- *Corresponding Author:
- H. M. Khan
Institute of Pharmacy, Lahore College for Women University, Lahore-54000, Pakistan
E-mail: humairaphd@hotmail.com
| Date of Submission | 03 June 2016 |
| Date of Revision | 30 November 2016 |
| Date of Acceptance | 23 January 2017 |
| Indian J Pharm Sci 2017; 79(1): 124-130 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Isoniazid and rifampicin are first line drugs used for the treatment of tuberculosis but their use is associated with potentially serious toxic manifestation in the liver leading to necrosis, cirrhosis and hepatitis causing poor patient compliance. The present study was conducted to evaluate hepatoprotective activity of Fumaria parviflora leaf extract in isoniazid and rifampicin-induced hepatotoxic rats. Acute toxicity was conducted with single oral doses of F. parviflora leaf extracts at 300, 2000 and 5000 mg/kg and rats were observed for changes in body weight, hematological parameters, behavioral changes and signs of toxicity. Hepatoprotective activity of F. parviflora leaf extract (100, 200 and 300 mg/kg, p.o.) was evaluated on isoniazid and rifampicin (50 mg/kg, i.p.) induced hepatotoxic rats using silymarin as a reference drug (100 mg/kg, p.o.). F. parviflora leaf extract was found to be safe up to 5 g/kg. From biochemical and histopathological parameters it was found that pretreatment of rats with F. parviflora leaf extract an hour prior to start isoniazid and rifampicin resulted in a significant decline in the serum levels of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase and total bilirubin as well as normal histology of liver biopsy specimens. It was concluded that F. parviflora leaf extract has significant hepatoprotective activity at the dose of 200 mg/kg comparable with that of silymarin. The plant extract appeared to have the potential to be used as a dietary supplement to antiTB therapy to protect against the hepatotoxicity of isoniazid and rifampicin.
Keywords
Tuberculosis, haematological parameters, hepatoprotective activity, Fumaria parviflora and histopathological parameters
Liver is the body’s largest gland, which plays a role in digestion, metabolism and storage of nutrients within the body, detoxification of drugs and toxins, regulation of homeostasis and providing defence against diseases [1]. Drug-induced hepatotoxicity has become a leading cause of liver maladies accounting for 2-5% hospitalizations for jaundice, 40% cases of acute hepatitis and 15-30% cases of fulminant hepatic failure [2].
Tuberculosis (TB), a deadly infectious disease is one of the leading causes of death. Pakistan ranks 5th amongst TB high-burden countries worldwide and has annual emergence of about 420 000 cases. Isoniazid (INH) and rifampicin (RMP), the 1st line drugs for treatment of TB are potentially hepatotoxic producing many metabolic and morphological aberrations in liver with incidence rate of about 2-28%. INH mediated oxidative damage is attributed to metabolic conversion of INH to its toxic metabolites that is exacerbated by RMP, a cytochrome P450 inducer, leading to poor patient compliance and emergence of multiple drug resistant TB [3]. In the ethnomedical traditional practices numerous medicinal plants and their formulations have been employed for alleviation of liver disorders. Conventional drugs used for the mitigation and cure of liver disorders are still inadequate. Plants possessing antioxidant activity are primarily sought for liver protective effects.
Fumaria parviflora lam. (fine leaf fumitory) is a herbaceous plant of the genus Fumaria belonging to the family Fumariaceae, found in Turkey, Indo-Pakistan and Iran. The plant is reported to have many biological properties such as in dermatological diseases [4], antidiarrheal, antispasmodic, bronchodilator [5], hypoglycemic [6], antiinflammatory [7], anthelmintic [8] and male fertility enhancing property [9]. The present study was conducted to evaluate acute toxicity and hepatoprotective activity of F. parviflora leaf extract against INH and RMP-induced liver damage.
