- *Corresponding Author:
- Hui-Zhen Zhang
School of Pharmacy, Chongqing Engineering Research Center of Pharmaceutical Sciences, Chongqing Medical and Pharmaceutical College, Chongqing 401331, China
E-mail: zhanghuizhen@lyu.edu.cn
| Date of Submission | 11 August 2017 |
| Date of Revision | 28 March 2018 |
| Date of Acceptance | 27 September 2018 |
| Indian J Pharm Sci 2018;80(6):1045-1056 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A series of novel diphenylpiperazine 1,2,3-triazole derivatives were designed, synthesized and characterized by proton nuclear magnetic resonance, carbon-13 nuclear magnetic resonance, infrared and mass spectroscopy. The prepared compounds were investigated in vitro for antibacterial, antifungal and cytotoxic activities. Preliminary results showed that nitroimidazole piperazine 14b was active against bacteria Bacillus subtilis, Micrococcus luteus, Bacillus proteus, Escherichia coli and B. typhi with MIC values of 25 μg/ml, but showed moderate activity against Candida albicans and Saccharomyces cerevisiae (MIC- 50 μg/ml). Moreover, the 1,2,3-triazole-linked nitroimidazole piperazine compound 15b and benzimidazole piperazine 15e were found to be effective in vitro against the PC-3 cell line and the cell survival rates were only 67 and 60 %, respectively at a concentration of 100 μM in a dose-dependent manner. Further, molecular docking experiments suggested that the nitro and hydroxyl groups of compound 14b could insert into base-pairs of DNA hexamer duplex by the formation of hydrogen bonds with guanine of DNA.
Keywords
Diphenylpiperazine, 1,2,3-triazole, antibacterial, antifungal, cytotoxicity
Increasing emergence of bacterial and fungal infections and new cancer cell lines has become growing threats to human health in the past few decades. More and more diseases and infections caused by some resistant microorganisms or cancers have failed to respond to conventional treatment, and in some cases, even last resort defenses have become ineffective. This situation has stimulated an urgent need to develop novel compounds with intriguing activities, broad biological spectra, fewer adverse effects, lower toxicity, especially with mechanisms of action distinct from the wellknown clinical agents [1].
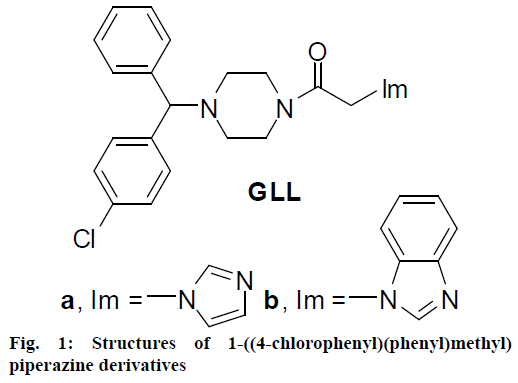
It is well known that piperazine nucleus is an important fragment for bioactive compounds. This skeleton is able to form hydrogen bonds, coordinate with metal ions, accept protons and perform quaternization, and could not only regulate physicochemical properties of desired molecules but also interact with various enzymes and receptors in biological system which exerts broad bioactive spectrum [2,3]. Therefore, piperazine fragment has been widely presented in clinical drugs especially in antimicrobial field. The introduction of diphenyl moiety into molecules made them easily penetrate the cell membrane with improved liposolubility and enhanced biological activities [4,5]. Recent research has revealed that diphenylpiperazine fragment possessed multiple pharmacological activities and compounds with this fragment included antihistamines, cetirizine and buclizine, antihypertensives, cinnarizine and dipfluzine, antimigraine agent lomerizine, anticancer agents and so on. Notably, this structure could directly react with the multidrug resistance-associated protein and inhibit its transportation to exhibit strong bioactivities [6]. Therefore, several investigators explored this scaffold to synthesize novel molecules with distinct mechanisms of action. Previous studies from our laboratory reported that diphenylpiperazine GLLa bearing imidazole ring exhibited equivalent bioactivity against Staphylococcus aureus similar to the standard chloromycin with a minimum inhibitory concentration (MIC) value of 3.1 μg/ml, and also showed desirable antifungal activities against Candida albicans and Saccharomyces cerevisiae with MIC values between 3.1 and 12.5 μg/ml, which were nearly close to fluconazole. The substitution of imidazole ring with benzimidazole moiety yielded compound GLLb, which resulted in good activity against the human prostatic carcinoma (PC-3) cell line [7]. This compelled the current investigation to prepare diphenylpiperazine-derived compounds with intriguing bioactivities. 1,2,3-Triazole, an unique electron-rich ring, endows its derivatives with extensive potential in medicinal chemistry [8]. This investigation dealt with the development of diphenylpiperazine 1,2,3-triazole derivatives as newer agents, which expected to play a large role in the treatment of fungal and bacterial infections, and cancer (Figure 1).
With the introduction of click chemistry, 1,2,3-triazole derivatives have opened up a new opportunity to azole compounds with interesting activity. 1,4-Disubstituted 1,2,3-triazoles could be easily employed as hydrogen bond acceptor due to the large dipole moment, which are favourable for binding to biological target sites and improving solubility [9]. Furthermore, 1,2,3-triazole could also act as an attractive bridge group to connect two pharmacophores and biologically beneficial fragments into one molecule to generate innovative multifunctional compounds [10]. Noticeably, the bioisosteric replacement between 1,2,3-triazole moiety and its bioisoster 1,2,4-triazole has received special attention in medical chemistry, which represented an efficient concept for the discovery and development of novel triazole drugs, significantly extending the chemical space of triazole scaffolds possessing potential activities or enhancing biological activities [11].
In view of such exciting properties and as an extension of earlier studies on the development of diphenylpiperazine azoles, 1,2,3-triazole was introduced into the diphenylpiperazine scaffold with the aim to investigate the effect on antimicrobial and anticancer activities. Various azolyl rings like 1,2,4-triazolyl [12] and its bioisosters imidazolyl [13] and benzimidazolyl [14-18] groups were introduced into the above modified structures to investigate the effect of azolyl rings on biological activities [19]. Moreover, aromatic 2,4-dichlorophenyl and naphthalenyl groups were incorporated to improve the pharmacological properties. Additionally, bisphenol A and enoxacin as important pharmacophores were present in the target molecules to study their effects on the bioactivity. The newly synthesized diphenylpiperazine 1,2,3-triazole derivatives were screened for antibacterial and antifungal activities as well as antitumor activity against PC-3 in vitro. Moreover, molecular docking studies were employed to study the interaction behaviours between the prepared compound and DNA hexamer duplex [20].
Materials and Methods
Melting points (MPs) were recorded on X-6 melting point apparatus and uncorrected. Thin-layer chromatography (TLC) was performed on pre-coated silica gel plates. Fourier-transform infrared spectra were carried out on Bruker RFS100/S spectrophotometer (Bio-Rad, Cambridge, MA, USA) using KBr pellets in the 400-4000 cm-1 range. Proton nuclear magnetic resonance (1H NMR) and 13C NMR spectra were recorded on a Bruker AV 300 spectrometer using tetramethylsilane (TMS) as an internal standard. The following abbreviations were used to designate aryl groups, Pip for piperazine. Mass spectra were recorded on Finigan Trace GC/MS. All chemicals and solvents were commercially available and were used without further purification.
