- *Corresponding Author:
- Krishnapriya Mohanraj
Department of Pharmaceutical Chemistry, Bombay College of Pharmacy, Kalina, Santacruz (E), Mumbai-400 098, India
E-mail: krishnapriyamohanraj@gmail.com
| Date of Submission | 13 June 2013 |
| Date of Revision | 2 December 2013 |
| Date of Acceptance | 8 December 2013 |
| Indian J Pharm Sci 2014;76(1):46-53 |
Abstract
A green chemistry approach for organic synthesis is described here, which involves microwave exposure of reactants in presence or absence of solvents. A novel and simple method has been developed for the synthesis of some benzotriazole derivatives under microwave irradiation. In addition, these compounds were synthesised also by conventional heating procedures for comparison. All the compounds synthesised were characterised by melting point, TLC, IR and 1 H NMR spectroscopy. Comparison between conventional and microwave-assisted synthesis was done by comparing total reaction time and percentage yield. The results suggest that microwave-assisted syntheses lead to higher yields within very short reaction times. On antifungal evaluation by cup plate method, all compounds showed antifungal activity. One compound showed activity similar to and two compounds showed better activity than standard antifungal drug flucanazole.
Keywords
Benzotriazole derivatives, conventional synthesis, microwave-assisted synthesis
Microwave-assisted synthesis is a branch of green chemistry. Microwave-assisted synthesis has gained much attention in recent years. Microwave irradiation-assisted chemical transformations are pollution free, eco-friendly and offer high yields together with simplicity in processing and handling [1-5].
Heating reactions with traditional equipment, such as oil baths, sand baths and heating mantles, is not only slow but it creates a hot surface on the reaction vessel where products, substrates and reagents often decompose over time. Microwave energy, in contrast, is introduced into the chemical reactor remotely and passes through the walls of the reaction vessel, heating the reactants and solvents directly. Microwave dielectric heating drives chemical reactions by taking advantage of the ability of some liquids and solids to transform electromagnetic radiation into heat wherein chemical reactions are accelerated because of selective absorption of microwave energy by the polar molecules [6]. A properly designed vessel allows the temperature increase to be uniform throughout the sample, leading to fewer by-products and/or product decomposition. The use of microwave energy instead of conventional heating often results in good yields in a short time as compared with reaction by classical synthetic methods [7-9].
Nowadays, microwave-assisted organic synthesis is gaining widespread acceptance in drug discovery laboratories. The rapid acceptance of this technology parallels the rising cost of R&D and decrease in the number of Food and Drug Administration (FDA) approvals, which have led to what is termed as a productivity crisis. Reducing the cost of failure, either by failing candidates sooner or by improving the overall probability of success, is the most powerful solution to improving R&D productivity. Microwave technology, by accelerating chemical reactions from hours or days to minutes, provides quick results. From time to time, microwave heating enables chemistries that were not previously possible by classical methods, expanding the realm of structures accessible to the chemist [10].
Katritzky and Singh reported the recent applications of microwave technology in well-known cyclisation reactions for heterocyclic ring formation and in other important reactions such as nucleophilic substitution, hetero-Diels-Alder reactions, 1,3-dipolar cycloaddition [11]. Benzotriazole is also a heterocyclic compound that can be a good leaving group, acts as an ambient anion director, an electron donor, radical or carbanion precursor. Benzotriazole is easy to introduce into molecules by a variety of condensations, additions and substitution reactions. Benzotriazole derivatives are of biological, chemical and industrial importance. These derivatives exhibit a good degree of analgesic, antiinflammatory, diuretic, antiviral and antihypertensive activities [12].
Materials and Methods
Synthesis was carried out using modified reported procedures for conventional synthesis [13-17] and reactions were modified to improve the yield and purity of products. For comparison, microwaveassisted synthesis was also carried out. All solvents and reagents used for synthesis were of general reagent grade. The reactions were monitored by thin layer chromatography (TLC) with Merck precoated silica plates (GF 254). Melting points were recorded in open capillaries in a thiel’s tube melting point apparatus and are uncorrected. Infrared (IR) was recorded (KBr disc method) on a Jasco Fourier transform infrared spectroscopy FT-IR 5300 spectrophotometer. 1H NMR spectra were recorded on Varian USA Mercury Plus (300 MHz) or Bruker (300 MHz) NMR spectrophotometer. Chemical shifts are reported in ppm down field from tetramethylsilane (TMS), which is used as the internal standard. MWassisted reactions were carried out in a domestic microwave oven (Samsung M183DN) at 180 and 300 W.