Materials and Methods
Fresh Leaves of F. parviflora were collected from the wheat fields of Bhera, district of Sargodha division. The plant was authenticated at the Department of Botany, University of Sargodha, Pakistan. A voucher No. 6030 was preserved in the herbarium, Department of Botany, University of Sargodha for future research reference. INH (Novartis Pharma Pakistan, Ltd., Batch No. 308), RMP (Novartis Pharma Pakistan, Ltd., Batch No. J0039) and silymarin (Abbott Laboratories, Pakistan, Batch No. 036N) were used in study. The percent purity of INH and RMP was ascertained according to the method described in British Pharmacopoeia [10] and found to be of standard quality. Stock solutions of INH and RMP were prepared having 10 mg/ml concentration in sterilized distilled water using dimethyl sulfoxide (DMSO) as the solvent.
Preparation of plant extract
Five kilograms of fresh leaves of F. parviflora Lam. were washed under running tap water, followed by rinsing with distilled water, shade-dried and pulverized in a mechanical grinder. Plant powder so obtained was subjected to maceration with 70% methanol (BDH Labs, CAT No: 443847D) followed by filtration by vacuum filter assembly (Sigma Aldrich Z290424) using 0.1 μ pore size filter paper. The methanol extract obtained was concentrated to dryness using rotary evaporator (Buchi Rotavapor R-3) at 50-55° [11]. The % yield was calculated to be about 12% (w/w). The extract was stored at 4° and dissolved in sterile distilled water prior to use.
Experimental animals
Healthy and active Wistar rats of either sex, weighing 150-200 g, were purchased from University of Health Sciences, Lahore and kept in large propylene cages (55×32.7×19 cm) with paddy husk as bedding in animal house of Lahore College for Women University, Lahore under standard environmental conditions (12 h light and dark cycle, 22±3° temperature and 35-60% humidity) according to the guidelines provided by Institute of laboratory animal research for the care and use of laboratory animals [12]. Study protocol was approved by research animal care and use committee, Institute of Pharmacy, Lahore College for Women University, Pakistan (Dir/LCWU/756-AE Dated 4-12- 15). Rats were acclimatized to laboratory condition for 1 w prior to commencement of experiment.
Acute toxicity
Acute toxicity of F. parviflora leaf extract was determined in Wistar rats with single oral dose at 300, 2000 and 5000 mg/kg according to OECD- 423 guidelines [13]. Rats were observed daily for 14 d for change in mean body weight, haematological parameters, behavioural changes and signs of toxicity including convulsions, twitching, rigidity, jumping, sedation, loss of righting reflex, catatonia, ataxia, loss of traction, cyanosis, blanching reddening and mortality. Body weights of rats and liver weights were recorded in each group on different days and statistical means compared for any difference.
Hepatoprotective activity
Experimental rats (n=30) were divided randomly into six groups, each having five rats. Treatment of each group carried out was as: group-1 was the control group and animals in this group were administered normal saline (1 ml, p.o.) once daily for 15 d. Group-2 was referred to as the induced group and animals of this group received INH and RMP (50 mg/kg each, i.p.) once daily for 15 d. Group-3, the reference group, in which animals were administered silymarin (100 mg/ kg, p.o.), an hour prior to INH and RMP (50 mg/kg each, i.p.), once daily for 15 d. Group-4, the treated group A, in which animals were administered dried methanol extract of F. parviflora (100 mg/kg, p.o.), an hour prior to INH and RMP (50 mg/kg each, i.p.), once daily for 15 d. Group-5, was referred to as the treated group B, the animals of which received dried methanol extract of F. parviflora (200 mg/kg, p.o.), an hour prior to INH and RMP (50 mg/kg each, i.p.), once daily for 15 d. Group-6, the treated group C in which animals were administered dried methanol extract of F. parviflora (300 mg/kg, p.o.), an hour prior to INH and RMP (50 mg/kg each, i.p.), once daily for 15 d.