Synthesis
1-(Prop-2-ynyl)-1H-1,2,4-triazole 2a was prepared according to the literature, starting from 1H-1,2,4- triazole (2.00 g, 29.0 mmol) and 3-bromo-1-propyne 1 (2.10 g, 17.6 mmol). Yield: 71 %; MP: 56-57° [21]. 4-Nitro-1-(prop-2-ynyl)-1H-imidazole 2b was prepared starting from 4-nitro-1H-imidazole (2.00 g, 17.6 mmol) and 3-bromo-1-propyne 1 (2.10 g, 17.6 mmol). Yield: 71 %; MP: 126-128° [22]. 2-Methyl- 5-nitro-1-(prop-2-ynyl)-1H-imidazole 2c was prepared starting from 2-methyl-5-nitro-1H-imidazole (2.00 g, 15.7 mmol) and 3-bromo-1-propyne 1 (1.85 g, 15.7 mmol). Yield: 80 %; MP: 130-132° [22].
Synthesis of 2-phenyl-1-(prop-2-ynyl)-1H-imidazole 2d
To a solution of 2-phenyl-1H-imidazole (1.00 g, 6.9 mmol) and sodium hydride (0.334 g, 13.8 mmol) in dry tetrahydrofuran 10 ml was added 3-bromo-1- propyne 1 (1.20 g, 10.2 mmol) at 40°. After 5 h, the reaction system was added 1 N hydrochloric acid to pH=7 at 0°. The solvent was removed and the residue was extracted with chloroform (3×20 ml), dried over anhydrous sodium sulphate, and then the solvent chloroform was removed in vacuo to give compound 2d as oil. Yield: 70 %; 1H NMR (CDCl3, 300 MHz) δ: 7.50- 7.48 (m, 5H, Ar-H), 7.02 (s, 1H, imidazole-H), 6.92 (s, 1H, imidazole-H), 3.11 (s, 2H, CH2), 1.78 (s, 1H, CH) ppm; MS (m/z): 182 [M]+. Compound 1-(prop-2-ynyl)- 1H-benzo[d]imidazole 2e was prepared according to the procedure described for compound 2d, starting from benzimidazole (1.20 g, 10.2 mmol) and 3-bromo- 1-propyne (1.20 g, 10.2 mmol). The pure product 2e was obtained as yellow oil. Yield: 19 % [23].
Synthesis of 2,4-dichloro-1-(prop-2-ynyloxy) benzene 3a
A suspension of 2,4-dichlorophenol (1.00 g, 6.1 mmol), potassium carbonate (1.64 g, 11.9 mmol) and tetrabutylammonium iodide (trace) was stirred in acetone/water (1/1, 10 ml). After 2 h, 3-bromo-1- propyne (2.10 g, 17.6 mmol) was added and the reaction system was stirred at room temperature continuously. After the reaction was completed (monitored by TLC, eluent, petroleum ether, Rf=0.5), the solvent was removed and the residue was extracted with ethyl acetate (3×20 ml), dried over anhydrous sodium sulphate and purified by silica gel column chromatography (eluent, petroleum ether) to afford compound 3a as white solid. Yield: 60 %; MP: 56-58° [24]. Compound 2-(prop- 2-ynyloxy)naphthalene 3b was prepared according to the procedure described for compound 3a, starting from naphthalen-2-ol (1.5 g, 10.4 mmol) and 3-bromo- 1-propyne (2.1 g, 17.6 mmol). The pure product 3b was obtained as yellow solid. Yield: 65 %; MP: 69-71° [25]. Compound 4,4'-(propane-2,2-diyl)bis((prop- 2-ynyloxy)benzene) 4 was prepared according to the procedure described for compound 3a, starting from bisphenol A (1.35 g, 5.9 mmol) and 3-bromo-1-propyne (2.10 g, 17.6 mmol). The pure product 4 was obtained as yellow solid. Yield: 54 %; MP: 70-71° [25].
Synthesis of 1-ethyl-6-fluoro-4-oxo-7-(4- (prop-2-ynyl)piperazin-1-yl)-1,4-dihydro-1,8- naphthyridine- 3-carboxylic acid 5
A suspension of enoxacin (0.20 g, 0.625 mmol) and potassium carbonate (0.087 g, 0.625 mmol) was stirred in acetonitrile (20 ml). 3-Bromo-1-propyne (0.12 g, 1.00 mmol) was added at 0°, and after 1 h, the reaction system was stirred at room temperature continuously. After the reaction was completed (monitored by TLC, eluent, chloroform/methanol, 15/1, V/V, Rf=0.5), the reaction system was filtered, and the residue was extracted with acetonitrile (3×20 ml), dried over anhydrous sodium sulphate and purified by silica gel column chromatography (eluent, chloroform/ methanol, 30/1, V/V) to afford compound 5 as white solid. Yield: 50 %; MP: 260°; 1H NMR (CDCl3, 300 MHz) δ: 15.06 (s, 1H, COOH), 8.70 (s, 1H, enoxacin-2-H), 8.12 (d, J=12.0 Hz, 2H), 4.45-4.27 (q, J=12.0 Hz, 2H, CH2CH3), 3.94 (s, 4H, Pip), 3.41 (s, 2H, CH2), 2.75 (s, 4H, Pip), 2.30 (s, 1H, CH), 1.51 (t, J=6.0 Hz, 3H, CH2CH3) ppm; MS (m/z): 359 [M]+.
Compound (4-chlorophenyl)(phenyl)methanol 7 was prepared according to the procedure described in the literature, starting from (4-chlorophenyl) (phenyl)methanone (10.02 g, 46.4 mmol) and sodium borohydride (2.01 g, 53 mmol). The pure product 7 was obtained as white solid. Yield: 95 %; MP: 65-67° [7]. Compound 1-chloro-4-(chloro(phenyl)methyl)benzene 8 was prepared according to the procedure described in the literature, starting from (4-chlorophenyl) (phenyl)methanol (9.03 g, 41.1 mmol) and thionyl chloride (6.16 g, 52 mmol). The pure product 8 was obtained as yellow oil. Yield: 95 % [7]. Compound 1-((4-chlorophenyl)(phenyl)methyl)piperazine 9 was prepared according to the procedure described in the literature, starting from 1-chloro-4-(chloro(phenyl) methyl)benzene (6.02 g, 25.3 mmol) and piperazine (10.00 g, 116 mmol). The pure product 9 was obtained as white solid. Yield: 62 %; MP: 66-68° [7].
Compound 1-chloro-3-(4-((4-chlorophenyl)(phenyl) methyl)piperazin-1-yl)propan-2-ol 10 was prepared according to the procedure described in the literature, starting from 1-[(4-chlorophenyl)(phenyl)methyl] piperazine (5.02 g, 17.4 mmol) and 2-(chloromethyl) oxirane (4.81 g, 52.2 mmol). The pure product 10 was obtained as white solid. Yield: 40 %; MP: 70-71° [7]. Compound 2-chloro-1-(4-((4-chlorophenyl)(phenyl) methyl)piperazin-1-yl)ethanone 11 was prepared according to the procedure described in the literature, starting from 1-[(4-chlorophenyl)(phenyl)methyl] piperazine (7.02 g, 24.4 mmol) and 2-chloroacetyl chloride (6.03 g, 53.0 mmol). The pure product 11 was obtained as yellow oil. Yield: 45 % [7].