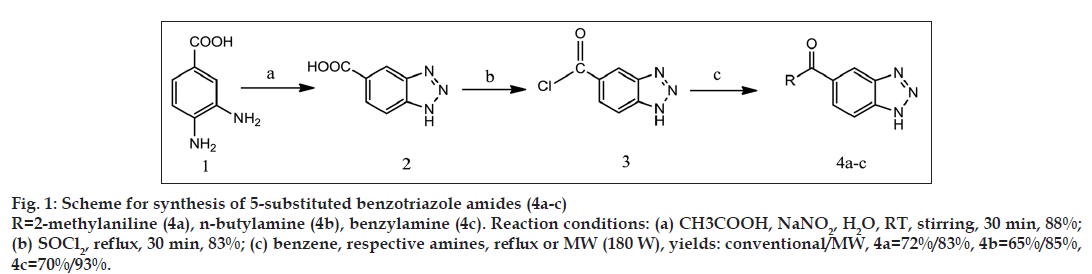
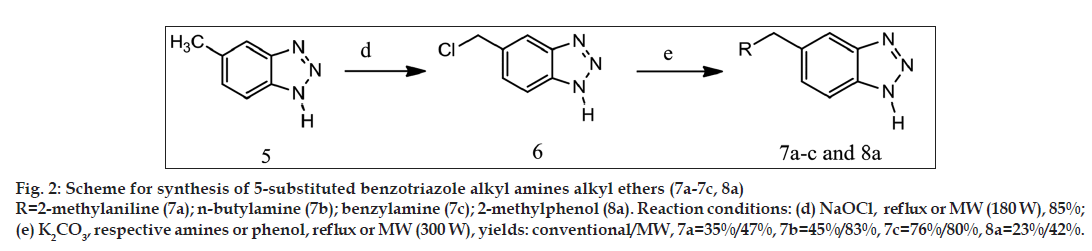
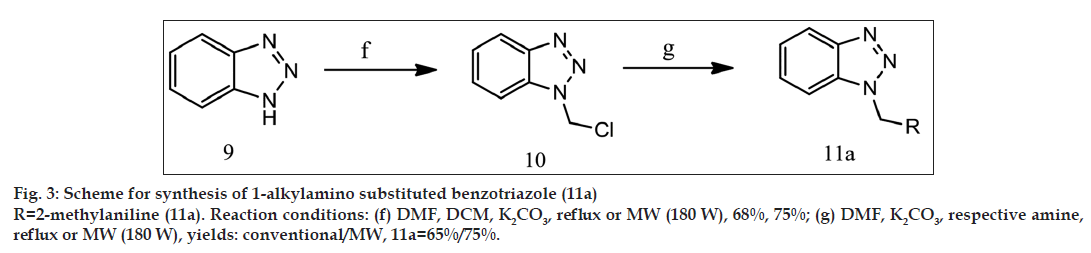
General synthetic scheme was used to synthesise 1 and 5 substituted benzotriazole analogues as shown in figs. 1-3.
Synthesis of benzotriazole-5-carboxylic acid (2)
Suspension of 3,4-diaminobenzoic acid (2 g, 13.15 mmol) was made in glacial acetic acid (5 ml, 75.36 mmol) and stirred magnetically. Solution of sodium nitrite (1 g, 16.66 mmol) was made in 5 ml of water. This was added to the suspension of 3,4-diaminobenzoic acid in glacial acetic acid in one portion while stirring. By this addition, temperature of reaction mixture increased slightly. Stirring was continued till the temperature was reached to room temperature. After completion of reaction (30 min) product was collected by filtering the reaction mixture. Product was washed with cold water to remove excess of acetic acid, and remaining residue was dried. The obtained product is pale brown coloured amorphous powder [13]. Percentage yield: 88%, melting point (MP): 299º. IR: 3510 cm-1 (N–H stretch), 3400–2400/cm (O–H stretch of carboxylic acid), 3030/cm (Aromatic C-H stretch), 1707/cm (C=O stretch of α-β unsaturated acids), 1304/cm (C–N bending), 1H NMR: 8.543 δ (s, 1H, aromatic proton of benzotriazole, Hd), 8.033 δ (d, 1H, aromatic proton of benzotriazole, Hc), 7.966 δ (d, 1H, aromatic proton of benzotriazole, Hb), 2.520 δ (s, 1H, N–H of benzotriazole).
Synthesis of benzotriazole-5-carbonyl chloride (3)
Benzotriazole-5-carboxylic acid (1.5 g, 9.20 mmol) and thionyl chloride (6 ml, 9.78 g, 82.10 mmol) were refluxed together in a 25 ml RBF fitted with calcium chloride guard tube. After completion of reaction (30 min) excess of thionyl chloride was removed by distillation (boiling point of thionyl chloride: 74º); remaining residue was further washed with 20% sodium bicarbonate solution (3×10 ml) to remove traces of thionyl chloride in the form of acid. One water wash (1×10 ml) was given and the remaining residue was dried [14]. The obtained product is dark brown coloured amorphous powder. Percent yield: 83%, MP: 151º.
