Engineering (RD&E) Fund through National Nanotechnology Center (NANOTEC), National Science and Technology Development Agency (NSTDA), Thailand (Grant No. NM-B22-NE6-17-50-13) and the Faculty of Pharmacy, Silpakorn University. We appreciate Dr. Sumalee Wannachaiyasit and Dr. Nalinee Poolsup for their invaluable comments during the preparation of the manuscript.
References
- Ravivarapu HB, Moyer KL, Dunn RL. Sustained suppression of pituitary-gonadal axis with and injection, in situ forming implant of leuprolide acetate. J Pharm Sci 2000;8:732-41.
- Hansen CM, Just L. Prediction of environmental stress cracking in plastics with Hansen solubility parameters. Ind Eng Chem Res 2001;40:21-5.
- Akhter S, Barry BW. Absorption through human skin of ibuprofen and lurbiprofen; effect of dose variation, deposited drug ilm, occlusion and the penetration enhancer N-methyl-2-pyrrolidone. J Pharm Pharmacol 1985;37:27-37.
- Sasaki H, Kojima M, Mori Y, Nakamura J, Shibasaki J. Enhancing effect of pyrrolidone derivatives on transdermal drug delivery I. Int J Pharm 1988;44:15-24.
- Koizumi A, Fujii M, Kondoh M, Watanabe Y. Effect of N-methyl-2-pyrrolidone on skin permeation of estradiol. Eur J Pharm Biopharm 2004;57:473-8.
- Polson AM, Garrett S, Stoller NH, Bandt CL, Hanes PJ, Killoy WJ, et al. Multi-center comparative evaluation of subgingivally delivered sanguinarine and doxycycline in the treatment of periodontitis. II. Clinical results. J Periodontol 1997;68:119-26.
- Kranz H, Bodmeier R. Structure formation and characterization of injectable drug loaded biodegradable devices: In situ implants versus in situ microparticles. Eur J Pharm Sci 2008;34:164-72.
- Schmolka IR. Artiicial skin I. preparation and properties of pluronic F-127 gels for treatment of burns. Biomed Mater Res 1972;6:571-82.
- Guzman M, Aberturas MR, Garcia F, Molpeceres J. Gelatin gels and polyoxyethylene polyoxypropylene gels: Comparative study of their properties. Drug Dev Ind Pharm 1994;20:2041-8.
- Rassing J, Atwood D. Ultrasonic velocity and light scattering studies on polyoxyethylene-polyoxypropylene copolymer pluronic F-127 in aqueous solution. Int J Pharm 1983;13:47-55.
- Wanka G, Hoffmann H, Ulbricht W. Phase diagrams and aggregation behavior of poly (oxyethylene)-poly (oxypropylene)-poly (oxyethylene) triblock coplymers in aqueous solutions. Macromolecules 1990;27:4115-59.
- Wanka G, Hoffmann H, Ulbricht W. The aggregation behavior of poly (oxyethylene)-poly (propylene oxide)-poly (ethylene oxide) block copolymers in aqueous solution. Colloid Polym Sci 1990;268:101-17.
- Alxandridis P, Holtzwarth JF, Hatton TA. Micellization of poly (ethylene oxide)-poly (propyleneoxide) poly (ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules 1994;27:2414-25.
- Jorgenson EB, Hvidt S, Brown W, Schillen K. Effects of salts on micellization and gelation of triblock copolymer studied by rheology and light scattering. Macromolecules 1997;30:2355-64.
- Juhasz J, Lenaerts V, Raymond P, Ong H. Diffusion of rat atrial natriuretic factor in thermoreversible poloxamer gels. Biomaterials 1989;10:265-8.
- Dimitrova E, Bogdanova S, Mitcheva M, Tanev I, Minkov E. Development of model aqueous ophthalmic solution of indomethacin. Drug Dev Ind Pharm 2000;26:1297-301.
- Barichello JM, Morishita M, Takayama K, Chib Y, Tokiwa S, Nagai T. Enhanced rectal absorption of insulin loaded Pluronic F-127 gels containing unsaturated fatty acids. Int J Pharm 1999;183:125-32.
- Shin SC, Cho CW. Physicochemical characterizations of piroxicam-poloxamer solid dispersion. Pharm Dev Technol 1997;2:403-7.
- Desai PR, Jain NJ, Sharma RK, Bahadur P. Effects of additives on micellization of PEO/PPO/PEO block copolymer F127 in aqueous solution. Colloids Surf A: Physicochem Eng Aspects 2001;178:57-69.
- Choi H, Lee M, Kim M, Kim CK. Effect of additives on the physicochemical properties of liquid suppository bases. Int J Pharm 1999;190:13-9.
- Yong CS, Choi J, Quan QZ, Rhee JD, Kim CK, Lim SJ, et al. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int J Pharm 2001;226:195-205.
- Miller SC, Donovan MD. Effect of poloxamer 407 gel on the miotic activity of pilocarpine nitrate in rabbits. Int J Pharm 1982;12:147-52.
- Jeong B, Lee DS, Shon JI, Bae YH, Kim SW. Thermoreversible gelation of poly (ethylene oxide) biodegradable polyester block copolymers. J Polym Sci Polym Chem 1999;37:751-60.
- Martin A. Physical Pharmacy. Philadelphia: Lea and Febiger; 1993. p. 393-422.