Biochemical parameters
Twenty-four hours post administration of the last dose of INH and RMP, blood samples (4 ml) of all rats were collected in heparinized tubes by cardiac puncture according to guidelines provided by laboratory animal science association [14]. The sera separated by centrifugation at 3000 rpm for 15 min were assayed for estimation of liver marker enzymes including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and total bilirubin in auto-analyser (Humalyzer-3500) by using reagent kits for ALT (Lot No. 14405), AST (Lot No. SA- 170), ALP (Lot No. SA-173) and total bilirubin (Lot No. 45590) manufactured by Randox and Human Diagnostic Division. Standard spectrophotometric methods as described by Wilkinson [15] were used to estimate ALT and AST in serum by measuring the decrease in absorbance of NADH at 340 nm, which is oxidized to NAD in the reaction involving conversion of L-alanine and L-aspartate to pyruvate and oxaloacetate. The ALP was estimated by measuring the concentration of p-nitro phenol formed from the hydrolysis of p-nitro phenyl phosphate by enzyme in the presence of oxidizing agent (Mg+²) and measured by absorbance at 405 nm proportional to ALP activity [16]. For estimation of bilirubin in serum Jendrassik Grof Method was used in which diazotized sulfanilic acid in acidic medium was converted to pink coloured azobilirubin by bilirubin and absorbance measured at 578 nm proportional to bilirubin concentration [17].
Histopathological grading
Rats were euthanized under high dose of anaesthetic ether and dissected to expose the liver. Liver specimens were removed, labelled, preserved in 10% phosphate buffered formalin, dehydrated with 70% isopropyl alcohol, impregnated and embedded in molten paraffin wax. Paraffin blocks prepared were cut into 3-4 μ thick sections using rotary microtome. These sections were de-paraffinized and stained with haematoxylin and eosin (H and E) [18]. Liver biopsy specimens were scored and graded using the Knodell histology activity index (HAI) [19].
Statistical analysis
The obtained data were statistically analysed using one way analysis of variance (ANOVA) test to determine overall effects of the treatments using statistical package SPSS Version 17.0. Post individual comparison was carried out with Fisher’s Least Significant Difference (LSD) test. Results were presented as mean±SD. P≤0.05 were considered statistically significant.
Results and Discussion
Single oral administration of F. parviflora leaves extract to experimental animals at dose rate of 300, 2000 and 5000 mg/kg revealed non-significant difference in mean body weight and haematological parameters among control group and treated groups. Rats treated with the extract did not show any behavioural changes and no signs of toxicity. No mortality occurred in any of treated and control groups over a period of 14 d.
According to anatomical variable (liver weight/ corporal weight relationship), reference group and treated groups A, B and C showed a preserved hepatic function, in a clear opposition to the liver damage induced by the combination of INH and RMP. On statistical analysis of data in different groups at selected days non-significant difference was observed between controls and treated whereas significant with induced group (Table 1). Hepatoprotective activity of plant extract was evaluated based upon biochemical and histological lesions.