Synthesis of N-o-tolyl-1H-benzo[d][1,2,3]triazole-5- carboxamide (compound 4a)
Benzotriazole-5-carbonyl chloride (1 g, 5.50 mmol) was mixed with 5 ml benzene; to this mixture equimolar proportion of o-toluidine in 10 ml of benzene was added and reaction was carried out by reflux using heating mantle for conventional synthesis and by microwave irradiation (180 W) using microwave oven for microwave-assisted synthesis. After completion of reaction (reflux=4 h, microwave=4 min 30 s), 10% hydrochloric acid was added to reaction mixture to remove excess of o-toluidine as its hydrochloride salt. Benzene layer was further washed with water (3×10 ml). Benzene layer was passed through anhydrous sodium sulphate [15]. Product was obtained as a light brown crystalline powder by removal of benzene by distillation. Percentage yield: 72% by reflux and 83% by microwave method, MP: 218º by reflux and 220º by microwave method. IR: 3254 cm-1 (N–H stretch), 3047 cm-1 (C–H stretch, aromatic) 2914 cm-1 (C–H stretch, aliphatic), 1649 cm-1 (C=O stretch of α, β unsaturated amide), 1608 cm-1 (C=C stretch), 1319/ cm (C–N bending), 1H NMR: 10.078 δ (s, 1H, O=C–N–H), 8.623 δ (s, 1H, aromatic proton of benzotriazole, Hd), 8.049 δ (d, 1H, aromatic proton of benzotriazole, Hc), 7.992 δ (d, 1H, aromatic proton of benzotriazole, Hb), 7.381 δ (d, 1H, Ar–H), 7.284 δ (t, 1H, Ar–H), 7.211 δ (t, 1H, Ar–H), 7.159 δ (d, 1H, Ar–H), 2.266 δ (s, 4H, -CH3, N–H of benzotriazole).
Other compounds 4b-c were synthesised in the similar manner by treating benzotriazole-5-carbonyl chloride with respective amines. Yields and melting points are given in Table 1.
| Compound | % Yield | MP (º) | Total reaction time | |||
|---|---|---|---|---|---|---|
| Conventional (%) | MW (%) | Conventional | MW | Conventional | MW | |
| 2 | 88 | - | 299 | - | 30 min | - |
| 3 | 83 | - | 151 | - | 30 min | - |
| 4a | 72 | 83 | 218 | 220 | 4 h | 4 min 30 s |
| 4b | 65 | 85 | 210 | 211 | 4 h 15 min | 4 min 10 s |
| 4c | 70 | 93 | 230 | 228 | 3 h 30 min | 4 min |
| 6 | 72 | 85 | 180 | 182 | 2 h 45 min | 4 min 20 s |
| 7a | 35 | 47 | 201 | 202 | 3 h | 11 min 20 s |
| 7b | 45 | 83 | 185 | 188 | 5 h 30 min | 4 min 20 s |
| 7c | 76 | 80 | 177 | 178 | 5 h | 6 min 30 s |
| 8a | 23 | 42 | 205 | 206 | 5 h 15 min | 6 min 10 s |
| 10 | 68 | 75 | 134 | 135 | 6 h | 4 min 20 s |
| 11a | 65 | 75 | 123 | 125 | 5 h 30 min | 3 min 10 s |
For all synthesis 180 W MW was used except for 7a, 7b, 7c and 8a, for which 300 W MW was used.
Table 1: Yields, Melting Points and Total Reaction Time for Synthesised Benzotriazole Derivatives
N-butyl-1H-benzo[d][1,2,3]triazole-5-carboxamide (4b)
MP 210º, IR: 3278/cm (N–H stretch), 3047/cm (C–H stretch, aromatic) 2934/cm (C–H stretch, aliphatic), 1655/cm (C=O stretch of α, β unsaturated amide), 1608/cm (C=C stretch), 1325/cm (C–N bending), 1H NMR: 8.500 δ (t, 1H, O=C–N–H), 8.366 δ (s, 1H, aromatic proton of benzotriazole, Hd), 7.817 δ (d, 1H, aromatic proton of benzotriazole, Hc), 7.750 δ (d, 1H, aromatic proton of benzotriazole, Hb), 3.300 δ (q, 2H, -CH2-), 2.520 δ (s, 1H, N-H of benzotriazole), 1.547 δ (p, 2H, -CH2-), 1.362 δ (sextet, 2H, -CH2-), 0.928 δ (t, 3H, -CH3).