Synthesis of 1-azido-3-(4-((4-chlorophenyl)(phenyl) methyl)piperazin-1-yl)propan-2-ol 12
A suspension of compound 10 (2.35 g, 6.2 mmol) and sodium azide (0.61 g, 9.3 mmol) was stirred in acetone/water (1/1, V/V, 30 ml) at 40° overnight. After the reaction was completed, the solvent was removed in vacuo and the residue was extracted with ethyl acetate (3×20 ml), dried over anhydrous sodium sulphate, and then the solvent ethyl acetate was removed in vacuo to give compound 12 as yellow oil. Yield: 95 %; 1H NMR (CDCl3, 300 MHz): δ 7.45-7.35 (m, 9H, Ar-H), 4.23 (s, 1H, Ar2CH), 3.65 (m, 2H), 2.91 (m, 2H), 2.74-2.62 (m, 8H, Pip) ppm.
Compound 2-azido-1-(4-((4-chlorophenyl)(phenyl) methyl)piperazin-1-yl)ethanone 13 was prepared according to the procedure described for compound 12, starting from compound 11 (2.01 g, 5.5 mmol) and sodium azide (1.07 g, 16.5 mmol). The pure product 13 was obtained as oil. Yield: 87 %; 1H NMR (CDCl3, 300 MHz): δ 7.43-7.22 (m, 9H, Ar-H), 4.31 (s, 1H, Ar2CH), 4.02 (s, 2H, COCH2), 3.70 (bs, 2H, Pip), 3.52 (bs, 2H, Pip), 2.50-2.41 (bs, 4H, Pip) ppm.
Synthesis of 1-(4-((1H-1,2,4-triazol-1-yl)methyl)-1H- 1,2,3-triazol-1-yl)-3-(4-((4-chloro phenyl)(phenyl) methyl)piperazin-1-yl)propan-2-ol 14a
To a solution of compound 12 (0.10 g, 0.66 mmol) in a t-BuOH/acetone/H2O (10 ml, 1/1/1, v/v/v) mixture were added sodium ascorbate (0.4 eq.) and copper (II) sulphate pentahydrate (0.2 eq.) successively. Here after compound 2a (0.245 g, 0.66 mmol) was added, and the mixture was stirred at 60°. After the reaction came to the end (monitored by TLC, eluent, chloroform/methanol, 50/1, V/V, Rf=0.3), the solvent was removed and the residue was extracted with chloroform (3×20 ml), dried over anhydrous sodium sulphate and purified by silica gel column chromatography (eluent, chloroform/ methanol, 100/1, V/V) to afford compound 14a as white solid. Yield: 60 %; MP: 127-128°; IR (KBr): ν 3059, 3027, 2967, 2921, 2860, 1658 (C=O), 1495, 1457, 1423, 1238, 1137, 1049, 850, 744, 702 cm-1; 1H NMR (CDCl3, 300 MHz) δ: 8.20 (s, 1H, 1,2,4-triazole 3-H), 7.94 (s, 1H, 1,2,4-triazole 5-H), 7.81 (s, 1H, 1,2,3-triazole 5-H), 7.34-7.26 (m, 9H, Ar-H), 5.50 (s, 2H, CH2-1,2,4-triazole), 4.52 (d, 1H, J=12.0 Hz, 1,2,3-triazole-CH2), 4.32-4.25 (m, 1H, CH(OH)), 4.20 (s, 1H, Ar2CH), 4.13-4.08 (m, 1H, 1,2,3-triazole-CH2), 2.69 (bs, 2H, NCH2CH(OH)), 2.46-2.18 (m, 8H, Pip) ppm; MS (m/z): 515 [M+Na]+.
Compound 1-(4-((4-chlorophenyl)(phenyl)methyl) piperazin-1-yl)-3-(4-((4-nitro-1H-imidazol-1-yl) methyl)-1H-1,2,3-triazol-1-yl)propan-2-ol 14b was prepared according to the procedure described for compound 14a, starting from compound 12 (0.50 g, 1.29 mmol) and compound 2b (0.20 g, 1.29 mmol). The pure product 14b was obtained as white solid. Yield: 72 %; MP: 117-118°; IR (KBr): ν 3143, 3027, 2952, 2882, 1545, 1490, 1451, 1337, 1135, 1052, 823, 757, 702 cm-1; 1H NMR (CDCl3, 300 MHz) δ: 7.87 (s, 1H, 1,2,3-triazole 5-H), 7.85 (s, 1H, nitroimidazole- 5H), 7.58 (s, 1H, nitroimidazole-2H), 7.35-7.21 (m, 9H, Ar-H), 5.30 (s, 2H, CH2-nitroimidazole), 4.57 (d, 1H, J=13.7 Hz, 1,2,3-triazole-CH2), 4.34-4.27 (m, 1H, CH(OH)), 4.23 (s, 1H, Ar2CH), 4.13 (d, 1H, 1,2,3-triazole-CH2), 2.77 (bs, 2H, NCH2CH(OH)), 2.56-2.33 (m, 8H, Pip) ppm; MS (m/z): 559 [M+Na]+, 537 [M]+.
Compound 2-(4-((1H-1,2,4-triazol-1-yl)methyl)-1H- 1,2,3-triazol-1-yl)-1-(4-((4-chloro-phenyl)(phenyl) methyl)piperazin-1-yl)ethanone 15a was prepared according to the procedure described for compound 14a, starting from compound 13 (0.35 g, 0.95 mmol) and compound 2a (0.10 g, 0.95 mmol). The pure product 15a was obtained as white solid. Yield: 66 %; MP: 107-108°; IR (KBr): ν 3122, 3027, 2961, 2861, 1661 (C=O), 1561, 1506, 1487, 1451, 1419, 1138, 1051, 851, 703 cm-1; 1H NMR (CDCl3, 300 MHz) δ: 8.20 (s, 1H, 1,2,4-triazole 3-H), 7.94 (s, 1H, 1,2,4-triazole 5-H), 7.78 (s, 1H, 1,2,3-triazole 5-H), 7.34-7.26 (m, 9H, Ar-H), 5.50 (s, 2H, CH2-1,2,3-triazole), 5.19 (s, 2H, COCH2), 4.24 (s, 1H, Ar2CH), 3.63-3.54 (d, 4H, Pip), 2.41 (bs, 4H, Pip) ppm; MS (m/z): 509 [M+Na]+.