| Groups | Initial body weight (g) | Final body weight (g) | Body weight gain (g) | Liver weight (g) | Liver weight/final body weight (%) |
|---|---|---|---|---|---|
| Control | 158.2±5.76 | 192.4±6.58 | 34.2±8.32 | 3.4±0.14 | 1.77±0.12 |
| Induced | 159.4±7.99a | 165±7.76a | 6±1.41a | 4.76±0.21a | 2.88±0.06a |
| Reference | 160±6.04* | 194±3.08* | 34±7.11* | 3.39±0.03* | 1.75±0.02* |
| Treated A | 156.4±5.59* | 199±7.42* | 42.6±6.73* | 3.79 ±0.17* | 1.91±0.09* |
| Treated B | 153.4±4.93* | 200.8±8.98* | 47.4±7.83* | 3.73±0.19* | 1.86±0.1* |
| Treated C | 157.8±4.60* | 204.2±16.2* | 46.4±14.1* | 3.82±0.11* | 1.88±0.20* |
Table 1: Effect of F. Parviflora leaves extract on relationship between liver weight and corporal weight of control and experimental rats
The effect of F. parviflora leaves extract on liver biomarkers of experimental rats has been illustrated in Table 2. Induced group showed significant increase (P<0.05) in serum level of ALT up to 69.4±5.31 IU/l, AST up to 69±3.00 IU/l, ALP up to 153±4.69 IU/l and total bilirubin up to 1.98±0.34 mg/dl as compared to control group. Administration of F. parviflora leaves extract at 100, 200 and 300 mg/kg an hour prior to INH and RMP resulted in significant decrease (P<0.05) in elevated serum level of ALT (61.8±4.38 IU/l at 100 mg/kg, 29±3.74 IU/l at 200 mg/kg and 30.2±5.06 IU/l at 300 mg/kg), AST (60.8±4.20 IU/l at 100 mg/kg, 29±2.23 IU/l at 200 mg/kg and 26.8±3.63 IU/l at 300 mg/kg), ALP (88.2±3.03 IU/l at 100 mg/kg, 84±3.16 IU/l at 200 mg/kg and 88±5.09 IU/l at 300 mg/kg) and total bilirubin (1.12±0.59 mg/dl at 100 mg/kg, 0.62±0.29 mg/dl at 200 mg/kg and 0.60±0.29 mg/dl at 300 mg/kg). Plant extract at 200 mg/kg was found to be more potent than plant extract at 100 mg/kg body weight in reducing serum level of ALT, AST, ALP and total bilirubin (P<0.05) showing comparable efficacy with that of silymarin.