N-benzyl-1H-benzo[d][1,2,3]triazole-5-carboxamide (4c)
MP 228º, IR: 3288/cm (N–H stretch), 3024/cm (C–H, aromatic stretch), 2843/cm (C–H stretch, aliphatic), 1645/cm (C=O stretch of α, β unsaturated amide), 1568/cm (C=C stretch), 1332/cm (C–N bending), 1H NMR: 9.315 δ (t, 1H, O=C–N–H), 8.50 δ (s, 1H, aromatic proton of benzotriazole, Hd), 7.985 δ (d, 1H, aromatic proton of benzotriazole, Hc), 7.930 δ (d, 1H, aromatic proton of benzotriazole, Hb), 7.320 δ (m, 5H, Ar–H), 4.540 δ (d, 2H, -CH2-), 2.50 δ (s, 1H, N–H of benzotriazole).
Synthesis of 5-chloromethylbenzotriazole (6)
5-methylbenzotriazole (1 g, 7.518 mmol) and sodium hypochlorite (18.2 ml, 1.1 g, 15 mmol, 1:0.5:0.5, 6% w/v solution in water) were mixed together and reaction was carried out by reflux using heating mantle for conventional synthesis and by microwave irradiation (180 W) using microwave oven for microwave-assisted synthesis. After completion of reaction (Reflux=2 h 45 min, microwave= 4 min 20 s) product was extracted by ethyl acetate (3×15 ml). Ethyl acetate layer was passed through anhydrous sodium sulphate. Ethyl acetate was distilled off to get the desired product [16]. The obtained product is dark yellow coloured amorphous powder after recrystallisation by toluene. Percentage yield: 72% by reflux and 85% by microwave method; MP: 180º by reflux and 182º by microwave method.
Synthesis of 5[tolylaminomethyl][1,2,3]benzotriazole (7a)
5-chloromethylbenzotriazole (0.3 g, 1.79 mmol), o-toluidine (1 ml, 1 g, 9.34 mmol) and potassium carbonate (0.248 g, 1.8 mmol) were mixed together and reaction was carried out by reflux using heating mantle for conventional synthesis and by microwave irradiation (300 W) using microwave oven for microwave-assisted synthesis. After completion of reaction (reflux= 3 h, microwave= 11 min 20 s), 10% hydrochloric acid was added in reaction mixture to remove excess of o-toluidine as its hydrochloride salt; product was obtained by filtration and recrystallised by toluene as light brown coloured amorphous powder. Percentage yield: 35% by reflux and 47% by microwave method; MP: 201º by reflux and 202º by microwave method. IR: 3256/cm (N–H stretch), 3042/ cm (C–H stretch, aromatic), 2918/cm (C–H stretch, aliphatic), 1649/cm (C=C stretch), 1319/cm (C–N bending), 1H NMR: 8.19 δ (s, 1H, aromatic proton of benzotriazole, Hd), 7.890 δ (d, 1H, aromatic proton of benzotriazole, Hc), 7.780 δ (d, 1H, aromatic proton of benzotriazole, Hb), 7.555 δ (d, 1H, Ar–H), 7.420 δ (t, 1H, Ar–H), 7.210 δ (d, 1H, Ar–H), 7.110 δ (t, 1H, Ar–H), 4.33 δ (s, 2H, -CH2-), 3.96 δ (s, 1H, aliphatic N–H), 2.25 δ (s, 4H, -CH3, N–H of benzotriazole).
Other compounds 7b–c was synthesised in the similar manner by treating 5-chloromethylbenzotriazole with respective amines. Yields and melting points are given in Table 1.
5[butylaminomethyl][1,2,3]benzotriazole (7b)
MP 188º, 1H NMR: 8.499 δ (s, 1H, aromatic proton of benzotriazole, Hd), 7.817 δ (d, 1H, aromatic proton of benzotriazole, Hc), 7.777 δ (d, 1H, aromatic proton of benzotriazole, Hb), 3.727 δ (s, 2H, -CH2-), 2.789 δ (t, 2H, -CH2-), 2.396 δ (s, 1H, N–H of benzotriazole), 1.899 δ (s, 1H, aliphatic N–H), 1.547 δ (p, 2H, -CH2-), 1.322 δ (sextet, 2H, -CH2-), 0.928 δ (t, 3H, -CH3).