Compound 1-(4-((4-chlorophenyl)(phenyl)methyl) piperazin-1-yl)-2-(4-((4-nitro -1H-imidazol-1-yl) methyl)-1H-1,2,3-triazol-1-yl)ethanone 15b was prepared according to the procedure described for compound 14a, starting from compound 13 (0.24 g, 0.66 mmol) and compound 2b (0.10 g, 0.66 mmol). The pure product 15b was obtained as white solid. Yield: 60 %; MP: 127-128°; IR (KBr): ν 3059, 3027, 2967, 2921, 2860, 1658 (C=O), 1545, 1495, 1457, 1423, 1337, 1238, 1138, 1049, 850, 744, 702 cm-1; 1H NMR (CDCl3, 300 MHz) δ: 7.87 (s, 1H, 1,2,3-triazole 5-H), 7.82 (s, 1H, nitroimidazole 5-H), 7.57 (s, 1H, nitroimidazole 2-H), 7.35-7.27 (m, 9H, Ar-H), 5.32 (s, 2H, CH2-nitroimidazole), 5.24 (s, 2H, COCH2), 4.27 (s, 1H, Ar2CH), 3.65-3.57 (bs, 4H, Pip), 2.43 (bs, 4H, Pip) ppm; MS (m/z): 543 [M+Na]+.
Compound 1-(4-((4-chlorophenyl)(phenyl)methyl) piperazin-1-yl)-2-(4-((2-methyl-5-nitro- 1H-imidazol- 1-yl)methyl)-1H-1,2,3-triazol-1-yl)ethanone 15c was prepared according to the procedure described for compound 14a, starting from compound 13 (0.44 g, 1.21 mmol) and compound 2c (0.20 g, 1.21 mmol). The pure product 15c was obtained as white solid. Yield: 62 %; MP: 173-175 °; IR (KBr): ν 3059, 3027, 2967, 2921, 2860, 1658 (C=O), 1545, 1495, 1457, 1423, 1337, 1238, 1138, 1049, 850, 744, 702 cm-1; 1H NMR (CDCl3, 300 MHz) δ: 7.74 (s, 2H, 1,2,3-triazole 5-H, imidazole 4-H), 7.35-7.26 (m, 9H, Ar-H), 5.22 (s, 4H, imidazole-CH2, COCH2), 4.25 (s, 1H, Ar2CH), 3.63 (s, 2H, Pip), 3.55 (s, 2H, Pip), 2.50 (s, 3H, CH3), 2.40 (bs, 4H, Pip) ppm; MS (m/z): 535 [M]+.
Compound 1-(4-((4-chlorophenyl)(phenyl)methyl) piperazin-1-yl)-2-(4-((2-phenyl-1H-imidazol-1-yl) methyl)-1H-1,2,3-triazol-1-yl)ethanone 15d was prepared according to the procedure described for compound 14a, starting from compound 13 (0.38 g, 1.02 mmol) and compound 2d (0.19 g, 1.02 mmol). The pure product 15d was obtained as white solid. Yield: 62 %; MP: 96-98°; 1H NMR (CDCl3, 300 MHz) δ: 7.63- 7.10 (m, 17H, Ar-H, 1,2,3-triazole 5-H, imidazole-H), 5.39 (s, 2H, imidazole-CH2), 5.32 (s, 2H, COCH2), 4.23 (s, 1H, Ar2CH), 3.70 (bs, 2H, Pip), 3.50 (bs, 2H, Pip), 2.39 (bs, 4H, Pip) ppm; MS (m/z): 552 [M]+.
Compound 2-(4-((1H-benzo[d]imidazol-1-yl)methyl) -1H-1,2,3-triazol-1-yl)-1-(4-((4-chlorophenyl) (phenyl)methyl)piperazin-1-yl)ethanone 15e was prepared according to the procedure described for compound 14a, starting from compound 13 (0.35 g, 0.96 mmol) and compound 2e (0.15 g, 0.96 mmol). The pure product 15e was obtained as white solid. Yield: 60 %; MP: 93-95°; 1H NMR (CDCl3, 300 MHz) δ: 7.99 (s, 1H, benzimidazole-H), 7.79 (s, 1H, benzimidazole-H), 7.51 (s, 1H, 1,2,3-triazole 5-H), 7.44 (s, 1H, benzimidazole-H), 7.32-7.21 (m, 10H, Ar-H, benzimidazole-H), 5.47 (s, 2H, benzimidazole- CH2), 5.01 (s, 2H, COCH2), 4.20 (s, 1H, Ar2CH), 3.58 (s, 2H, Pip), 3.47 (s, 2H, Pip), 2.35 (bs, 4H, Pip) ppm; 13C NMR (CDCl3, 75 MHz) δ : 162.9, 143.9, 142.8, 141.1, 140.3, 133.5, 133.0, 129.0, 128.9, 128.8, 127.7, 127.6, 123.9, 123.2, 122.4, 120.4, 109.8, 75.0, 51.5, 51.1, 50.8, 45.4, 42.5, 40.5 ppm; MS (m/z): 526 [M]+.
Compound 1-(4-((4-chlorophenyl)(phenyl)methyl) piperazin-1-yl)-3-(4-((2,4-dichloro phenoxy)methyl)- 1H-1,2,3-triazol-1-yl)propan-2-ol 16a was prepared according to the procedure described for compound 14a, starting from compound 12 (0.39 g, 1.0 mmol) and compound 3a (0.20 g, 1.0 mmol). The pure product 16a was obtained as white solid. Yield: 70 %; MP: 82-83°; IR (KBr): ν 3145, 3027, 2967, 2921, 2860, 1545, 1518, 1490, 1451, 1238, 1138, 1049, 823, 757, 702 cm-1; 1H NMR (CDCl3, 300 MHz) δ: 7.86 (s, 1H, 1,2,3-triazole 5-H), 7.35-7.16 (m, 11H, Ar-H), 7.06-7.03 (d, 1H, J= 8.8 Hz, Ar-H), 5.3 (s, 2H, OCH2), 4.53 (d, 1H, J= 14.0 Hz, 1,2,3-triazole-CH2), 4.35-4.29 (m, 1H, CH(OH)), 4.20 (s, 1H, Ar2CH), 4.16-4.09 (m, 1H, 1,2,3-triazole-CH2), 2.68 (bs, 2H, NCH2CH(OH)), 2.43-2.18 (m, 8H, pip) ppm; MS (m/z): 588 [M]+.
Compound 1-(4-((4-chlorophenyl)(phenyl)methyl) piperazin-1-yl)-3-(4-((naphthalen-2-yloxy)methyl)- 1H-1,2,3-triazol-1-yl)propan-2-ol 16b was prepared according to the procedure described for compound 14a, starting from compound 12 (0.65 g, 1.7 mmol) and compound 3b (0.31 g, 1.7 mmol). The pure product 16b was obtained as white solid. Yield: 85 %; MP: >260°; IR (KBr): ν 3145, 3027, 2967, 2921, 2860, 1599, 1545, 1518, 1451, 1238, 1138, 1049, 823, 757, 702 cm-1; 1H NMR (CDCl3, 300 MHz) δ: 7.85 (s, 1H, 1,2,3-triazole 5-H), 7.76-6.82 (m, 16H, Ar-H), 5.3 (s, 2H, OCH2), 4.53 (d, 1H, J=14.0 Hz, 1,2,3-triazole-CH2), 4.35-4.29 (m, 1H, CH(OH)), 4.20 (s, 1H, Ar2CH), 4.16-4.09 (m, 1H, 1,2,3-triazole-CH2), 2.68 (bs, 2H, NCH2CH(OH)), 2.43-2.18 (m, 8H, Pip) ppm; MS (m/z): 569 [M]+.