- Ivanova R, Alexandridis P, Lindman B. Interaction of poloxamers block copolymers with cosolvents and surfactants. Colloids Surf A Physic Eng Aspects 2001;183-185:41-53.
- Eberlein-Konig B, Schafer T, Huss-Marp J, Darsow U, Mohrenschlager M, Herbert O, et al. Skin surface pH, stratum corneumhydration, trans-epidermal water loss and skin roughness related to atopic eczema and skin dryness in a population of primary school children. Acta Derm Venereol 2000;80:188-91.
- Pandit N, Trygstad T, Croy S, Bohorquez M, Koch C. Effect of salts on the micellization, clouding, and solubilization behavior of Pluronic F127 solutions. J Colloid Interf Sci 2000;222:213-20.
- Rhee YS, Shin YH, Park CW, Chi SC, Park ES. Effect of lavors on the viscosity and gelling point of aqueous poloxamer solution. Arch Pharm Res 2006;29:1171-8.
- Maheshwari M, Miglani G, Mali A, Paradkar A, Yamamura S, Kadam S. Development of tetracycline-serratiopeptidase-containing periodontal gel: Formulation and preliminary clinical study. AAPS Pharm Sci Tech 2006;7:E1-10.
- Maheshwari M, Paradkar A, Yamamura S, Kadam S. Preparation and characterization of pluronic?colloidal silicon dioxide composite particles as liquid crystal precursor. AAPS PharmSciTech 2006;7:E1-7.
- Malmsten M, Lindman B. Self-assembly in aqueous block copolymer solutions. Macromolecules 1992;25:5440-5.
- Gilbert JC, Richardson JL, Davies MC, Palin KJ, Hadgraft J. The effect of solutes and polymers on the gelation properties of pluronic F-127 solutions for controlled drug delivery. J Control Release 1987;5:113-8.
- Scherlund M, Malmsten M, Holmqvist P, Brodin A. Thermosetting microemulsions and mixed micellar solutions as drug delivery systems for periodontal anesthesia. Int J Pharm 2000;194:103-6.
- Hansen CM, Just L. Prediction of environmental stress cracking in plastics with Hansen solubility. Ind Eng Chem Res 2001;40:21-5.
 and gel melting
temperature
and gel melting
temperature  n=3.
n=3.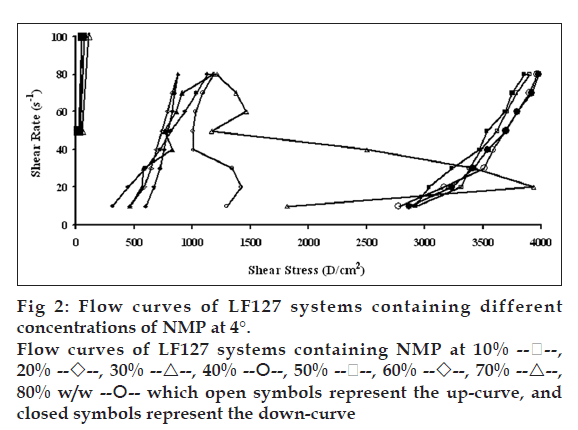
 20%
20%  30%
30%  40%
40%  50%
50%  60%
60%  70%
70%  80% w/w
80% w/w  which open symbols represent the up-curve, and
closed symbols represent the down-curve
which open symbols represent the up-curve, and
closed symbols represent the down-curve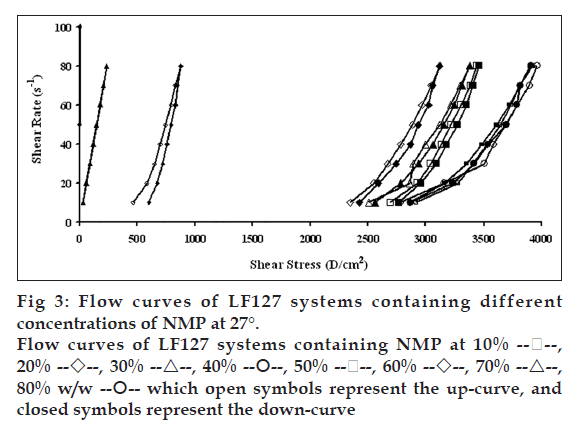
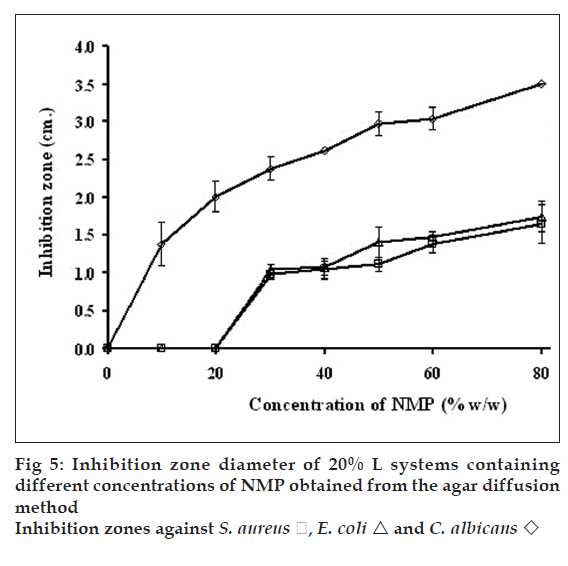
 E. coli
E. coli  and C. albicans
and C. albicans