5[benzylaminomethyl][1,2,3]benzotriazole (7c)
MP 178º, IR: 3383/cm (N-H stretch), 2926/cm (C–H stretch), 1608/cm (C=C stretch), 1299/cm (C–N bending), 1H NMR: 7.918 δ (s, 1H, aromatic proton of benzotriazole, Hd), 7.660 δ (d, 1H, aromatic proton of benzotriazole, Hc), 7.636 δ (s, 1H, aromatic proton of benzotriazole, Hb), 7.318 δ (t, 3H, Ar–H), 7.192 δ (d, 2H, Ar–H), 3.711 δ (s, 4H, -CH2-,-CH2-), 2.583 δ (s, 2H, N–H of benzotriazole, aliphatic N–H).
Synthesis of 5(o-tolyloxymethyl)[1,2,3]benzotriazole (8a)
5-chloromethylbenzotriazole (0.3 g, 1.79 mmol), o-cresol (1 ml, 1.04 g, 10.8 mmol) and potassium carbonate (0.248 g, 1.8 mmol) were mixed together and reaction was carried out by reflux using heating mantle for conventional synthesis and by microwave irradiation (300 W) using microwave oven for microwave-assisted synthesis. After completion of reaction (reflux= 5 h 15 min, microwave= 6 min 10 s); 10% aqueous solution of NaOH was added to remove excess of o-cresol; product was obtained as light brown coloured amorphous powder by filtration and recrystallisation by toluene. Yield: 23% by reflux and 42% by microwave method; MP: 205º by reflux and 206º by microwave method. IR: 3385/cm (N–H stretch), 2928/cm (C–H stretch), 1562/cm (C=C stretch), 1288/cm (C–N bending), 1259/cm (C–O bending), 1H NMR: 8.225 δ (s, 1H, aromatic proton of benzotriazole, Hd), 7.962 δ (d, 1H, aromatic proton of benzotriazole, Hc), 7.845 δ (d, 1H, aromatic proton of benzotriazole, Hb), 7.381 δ (d, 1H, Ar–H), 7.284 δ (t, 1H, Ar–H), 7.211 δ (d, 1H, Ar–H), 7.159 δ (t, 1H, Ar-H), 4.165 δ (s, 2H, -CH2-), 2.266 δ (s, 4H, N–H of benzotriazole, -CH3).
Synthesis of 1-chloromethylbenzotriazole (10)
Benzotriazole (2 g, 16.8 mmol) was taken in 50 ml RBF and 10 ml of DMF was added to RBF. Dichloromethane (6.8 g, 5 ml, 80 mmol) and potassium carbonate (2.31 g, 16.8 mmol) were added in flask. Reaction was carried out by reflux using heating mantle for conventional synthesis and by microwave irradiation (180 W) using microwave oven for microwave-assisted synthesis and monitored by TLC (chloroform). After TLC showed completion of reaction (reflux= 6 h, microwave= 4 min 20 s) product was precipitated by transferring the reaction mixture to 250 ml beaker containing ice cold water (25 ml). Product was obtained by filtering the solution and it was recrystallised by hot water to remove impurity [17]. The obtained product is white crystalline powder. Yield: 68% by reflux and 75% by microwave method; MP: 134º by reflux and 135º by microwave method.
Synthesis of 1-[tolylaminomethyl][1,2,3] benzotriazole (11a)
1-(chloromethyl)-1H-benzotriazole (1 g, 6 mmol) was taken in 50 ml RBF. 10 ml of DMF was added in flask. o-toluidine (1.92 g, 1.92 ml, 18 mmol) and potassium carbonate (0.82 g, 6 mmol) were added in reaction mixture and reaction was carried out by reflux using heating mantle for conventional synthesis and by microwave irradiation (180 W) using microwave oven for microwave-assisted synthesis and monitored by TLC (chloroform). After TLC showed completion of reaction (reflux=5 h 30 min, microwave=3 min 10 s), 10% hydrochloric acid was added to remove excess of o-toluidine as its hydrochloride salt. Desired product was extracted by chloroform (3×10 ml) and recrystallised by hot water [17]. The obtained product is white crystalline powder. Yield: 65% by reflux and 75% by microwave method; MP: 123º by reflux and 125º by microwave method. 1H NMR: 8.060 δ (d, 2H, aromatic proton of benzotriazole), 7.906 δ (t, 2H, aromatic proton of benzotriazole), 7.556 δ (d, 1H, Ar–H), 7.530 δ (t, 1H, Ar–H), 7.387 δ (d, 1H, Ar–H), 7.427 δ (t, 1H, Ar-H), 4.895 δ (s, 2H, -CH2-), 4.011 δ (s, 1H, N–H), 2.198 δ (s, 3H, -CH3).