| Group | Treatment (mg/kg) | Biochemical parameters | |||
|---|---|---|---|---|---|
| ALT (IU/l) | AST | ALP | Total bilirubin (mg/dl) | ||
| (IU/l) | (IU/l) | ||||
| Control | Normal saline (1 ml) | 25.8±3.56 | 24.8±3.11 | 87.4±4.33 | 0.52±0.13 |
| Induced | INH+RMP(50+50) | 69.4±5.31a | 69±3.00a | 153±4.69a | 1.98±0.34a |
| Reference | Silymarin+INH+RMP (100+50+50) | 31.6±3.36* | 28.8±2.86* | 86±4.00* | 0.58±0.21* |
| Treated group A | FP+INH+RMP (100+50+50) | 61.8±4.38* | 60.8±4.20* | 88.2±3.03* | 1.12±0.59* |
| Treated group B | FP+INH+RMP (200+50+50) | 29±3.74* | 29±2.23* | 84±3.16* | 0.62±0.29* |
| Treated group C | FP+INH+RMP (300+50+50) | 30.2±5.06* | 26.8±3.63* | 88±5.09* | 0.60±0.29* |
Table 2: Effect of Fumaria Parviflora Leaves Extract on Liver Biomarkers in Control, Induced, Reference and Treated Groups
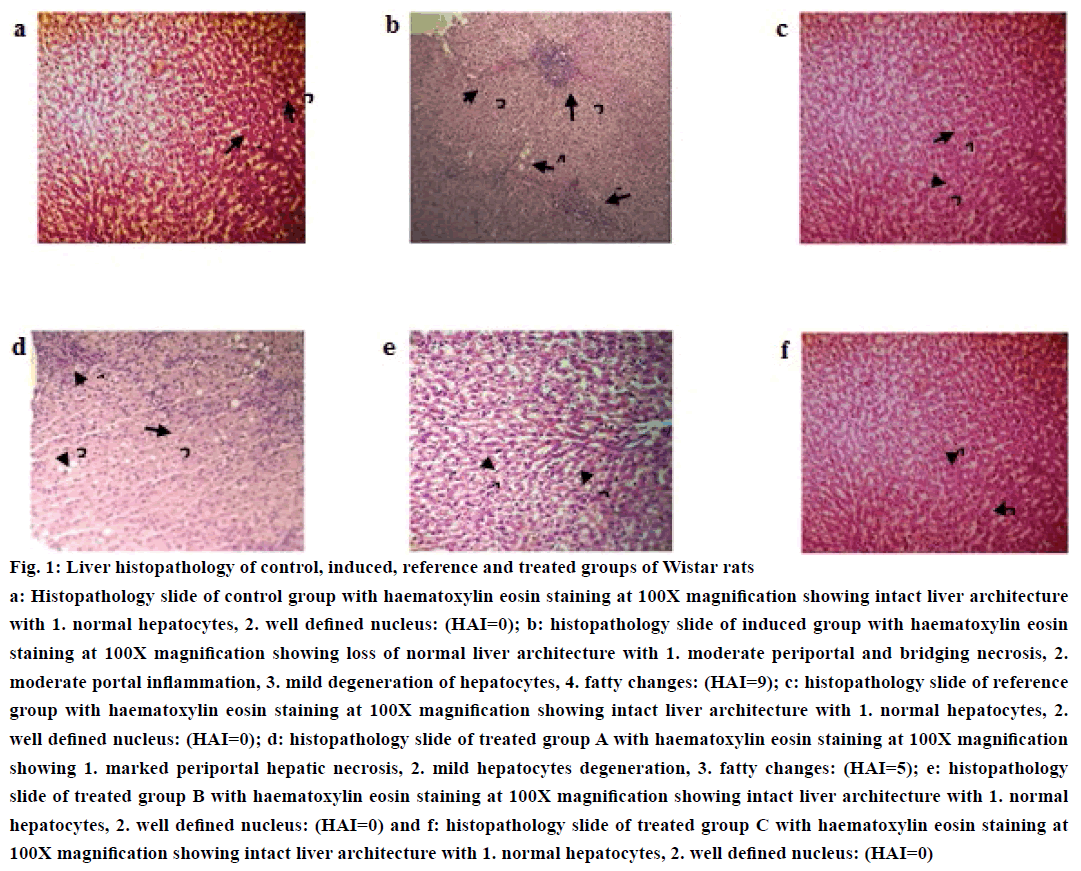
Histopathological findings were enumerated in the form of numerical scoring by using the HAI (Table 3). Histopathological scoring of transverse sections of liver biopsy specimens of control group showed intact liver architecture with normal hepatocytes and well defined nucleus with HAI=0.00±0.00 (Figure 1a) while the liver biopsy specimens of induced group showed moderate to marked periportal necrosis, moderate periportal and bridging necrosis, mild to moderate hepatocytes degeneration and fatty changes and moderate portal inflammation with HAI=8.4±0.89 (Figure 1b). Histological examination of transverse sections of liver specimens of treated group A showed moderate to marked periportal inflammation, mild degeneration of hepatocytes and fatty changes with HAI=4.4±0.55 while no pathological lesions were observed in histopathological sections of reference group and treated groups B and C (Figure 1c, d, e and f).