Compound 1-(4-((4-chlorophenyl)(phenyl)methyl) piperazin-1-yl)-2-(4-((2,4-dichloro phenoxy)methyl)- 1H-1,2,3-triazol-1-yl)ethanone 17a was prepared according to the procedure described for compound 14a, starting from compound 13 (0.37 g, 1.0 mmol) and compound 3a (0.20 g, 1.0 mmol). The pure product 17a was obtained as white solid. Yield: 70 %; MP: 112- 114°; IR (KBr): ν 3145, 3027, 2967, 2923, 1664 (C=O), 1483, 1416, 1238, 1136, 1056, 804, 758 cm-1; 1H NMR (CDCl3, 300 MHz) δ: 7.83 (s, 1H, 1,2,3-triazole 5-H), 7.36-7.16 (m, 11H, Ar-H), 7.05-7.03 (d, 1H, J=8.8 Hz, Ar-H), 5.26 (s, 2H, OCH2), 5.19 (s, 2H, COCH2), 4.23 (s, 2H, Ar2CH), 3.63-3.53 (d, 4H, Pip), 2.39 (s, 4H, Pip) ppm; 13C NMR (CDCl3, 75 MHz) δ: 163.02 (C=O), 152.57, 143.60, 141.10, 140.36, 132.99, 130.02, 129.05, 128.88, 128.81, 127.70, 127.64, 127.56, 126.45, 124.76, 123.99, 115.10, 75.00, 63.41, 51.55, 51.12, 50.88, 45.41, 42.43 ppm; MS (m/z): 595 [M+Na]+.
Compound 1-(4-((4-chlorophenyl)(phenyl)methyl) piperazin-1-yl)-2-(4-((naphthalen-2-yloxy) methyl)- 1H-1,2,3-triazol-1-yl)ethanone 17b was prepared according to the procedure described for compound 14a, starting from compound 13 (0.63 g, 1.7 mmol) and compound 3b (0.31 g, 1.7 mmol). The pure product 17b was obtained as white solid. Yield: 85 %; MP: >260°; IR (KBr): ν 3145, 3059, 3027, 2960, 2922, 2862, 1665 (C=O), 1599, 1510, 1451, 1238, 1141, 1049, 838, 757 cm-1; 1H NMR (CDCl3, 300 MHz) δ: 7.83 (s, 1H, 1,2,3-triazole 5-H), 7.78-6.85 (m, 16H, Ar-H), 5.34 (s, 2H, OCH2), 5.20 (s, 2H, COCH2), 4.21 (s, 1H, Ar2CH), 3.63-3.54 (d, 4H, Pip), 2.39 (s, 4H, Pip) ppm; 13C NMR (CDCl3, 75 MHz) δ: 163.15(C=O), 156.18, 144.45, 141.19, 140.41, 134.45, 133.67, 129.54, 129.22, 129.09, 128.91, 128.84, 127.76, 127.63, 126.92, 126.44, 124.41, 123.88, 118.85, 107.35, 75.03, 62.06, 51.65, 51.19, 50.91, 45.58, 42.52 ppm; MS (m/z): 575 [M+Na]+.
Compound 2,2'-(4,4'-(4,4'-(propane-2,2-diyl)bis(4,1- phenylene))bis(oxy)bis(methylene)bis(1H-1,2,3- triazole-4,1-diyl))bis(1-(4-((4-chlorophenyl)(phenyl) methyl)piperazin-1-yl)ethanone) 18 was prepared according to the procedure described for compound 14a, starting from compound 13 (0.98 g, 2.64 mmol) and compound 4 (0.4 g, 1.32 mmol). The pure product 18 was obtained as white solid. Yield: 69 %; MP: >260°; IR (KBr): ν 3144, 3060, 3028, 2965, 2868, 1664 (C=O), 1607, 1509, 1451, 1237, 1138, 1048, 830, 758 cm-1; 1H NMR (CDCl3, 300 MHz) δ: 7.78 (s, 2H, 1,2,3-triazole 5-H), 7.35-6.85 (m, 28H, Ar-H), 5.20 (s, 8H, OCH2, COCH2), 4.23 (s, 2H, Ar2CH), 3.62-3.53 (d, 8H, N(CH2CH2)2NCO), 2.39 (s, 8H, N(CH2CH2)2NCO) ppm; 13C NMR (CDCl3, 75 MHz) δ: 163.40(C=O), 156.38, 144.94, 143.87, 141.42, 140.63, 133.26, 129.31, 129.12, 129.05, 128.01, 127.98, 127.78, 124.55, 114.40, 75.26, 62.29, 51.85, 51.40, 51.10, 45.80, 42.73, 41.95, 31.22 ppm; MS (m/z): 382 [M]+.
Synthesis of 7-(4-(2-(4-((4-chlorophenyl)(phenyl) methyl)piperazine-1-yl)-2-oxoethyl)piperazin-1-yl)- 1-ethyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthy ridine-3-carboxylic acid 19
To a solution of enoxacin (0.20 g, 0.625 mmol) and potassium carbonate (0.087 g, 0.625 mmol) in acetonitrile 20 ml of compound 11 (0.23 g, 0.625 mmol) was added at 0°. After 1 h, the mixture was stirred at room temperature for 12 h, the solvent was removed and the residue was extracted with chloroform (320 ml), dried over anhydrous sodium sulphate and purified by silica gel column chromatography (eluent, ethyl acetate/ petroleum ether, 2/1, V/V) to afford compound 19 as white solid. Yield: 49 %; MP: 165-167°; 1H NMR (CDCl3, 300 MHz) δ: 15.04 (s, 1H, COOH), 8.56 (s, 1H, enoxacin-2-H), 8.10 (d, J=12.0 Hz, 2H), 7.35- 7.22 (m, 9H, Ar-H), 4.43-4.36 (q, J=6.0 Hz, 2H, CH2CH3), 4.23 (s, 1H, Ar2CH), 3.88 (s, 4H, Pip), 3.60 (s, 4H, Pip), 3.25 (s, 2H, COCH2), 2.69 (s, 4H, Pip), 2.38 (s, 4H, Pip), 1.49 (t, J=6.0 Hz, 3H, CH2CH3) ppm; MS (m/z): 647 [M]+.
Antibacterial and antifungal assays
MIC (μg/ml) is defined as the lowest concentration of target compounds that completely inhibit the growth of bacteria, by means of standard two-fold serial dilution method in 96-well microtiter plates according to the National Committee for Clinical Laboratory Standards (NCCLS). The tested microorganism strains were provided by the School of Pharmaceutical Sciences, Southwest University and the College of Pharmacy, Third Military Medical University. Chloromycin and fluconazole, were used as control drugs. Dimethylsulfoxide (DMSO) with bacterial culture and without test compounds was used as positive control to ensure that the solvent had no effect on bacteria growth. All the bacterial and fungal growth was monitored visually and spectrophotometrically, and the experiments were performed in triplicate.