Antifungal assay by cup plate method
Antifungal activity was determined using the cup plate method [18]. Before starting the study, all required apparatus such as petri plates, test tubes and cup borer were sterilized by autoclaving (121º. 115 lbs). Fungi culture of C. albicans was purified by subculturing on Sabouraud dextrose agar before carrying out the testing. From these grown colonies a single C. albicans colony was inoculated in 10 ml of Sabouraud dextrose broth and kept in incubator at 37º.
From this inoculate, 1 μl of inoculum was transferred to sterile petri plates. Solution of 400 ml of Sabouraud dextrose agar was made in distilled water and sterilized by autoclave. Twenty to thirty millilitres of this agar solution was poured in to each petri plate containing fungal culture. These plates were allowed to cool and kept in refrigerator for 20-30 min. Four cups were bored on each plate by a sterile cup borer. One hundred microlitres of respective test solution, or standard drug flucanazole (10, 50 and 100 μg/ml in 50% v/v methanol in water) was added in each cup. Vehicle control (50% v/v methanol in water) was also tested. After addition of test solutions, plates were kept in incubator at 37º for 48 h. After completion of incubation, zones of inhibition were measured. Mean% zone of inhibition for synthesized compounds against C. albicans is given in Table 2.
Results and Discussion
All benzotriazole derivatives were synthesised by both conventional synthesis and microwave-assisted synthesis by synthetic schemes (Fig. 1-3). 5-substituted benzotriazole amides (4a-c) were synthesised from benzotriazole-5-carbonyl chloride by modified reported procedure [14] with yields ranging from 65% to 72% when synthesised by conventional method and 83-93% when synthesised by using microwave irradiation. In reported procedure [14] aromatic carboxylic acids like benzoic acid were used to synthesise the corresponding acid chloride compounds. We have used benzotriazole-5-carboxylic acid to synthesise benzotriazole-5-carbonyl chloride intermediate, by fig. 1, for the first time.
5-chloromethylbenzotriazole (compound 6) was synthesised by modified reported procedure [16] with 72% yield when synthesised by conventional method and 85% yield when synthesised by using microwave-assisted synthesis. Reported procedure is for synthesis of benzyl chloride from toluene. We have used 5-methylbenzotriaozle for synthesis of 5-chloromethylbenzotriaozle. Higher proportion of sodium hypochlorite was used for better yields. If equimolar proportion of 5-methylbenzotriaozle and sodium hypochlorite was used then yield of product (compound 6) decreased to 65% and reaction time was also increased up to 5-6 h (conventional synthesis method).
The other 5-substituted benzotriazole derivatives (7a-c, 8a) were synthesised from 5-chloromethylbenzotriazole with yields ranging from 23% to 76% when synthesised by conventional method and 42-83% when synthesised using microwave irradiation. No such suitable reported procedure was found for synthesis of these benzotriazole derivatives. Thus we have developed new synthetic method for synthesis of 5-substituted benzotriazole derivatives; microwave-assisted synthesis led to improved yields and less reaction time.
1-[tolylaminomethyl][1,2,3]benzotriazole (11a) was synthesised from 1-chloromethylbenzotriazole using reported method [16] with 65% yield by conventional synthesis and 75% by microwave-assisted synthesis. Table 1 shows the comparison between percentage yields and total reaction time for all synthesised benzotriazole derivatives.
In all cases microwave-assisted synthesis gave better yields and was completed in much shorter period of time. All conventional syntheses of final benzotriazole derivatives were completed within 3-6 h. Intermediates 2 and 3 require very less time (30 min). Synthesis of benzotriazole-5-carboxylic acid (2) was not possible by microwave-assisted synthesis as it is a highly exothermic reaction. Synthesis of benzotriazole-5-carbonyl chloride (3) was not possible by microwave-assisted synthesis as it involves the use of thionyl chloride, which is not amenable to use under microwave irradiation. All microwave-assisted syntheses were completed within 3-6 min 30 s, except for compound 7a, which required 11 min 20 s.
Comparative analysis of percentage yields and total reaction time for all synthesised benzotriazole derivatives by both conventional method and microwave-assisted method was carried out to find out if microwave-assisted synthesis of benzotriazole derivatives adds any advantage or not. It was found that there is improvement in percentage yields of benzotriazole derivatives and also drastic reduction in total reaction time. By using microwave irradiation, reaction is possible within few minutes; and it also improves the yield. This would be highly advantageous for drug discovery laboratories where small amounts of different analogues have to be synthesised in short periods of time. This is very useful for combinatorial synthesis of new libraries of compounds. Microwave-assisted synthesis is quicker, high yielding, environment friendly and shows cleaner chemistry.