| Groups | Histopathologicallesions | HAI | Mean HAI | ||||
|---|---|---|---|---|---|---|---|
| Periportal +/- bridging necrosis | Intralobular degeneration and focal necrosis | Portal inflammation | Fibrosis | ||||
| Control group | 1 | 0 | 0 | 0 | 0 | 0 | 0.00±0.00 |
| 2 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | 0 | 0 | 0 | 0 | 0 | ||
| 5 | 0 | 0 | 0 | 0 | 0 | ||
| Induced group | 1 | 5 | 1 | 3 | 0 | 9 | 8.4±0.89a |
| 2 | 3 | 3 | 3 | 0 | 9 | ||
| 3 | 3 | 1 | 3 | 0 | 7 | ||
| 4 | 5 | 1 | 3 | 0 | 9 | ||
| 5 | 4 | 1 | 3 | 0 | 8 | ||
| Reference group | 1 | 0 | 0 | 0 | 0 | 0 | 0.00±0.00* |
| 2 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | 0 | 0 | 0 | 0 | 0 | ||
| 5 | 0 | 0 | 0 | 0 | 0 | ||
| Treated group A | 1 | 3 | 1 | 0 | 0 | 4 | 4.4±0.55* |
| 2 | 3 | 1 | 0 | 0 | 4 | ||
| 3 | 4 | 1 | 0 | 0 | 5 | ||
| 4 | 4 | 1 | 0 | 0 | 5 | ||
| 5 | 3 | 1 | 0 | 0 | 4 | ||
| Treated group B | 1 | 0 | 0 | 0 | 0 | 0 | 0.00±0.00* |
| 2 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | 0 | 0 | 0 | 0 | 0 | ||
| 5 | 0 | 0 | 0 | 0 | 0 | ||
| Treated group C | 1 | 0 | 0 | 0 | 0 | 0 | 0.00±0.00* |
| 2 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | 0 | 0 | 0 | 0 | 0 | ||
| 5 | 0 | 0 | 0 | 0 | 0 | ||
Table 3: Histological Scoring of Transverse Sections of Liver Specimens
Figure 1: Liver histopathology of control, induced, reference and treated groups of Wistar rats
a: Histopathology slide of control group with haematoxylin eosin staining at 100X magnification showing intact liver architecture with 1. normal hepatocytes, 2. well defined nucleus: (HAI=0); b: histopathology slide of induced group with haematoxylin eosin staining at 100X magnification showing loss of normal liver architecture with 1. moderate periportal and bridging necrosis, 2. moderate portal inflammation, 3. mild degeneration of hepatocytes, 4. fatty changes: (HAI=9); c: histopathology slide of reference group with haematoxylin eosin staining at 100X magnification showing intact liver architecture with 1. normal hepatocytes, 2. well defined nucleus: (HAI=0); d: histopathology slide of treated group A with haematoxylin eosin staining at 100X magnification showing 1. marked periportal hepatic necrosis, 2. mild hepatocytes degeneration, 3. fatty changes: (HAI=5); e: histopathology slide of treated group B with haematoxylin eosin staining at 100X magnification showing intact liver architecture with 1. normal hepatocytes, 2. well defined nucleus: (HAI=0) and f: histopathology slide of treated group C with haematoxylin eosin staining at 100X magnification showing intact liver architecture with 1. normal hepatocytes, 2. well defined nucleus: (HAI=0)
Drug-induced liver injury has become a critical health issue caused by many drugs such as antiretroviral drugs, antitubercular drugs, antipsychotics, antihyperlipidemics, NSAID’s, and immunosuppressants [20]. The current study was conducted to evaluate firstly, the acute toxicity followed by appraisal of hepatoprotective activity of methanol extract of F. parviflora leaves in amelioration of INH and RMP mediated hepatic damage.
In acute toxicity, the highest dose of the leaf extract (5 g/kg) used was proved to be safe without any signs of toxicity or mortality observed. According to the Organization for Economic Co-operation and Development (OECD) guidelines [13] for acute oral toxicity study, the plant having LD50 value of 5 g/kg or higher is considered as safe or nontoxic. However, in present study the dose of 100, 200 and 300 mg/kg was selected for evaluation of hepatoprotective activity of F. parviflora leaves extract which were far lower than the highest dose tested in experiment carried out for toxicity study.
Hepatotoxicity induced by INH and RMP is characterized by increase in serum level of liver biomarkers such as ALT, AST, ALP and total bilirubin as a result of hepatocellular dysfunction and loss of cellular integrity of cell membrane. F. parviflora leaves extract mediated suppression of the INH and RMP induced increased ALT, AST and ALP activity with concurrent depletion of raised total bilirubin in serum is an indication of stabilization of plasma membrane and biliary tract as well as repair of hepatocellular damage caused by INH and RMP [21].