Antibacterial assays
The prepared compounds 14-19 were evaluated for antibacterial activities against Gram-positive bacteria (S. aureus ATCC 6538, methicillin-resistant S. aureus N315 (MRSA), Micrococcus luteus and Bacillus subtilis ATCC 21216), Gram-negative bacteria (Escherichia coli ATCC 8099, Pseudomonas aeruginosa ATCC 27853, B. typhi and B. proteus ATCC 13315). The bacterial suspension was adjusted with sterile saline to a concentration of 1×105 CFU. Initially the compounds were dissolved in DMSO to prepare the stock solutions, then the tested compounds and reference drugs were prepared in Mueller-Hinton broth (Guangdong Huaikai Microbial Science & Tech Co., Ltd., Guangzhou, Guangdong, China) to obtain the required concentrations of 400, 200, 100, 50, 25, 12.5, 6.25, 3.1, 1.6, 0.8 and 0.4 μg/ml. These dilutions were inoculated and incubated at 37° for 24 h.
Antifungal assays
The newly synthesized compounds 14–19 were evaluated for antifungal activities against C. albicans ATCC 76615, Aspergillus fumigatus ATCC 96918, Calathea utilis, S. cerevisiae and A. flavus. A spore suspension in sterile distilled water was prepared from one day old culture of the fungi growing on Sabouraud agar (SA) media. The final spore concentration was 1-5×103 spore/ml. From the stock solutions of the tested compounds and reference antifungal drug fluconazole, dilutions in sterile RPMI 1640 medium (Neuronbc Laboraton Technology CO., Ltd, Beijing, China) were made resulting in eleven wanted concentrations (0.4 to 400 μg/ml) of each tested compound. These dilutions were inoculated and incubated at 35° for 24 h.
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay for cytotoxic activity
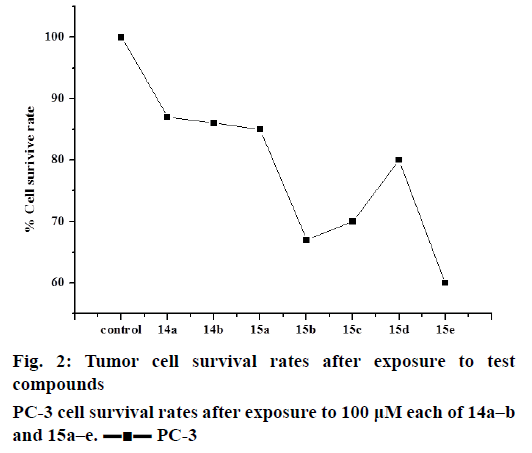
PC-3 cell line was purchased from the Shanghai Institutes for Biological Sciences, China. Ham’s F12 nutrient mixture, enriched with 10 % heat inactivated foetal bovine serum and 1 % of penicillin-streptomycin was used for cell culture tests. The cytotoxic activity was investigated using MTT assay [26]. Stock solutions (100 mM) of test compounds 14a–b and 15a–e were prepared in DMSO and stored at –20° prior to make dilutions for the biological assay. Cell suspensions were diluted to 105 cells/ml, suitably prepared and distributed in plates of culture with 96 wells (225 μl in each well), then incubated at 37° in a humid atmosphere with 5 % of CO2. After 24 h, 25 μl of the test compounds were added to each well. The plates were incubated again at 37° for 24 h. Then, 25 μl of MTT solution (5 mg/ml) was added to each well, and the mixture was incubated at 37° for 2 h. At the end of this period, the culture medium with the MTT excess was aspirated and after that, 100 μl of DMSO was added to each well to dissolve the formazan crystals. The optical density of the wells was measured at 490 nm. Results were evaluated by comparing the absorbance of the wells containing compound-treated cells with the absorbance of wells containing 0.1 % DMSO alone (solvent control). Conventionally, cell viability was estimated to be 100 % in the solvent control. All assays were performed in triplicate and mean ± SD values were used to estimate cell growth inhibition rate. The cell survival rates were shown in Figure 2.
Molecular docking
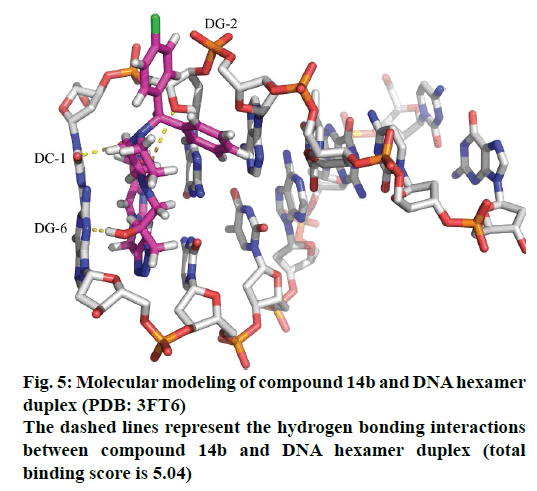
All the docking studies were carried out using Surflex- Docking 2.0 on a window 7 workstation. The crystal structure of DNA (PDB entry 3FT6) was downloaded from the protein data bank and used for docking studies. Water molecules were removed from protein PDB files, and hydrogen atoms were added. The 3D structures of compound 14b were first built using Surflex-Docking 2.0 sketch followed by energy minimization. At last we used the Surflex-Docking program to automatically dock the drugs into the binding pockets of DNA. In the docking process, 20 conformations were obtained, and among them the lowest free energy solution (or the highest total score) was chosen for our compounds modelling.
Results and Discussion
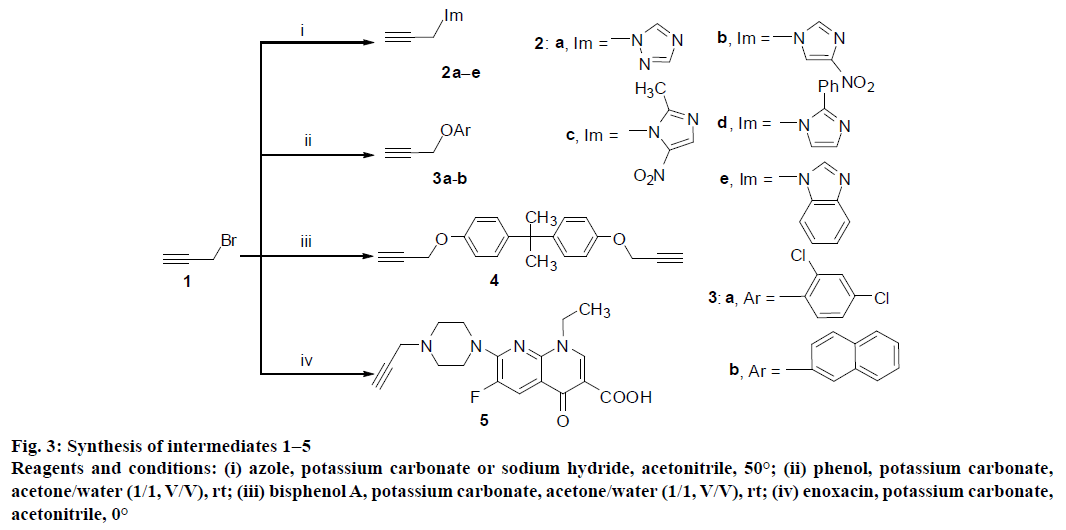
The target diphenylpiperazine 1,2,3-triazole derivatives were prepared via multi-step reactions and the synthetic process was outlined in Figures 3 and 4. Commercially available 3-bromoprop-1-yne was reacted with azoles in acetonitrile to produce compounds 1a−e, and while it was treated with phenol or bisphenol A in acetone/ water (1/1) in the presence of potassium carbonate, propargyl ether compounds 2a−b or 3 were obtained in yields of 54-65 %. Furthermore, 3-bromoprop-1- yne could react with enoxacin and sulfonamide via N-alkylation reaction to give the corresponding alkynes 4 and 5 (Figure 3).