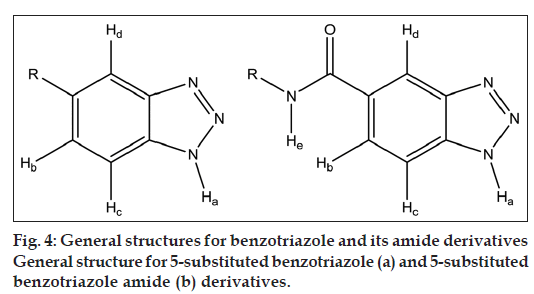
After spectral analysis of all synthesised benzotriazole derivatives it was observed that for all synthesised benzotriazole derivatives, IR spectra value of N–H stretching ranges between 3254 and 3385 cm-1, aromatic C–H stretching ranges between 3024 and 3047 cm-1, aliphatic C–H stretching ranges between 2843 and 2934 cm-1 and C=O stretching value for secondary amides of 5-substituted benzotriazole amides ranges between 1645 and 1655 cm-1. After analysing the 1H NMR data, it was observed that hydrogen attached to nitrogen of benzotriazole ring (Ha) showed δ value between 2.250 and 2.583 δ for all 5-substituted benzotriazole derivatives (fig. 4a).
For all 5-substituted benzotriazole derivatives aromatic protons Hb and Hc of benzotriazole ring showed doublet due to ortho coupling with each other (7-10 Hz). Hd appears more downfield (δ value ranges between 7.918 and 8.623 δ for all 5-substituted benzotriazole derivatives) compared with Hb and Hc since it is ortho to the triazole ring, which is electron withdrawing and hence deshielding. Hc appears more downfield (δ value ranges between 7.651 and 8.048 δ) compared with Hb (δ value ranges between 7.632 and 7.91) since it is ortho to the triazole ring.
For all 5-substituted benzotriazole amide derivatives (4a, 4b and 4c) amide proton (He) showed δ value ranging between 8.482 and 10.078 δ since it is attached to nitrogen, which is attached to C=O that is electron withdrawing group, hence deshielding due to anisotropic effect [18] (fig. 4b). For compounds 4a, 7a and 8a, which are all toluene derivatives, the CH3 of tolyl and the N-H of benzotriazole showed a single signal at around 2.25 δ of four proton intensity.
After performing a comparative statistical analysis (unpaired t test) of the % zones of inhibition of all synthesized compounds with those of vehicle control, it was observed that all test compounds showed antifungal activity (p<0.05) at 50 and 100 μg/ml concentration. Unpaired t test performed between the % zone of inhibition of fluconazole (standard) and test compounds, one compound (8a) was found to show similar activity while two compounds (7a and 7c) showed greater activity and other compounds (compound 2, 4a-c, 6, 7b, 10 and 11a) showed less activity than fluconazole (Table 2).
| Compound | Mean % zone of inhibition (mm) | |||
|---|---|---|---|---|
| Vehicle control | 10 µg/ml | 50 µg/ml | 100 µg/ml | |
| 2 | 0 | 12.03 ± 0.40* | 15.27 ± 0.43*† | 55.02 ± 0.87*† |
| 4a | 0 | 4.16 ± 0.34 | 23.70 ± 0.99*† | 58.38 ± 0.23*† |
| 4b | 0 | 4.16 ± 0.12 | 28.51 ± 0.12*† | 54.07 ± 0.49*† |
| 4c | 0 | 4.16 ± 0.53 | 27.40 ± 0.53*† | 54.30 ± 0.5*† |
| 6 | 0 | 0 | 14.81 ± 0.75*† | 54.91 ± 0.15*† |
| 7a | 0 | 0 | 34.24 ± 0.13*† | 67.67 ± 0.67*† |
| 7b | 0 | 12.5 ± 0.53* | 30 ± 0.09*† | 62.47 ± 0.91*† |
| 7c | 0 | 12.5 ± 0.14* | 27.40 ± 0.76*† | 67.67 ± 1.10*† |
| 8a | 0 | 0 | 36.36 ± 0.53*† | 66.11 ± 0.84* |
| 10 | 0 | 0 | 4.16 ± 0.23*† | 47.26 ± 0.46*† |
| 11a | 0 | 0 | 4.16 ± 0.45*† | 50.19 ± 0.34*† |
| Fluconazole | 0 | 12.5 ± 0.32* | 30 ± 0.87* | 65.55 ± 0.67* |
Comparison of test with vehicle control at *P<0.05, comparison of test compounds at 50 and 100 μg/ml concentrations with standard fluconazole at same concentrations+P<0.05, all values are mean±% RSD of a sample size of n=3
Table 2: Mean% Zone of Inhibition for Synthesized Compounds Against C. Albicans
The results of the study suggested that microwave-assisted syntheses led to higher yields within very short reaction times compared to the conventional methods. Due to development of resistance against existing azole and triazole antifungal agents, it became necessary to develop newer antifungal agents. Further research on 5-substituted benzotriazole analogues can be carried out to developed newer antifungal agents since these analogues showed better in vitro antifungal activity as compared to standard drug fluconazole.