Previous studies have demonstrated the effectiveness of aqueous and methanol extract of F. parviflora (500 mg/kg, p.o.) against PCM-induced hepatotoxicity [22] and against nimsulide-induced hepatotoxicity [23] and antihepatotoxic activity was appeared to be mediated through its stimulatory effect on antioxidant enzymes and inhibitory effect on microsomal mediated apoptosis. Authors have suggested that the hepatoprotective activity of plants is attributed to antioxidant activity of isoquinolone alkaloids present in them [24]. Antioxidant activity of methanol extract of F. parviflora has also been evaluated by Hamedeyazdan concluding that the plant extract possesses an ability to provide defence against oxidative stress caused by drugs, toxins and xenobiotics [25]. High Performance Liquid Chromatography-diode array detector (HPLC-DAD) analysis of four Fumaria species such as F. vaillantii, F. parviflora, F. jankae and F. rostellata has revealed the presence of many isoquinolone alkaloids in them especially protopine, allocryptopine, sanguinarine, chelidonine, stylopine, bicuculline, hydrastine and cheleritrine. F. parviflora was found to be the richest source of protopine containing about 288.27 mg of protopine/100 g of plant [26]. Protopine (11 mg/kg, p.o.) was found to be potent hepatoprotectant against PCM and CCl4-induced hepatotoxicity and hepatoprotective potential was due to its inhibitory effect on drugs metabolizing enzymes, activation of which leads to oxidative stress [27]. In a study by Rathi, protopine was isolated from whole plant of F. indica and was evaluated for its hepatoprotective potential against galactosamine-induced liver damage concluding that protopine (10-20 mg/kg, p.o.) plays an important role in mediating hepatoprotection [28].
From acute toxicity of F. parviflora leaves extract on experimental animals it was found that the plant extract is safe up to dosage of 5000 mg/kg suggesting that the F. parviflora leaves extract is safe for therapeutic purposes with wide safety dosage profile. From the biochemical and histopathological evaluation it is concluded that F. parviflora leaves extract has significant hepatoprotective activity against INH and RMP-induced hepatotoxicity and it has shown comparable efficacy with silymarin at dose of 200 mg/kg. Therefore it is reasonable to conclude that this plant extract has the potential to be a nutritional supplement to patients receiving antitubercular therapy to ameliorate the toxic effects of INH and RMP. However, the therapeutic efficacy and toxicity of active constituents of F. parviflora needs to be explored extensively and large scale clinical trials are to be conducted to ascertain its safety and efficacy in humans.
Acknowledgments
The authors are grateful to the authorities of Institute of Pharmacy, Lahore College for Women University, Lahore and Department of Pharmacology, University of Veterinary and Animal Sciences, Lahore for providing the necessary facility to carry out this research work. The authors also thank Prof. Amin Ullah Shah, Department of Botany, University of Sargodha, Pakistan for authenticating the plant sample.
Financial support and sponsorship
Nil.
References
- Tortora GJ, Derrickson BH. The digestive system. In: Roesch B, editor. Principles of Anatomy and Physiology, 12th ed. Hoboken: John Wiley and Sons 2008. p. 945-51.
- Kshirsagar A, Vetal Y, Ashok P, Bhosle P, Ingawale D. Drug induced hepatotoxicity: A comprehensive review. Int J Pharmacol 2008;7:1.
- Pandit A, Sachdeva T, Bafna P. Drug Induced Hepatotoxicity: A Review. J App Pharma Sci 2012;2:233-43.
- Jowkar F, Jamshidzadeh A, Yazdi MA, Pasalar M. The Effects of FumariaparvifloraExtract on Chronic Hand Eczema: A Randomized Double-Blind Placebo Controlled Clinical Trial. Iran Red Cres Med J 2011;13:824-8.
- Rehman NU, Bashir S, Al-Rehaily AJ, Gilani AH. Mechanisms Underlying the Antidiarrheal, Antispasmodic and Bronchodilator Activities of Fumariaparviflora and Involvement of Tissue and Species Specificity. J Ethnopharmacol 2012;144:128-37.
- Fathiazad F, Hamedeyazdan S, Khosropanah MK, Khaki A. Hypoglycemic Activity of FumariaParviflorain Streptozotocin-Induced Diabetic Rats. Adv Pharm Bull 2013;3:207-10.