Figure 3: Synthesis of intermediates 1−5
Reagents and conditions: (i) azole, potassium carbonate or sodium hydride, acetonitrile, 50°; (ii) phenol, potassium carbonate, acetone/water (1/1, V/V), rt; (iii) bisphenol A, potassium carbonate, acetone/water (1/1, V/V), rt; (iv) enoxacin, potassium carbonate, acetonitrile, 0°
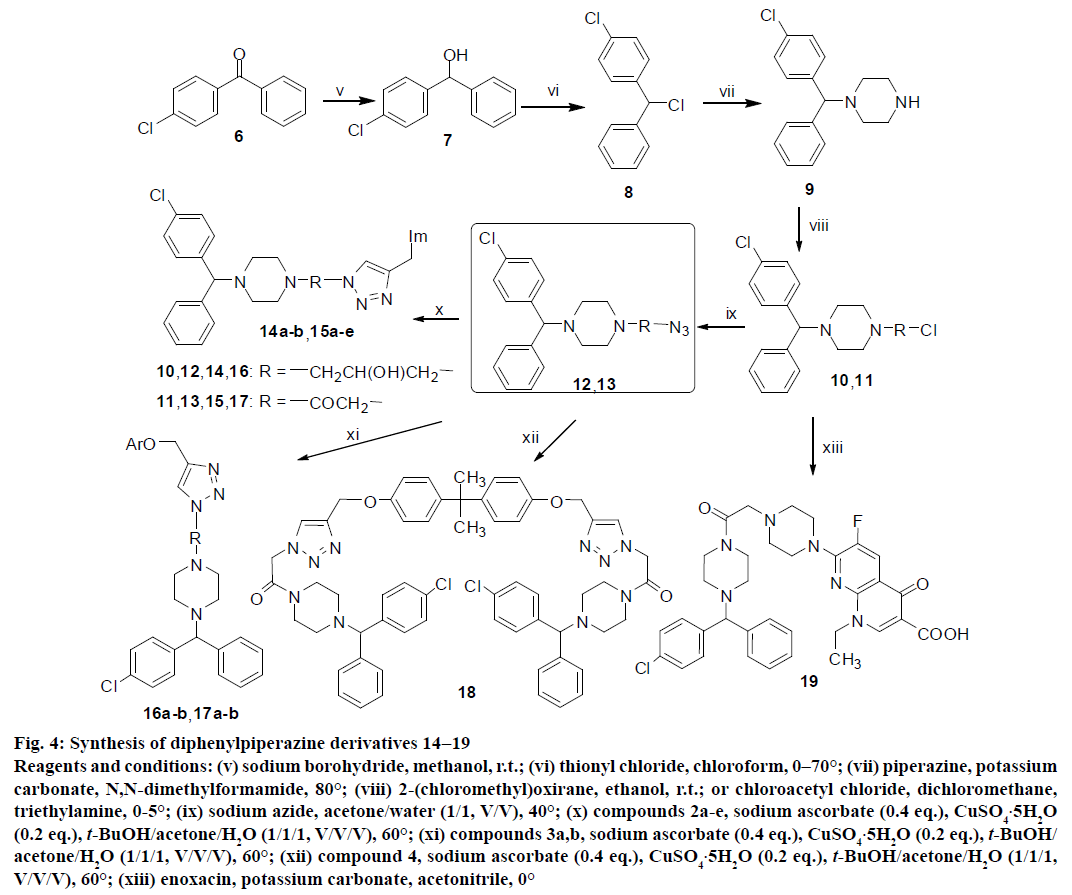
Figure 4: Synthesis of diphenylpiperazine derivatives 14−19
Reagents and conditions: (v) sodium borohydride, methanol, r.t.; (vi) thionyl chloride, chloroform, 0–70°; (vii) piperazine, potassium carbonate, N,N-dimethylformamide, 80°; (viii) 2-(chloromethyl)oxirane, ethanol, r.t.; or chloroacetyl chloride, dichloromethane, triethylamine, 0-5°; (ix) sodium azide, acetone/water (1/1, V/V), 40°; (x) compounds 2a-e, sodium ascorbate (0.4 eq.), CuSO4.5H2O (0.2 eq.), t-BuOH/acetone/H2O (1/1/1, V/V/V), 60°; (xi) compounds 3a,b, sodium ascorbate (0.4 eq.), CuSO4.5H2O (0.2 eq.), t-BuOH/acetone/H2O (1/1/1, V/V/V), 60°; (xii) compound 4, sodium ascorbate (0.4 eq.), CuSO4.5H2O (0.2 eq.), t-BuOH/acetone/H2O (1/1/1, V/V/V), 60°; (xiii) enoxacin, potassium carbonate, acetonitrile, 0°
The (4-chlorophenyl)(phenyl)methanone 6 was reduced by sodium borohydride to produce alcohol 7, and then further treated by thionyl chloride to give chloride 8. The latter was subsequently reacted with piperazine to afford 1-((4-chlorophenyl)(phenyl)methyl) piperazine 9. The N-alkylation of piperazine ring of compound 9 with 2-(chloromethyl)oxirane or chloroacetyl chloride afforded the compounds 10 and 11 in 40 and 45 % yields, respectively. Further substitution of compounds 10 and 11 with sodium azide was performed to provide the important intermediates 12 and 13. The 1,3-dipolar cycloaddition of azides with propargyl azoles, propargyl ethers or propargyl sulfonamide using catalytic amount of copper sulphate and sodium ascorbate in t-BuOH/ acetone/H2O (1/1/1, V/V/V) mixture at 60° afforded diphenylpiperazine 1,2,3-triazole derivatives 14a−b, 15a−e, 16a−b, 17a−b and 18 in moderate to good yields ranging from 60 to 85 %. However, the enoxacin contained diphenyl 1,2,3-triazole compound was not synthesized unfortunately. In order to exploration the important role of 1,2,3-triazole fragment to the bioactivity, the hydrophobic group ((4-chlorophenyl) (phenyl)methyl)piperazine was introduced into the C-7 position of enoxacin piperazine ring (19) to improve antimicrobial activity and broaden spectrum (Figure 4).