Acknowledgements
We thank the University of Mumbai and the All India Council for Technical Education (AICTE) for funding parts of this project. We thank M/s Agon Pharma Pvt. Ltd., Pune, Finornic Chemicals Pvt. Ltd., Ankleshwar, Amrutlal Bhurabhai and Co., Mumbai, Yasho Industries Pvt. Ltd., Mumbai, Abhilasha Pharma Pvt. Ltd., Ankleshwar, Nivika Chemo Pharma Pvt. Ltd., Ankleshwar, and M. K. Ranganekar Drug Testing and Training Lab, Mumbai for providing gift samples of 5-methylbenzotriazole, 3,4-diaminobenzoic acid, sodium hypochlorite, benzotriazole, fluconazole, Sabouraud dextrose broth and agar, and Candida albicans culture, respectively.
References
- Mahajan K, Fahmi N, Singh RV. Synthesis, characterization and antimicrobial studies of Sb(III) complexes of substituted thioimines. Indian J Chem 2007;46A:1221-5.
- Mahajan K, Swami M, Singh RV. Microwave synthesis, spectral studies, antimicrobial approach, and coordination behavior of antimony (III) and bismuth (III) compounds with benzothiazoline. Russ J Coord Chem 2009;35:179-85.
- Mohanan K., Kumari S, Rijulal G. Microwave assisted synthesis, spectroscopic, thermal, and antifungal studies of some lanthanide (III) complexes with a heterocyclic bishydrazone. J Rare Earths 2008;26:16-21.
- Garg R, Saini MK, Fahmi N, Singh RV. Spectroscopic and biochemical studies of some manganese (II), Oxovanadium (V) and Dioxomolybdenum (VI) complexes S/O and N donor agents synthesized under microwave conditions. Trans Met Chem 2006;31:362-7.
- Sharma K, Singh R, Fahmi N, Singh RV. Microwave assisted synthesis, characterization and biological evaluation of palladium and platinum complexes with azomethines. Spectrochim Acta A Mol Biomol Spectrosc 2010;75A:422-7.
- Varma RS. Solvent-free accelerated organic syntheses using microwaves. Pure Appl Chem 2001;73:193-8.
- Mavandadi F, Pilotti A. The impact of microwave-assisted organic synthesis in drug discovery. Drug Discov Today 2006;11:165-74.
- Vasudevan A. Microwave-assisted organic synthesis an enabling technology with disruptive potential. Drug Discov World 2008;Fall:83-90.
- Kidwai M. Dry media reactions. Pure Appl Chem 2001;73:147–51.
- Di Masi JA. The price of innovation: New estimates of drug development costs. J Health Econ 2003;22:151–85.
- Katritzky AR, Singh SK. Microwave-assisted heterocyclic synthesis. Arkivoc 2003;13:68-86.
- Swamy SN, Sarala BG, Priya BS, Gaonkar SL, Prasad JS, Rangappa KS. Microwave-assisted synthesis of N- alkylated benzotriazole derivatives; Antimicrobial studies. Bioorg Med Chem Lett 2006;16:999-1004.
- Howard DK, Randell DR. Compositions containing substituted benzotriazoles. United States Patent; Patented Nov. 26, 1968: Patent No. 3,413,227.
- Furniss BS, Hannaford AJ, Smith PW, Tatchell AR. Investigation and Characterisation of Organic Compounds. In: Furniss BS, editor. Vogel’s Textbook of Practical Organic Chemistry. 5th ed. UK: Longman Scientific and Technical publication; 1989. p. 1261-2.
- Loomis CL. Process of Chlorinating Toluene. United States Patent;Patented Oct 1, 1918: Patent no. 1,280,612.
- Pawar SS, Gorde PL, Kakde RB. Synthesis of new N1-substituted benzotriazole as anthelmintic agents. Arch Appl Sci Res 2010;2:80-5.
- Rex JH, Pfaller MA, Walsh TJ, Chaturvedi V, Espinel -Ingroff A, Ghannoum MA, et al. Antifungal Susceptibility Testing: Practical Aspects and Current Challenges. Clin Microbiol Rev 2001:14:643-58.
- Pavia DL, Lampman GM, Kriz GS. Chapter 3: Nuclear Magnetic Resonance: Part one: Basic concept. In: Pavia - Introduction to Spectroscopy, 3rd ed. USA: Thomson Learning Academic Resource Center; 2001. p. 152-4.