- Arzi A, Nazari Z, Salianeh SVA. Effect of FumariaParviflora hydro alcoholic extract on induced carageenan inflammation in male rat paw. Jentashapir J Health Res 2013;4:121-30.
- Naz I, Palomares-Rius JE, Blok SV, Khan MR, Ali S, Ali S. In vitroand in plantanematicidal activity of Fumariaparviflora(Fumariaceae) against the southern root-knot nematode Meloidogyne incognita. Plant Pathol 2013;62:943-52.
- Dorostghoal M, Seyyednejad SM, Khajehpour L, Jabari A. Effects of FumariaparvifloraLeaves Extract on Reproductive Parameters in Adult Male Rats. Iran J Reprod Med 2013;11:891-8.
- British Pharmacopoiea, London: HMSO Publication; 2007. p. 664.
- Tiwari P. Pharmaceutical Screening and Extraction. IntPharmaceutSci 2011;1:98-106.
- https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf.
- https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf.
- http://www.dilab.com/pdf/lasa_blood_sampling.pdf.
- Wilkinson JH, Baron DN, Moss DW, Walker PG. Standardization of clinical enzyme assays: a reference method for aspartate and alanine transaminases. J ClinPathol 1972;25:940-4.
- Tietz NW, Burtis CA, Duncan P, Ervin K, Petitclerc CJ, Rinker AD, et al. A reference method for measurement of alkaline phosphatase activity in human serum. ClinChem 1983;29:751-61.
- Cherian AG, Soldin SJ, Hill JG. Automated Jendrassik-Grof Method for Measurement of Bilirubin in Serum with the Greiner Selective Analyzer (GSA II D), and Comparison with the Method Involving Diazotized 2,4-Dichloroaniline. ClinChem 1981;27:748-52.
- Slaoui M, Fiette L. Histopathology procedures: from tissue sampling to histopathological evaluation. Methods MolBiol2011;691:69-82.
- Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981;1:431-5.
- Ahmad F, Tabassum N. Experimental models used for the study of antihepatotoxic agents. J Acute Dis 2012;1:85-9.
- Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. Antituberculosis drug induced hepatotoxicity: concise up-to-date review. J GastroenterolHepatol 2008;23:192-202.
- Gilani AH, Janbaz KH, Akhtar MS. Selective protective effect of an extract from FumariaParvifloraon paracetamol induced hepatotoxicity. Gen Pharmacol 1996;27:979-83.
- Tripatha M, Singh BK, Raisuddin S, Kakkar P. Abrogation of nimesulide induced oxidative stress and mitochondria mediated apoptosis by FumariaParvifloraLam. extract. J Ethnopharmacol 2011;136:94-102.
- Orhan IE, Sener B, Musharraf SG. Antioxidant and hepatoprotective activity appraisal of four selected Fumariaspecies and their total phenol and flavonoid quantities. ExpToxicolPathol2012;64:205-9.
- Hamedeyazdan F, Fathiazad H, Nazemiyeh S, Faezi SA. Phytochemical analysis and antioxidant activity of Fumariaparviflora. Res Pharm Sci 2012;7:S762.
- Paltinean R, Toiu A, Wauters JN, Frederich M, Tits M, Angenot L, et al. Identification and Determination of Alkaloids in FumariaSpecies from Romania. Digest J NanomatBiostruct 2013;8:817-24.
- Janbaz KH, Saeed SA, Gilani AH. An assessment of the potential of protopine to inhibit microsomal drug metabolizing enzymes and prevent chemical induced hepatotoxicity in rodents. Pharmacol Res 1998;38:215-9.
- Rathi A, Srivastava AK, Shirwaikar A, Rawat AKS, Mehrotra S. Hepatoprotective potential of FumariaindicaPugsley whole plant extracts, fractions and an isolated alkaloid protopine. Phytomedicine: Int J PhytotherapyPhytopharmacol 2008;15:470-7.