1,3-Dipolar cycloadditions of azide with alkyne to form five-membered 1,2,3-triazole ring are classic click reaction, and have been extensively researched. To further manifest this reaction, the reaction of compound 12 with compound 2a was employed as a model reaction, and some influential factors such as solvent and catalyst were optimized to improve the transformation yields for different substrates. As shown in Table 1, nearly no reaction occurred when CuSO4.5H2O (0.1 eq.) and sodium ascorbate (0.2 eq.) were employed as catalysts (entries 1 and 2). The highest yield (60 %) and shortest reaction time (1 h) were obtained when the ratio of catalyst was improved to CuSO4.5H2O (0.2 eq.) and sodium ascorbate (0.4 eq., entry 4). Therefore t-BuOH/acetone/H2O (1/1/1, V/V/V) were chosen as solvent medium, and CuSO4.5H2O (0.2 eq.) and sodium ascorbate (0.4 eq.) were employed as catalysts to perform this transformation.
| Entry | Solvent(V/V) | Catalyst | Time (h) | Yielda (%) |
|---|---|---|---|---|
| 1 | Acetone/water (1/1) | CuSO4×5H2O (0.1 eq.), sodium ascorbate (0.2 eq.) | 10 | Trace |
| 2 | t-BuOH/H2O (1/1) | CuSO4×5H2O (0.1 eq.), sodium ascorbate (0.2 eq.) | 10 | Trace |
| 3 | t-BuOH/H2O (1/1), | CuSO4×5H2O (0.15 eq.), sodium ascorbate (0.3 eq.) | 10 | 20 |
| 4 | t-BuOH/acetone/H2O (1/1/1) | CuSO4×5H2O (0.2 eq.), sodium ascorbate (0.4 eq.) | 1 | 60 |
Table 1: Screening of the Optimal Reaction Conditions for Compound 14a
All the new compounds were characterized by IR, 1H NMR, 13C NMR and MS spectra. Their spectral analyses were consistent with the assigned structures and listed in the experimental section. The mass spectra for compounds gave a major fragment of [M+H]+ or [M+Na]+ according to their molecular formula.
In IR spectra, all the synthesized compounds gave absorptions in 3026-3100 cm-1, which was attributed to the stretching vibration of C–H bonds of the aromatic ring. Compounds 15a–e, 17a–b and 18 exhibited strong absorption bands at 1658-1665 cm-1 region, which indicated the presence of carbonyl groups, whereas two absorption between 1545 and 1337 cm-1 were attributed to the NO2 group in compounds 14b and 15b. Moreover, the IR spectra of target compounds 16a–b and 17a–b also exhibited characteristic strong absorption at 1237- 1238 cm-1 attributable to the stretching vibration of C–O bonds. Notably, the characteristic C−N bands of piperazine ring in all piperazine derivatives appeared in the region between 1135-1141 cm-1 and 1048- 1056 cm-1. All the other absorption bands were also observed at the expected regions.
In 1H NMR spectra, compounds 14-18 gave singlets at 5.22-5.50 ppm assigned to the CH2 protons linked to C-4 of the 1,2,3-triazole ring. The singlets at 4.57- 4.09 ppm of compounds 14 and 16 were assigned to the CH2 protons linked with N-1 of the 1,2,3-triazole ring, while the N-CH2 protons in compounds 15, 17 and 18 gave higher shift signals at 5.19-5.24 ppm due to the presence of the strong electron-withdrawing carbonyl group. Moreover, for compounds 15 and 17 the CH2 protons of piperazine ring were observed at 3.63-3.53 ppm and 2.39-2.43 ppm, but only one signal at 2.56-2.18 ppm was found for compound 4. All the other aromatic and aliphatic protons appeared at the appropriate chemical shifts and integral values.
The in vitro antimicrobial screening of all the synthesized compounds was performed on four Grampositive bacteria (S. aureus ATCC 6538, MRSA, M. luteus ATCC 4698 and B. subtilis ATCC 21216), four Gram-negative bacteria (E. coli ATCC 8099, P. aeruginosa ATCC 27853, B. typhi and B. proteus ATCC 13315) and three fungi (C. albicans ATCC 76615, S. cerevisiae and A. flavus) using two fold serial dilution technique recommended by the NCCLS with clinically used antimicrobial drugs chloromycin, enoxacin and fluconazole serving as positive controls.
The synthesized compounds were also evaluated for cytotoxic activity on a human tumor cell line, PC-3 using the MTT assay. Results of each tested compound were reported as the percent survived cells out of the treated cells compared to the untreated control cells [7]. The cell survival rates were summarized in Figure 2.
The in vitro antifungal evaluation displayed that only some compounds exhibited inhibitory activity against C. albicans and S. cerevisiae. Diphenylpiperazine 1,2,3-triazole derivatives 14a, 14b and 15b exhibited moderate activity with MIC values of 50-100 μg/ ml against C. albicans and S. cerevisiae. Compound 19 bearing enoxacin fragment displayed relatively better antifungal activity on C. albicans, S. cerevisiae and A. flavus. It was noteworthy that the 4-nitro imidazole contained compound 14b and 15b exhibited 2-fold more potent activity against A. flavus in comparison to fluconazole (MIC=200 μg/ml; Table 2). The in vitro antibacterial screening demonstrated that some of the newly synthesized compounds could effectively inhibit the growth of all the tested microorganisms. Specially, nitroimidazole piperazine 14b gave good activity against B. subtilis, M. luteus, B. proteus, E. coli and B. typhi with MIC values of 25 μg/ml and it exhibited moderate inhibition of the growth of S. aureus and MRSA with MIC value of 50 μg/ml. The substitution of 4-nitro imidazole by triazole ring, which yielded compound 14a, resulted in decreased antibacterial activity. Furthermore, the replacement of hydroxyl group of compound 14 by carbonyl moiety, which gave compounds 15a−e, also led to weak activity against the tested bacterial strains (Table 3).
| Compounds | C. albicans | S. cerevisiae | A. flavus |
|---|---|---|---|
| 14a | 100 | 100 | 200 |
| 14b | 50 | 50 | 100 |
| 15a | 400 | 200 | 400 |
| 15b | 100 | 100 | 100 |
| 15c | 400 | 400 | 400 |
| 15d | 400 | 400 | 400 |
| 15e | 400 | 400 | 400 |
| 16a | 400 | 400 | 400 |
| 16b | >400 | >400 | >400 |
| 17a | 400 | 400 | 400 |
| 17b | >400 | >400 | >400 |
| 18 | >400 | >400 | >400 |
| 19 | 200 | 200 | 200 |
| Fluconazole | 3.1 | 6.2 | 200 |
Table 2: Antifungal Activity of Compounds 14–19
| Compounds | Gram-positive bacteria | Gram-negative bacteria | ||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus | MRSA | B. subtilis | M. luteus | B. proteus | E. coli | P. aeruginosa | B. typhi | |
| 14a | 100 | 100 | 200 | 200 | 200 | 100 | 200 | 100 |
| 14b | 50 | 50 | 25 | 100 | 25 | 25 | 25 | 25 |
| 15a | 400 | 200 | 400 | 400 | 200 | 200 | 200 | 200 |
| 15b | 200 | 200 | 100 | 100 | 400 | 200 | 200 | 100 |
| 15c | 200 | 200 | 200 | 400 | 100 | 400 | 400 | 400 |
| 15d | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 |
| 15e | 200 | 200 | 100 | 200 | 400 | 400 | 400 | 400 |
| 16a | 400 | 400 | 400 | 400 | >00 | 400 | 400 | 400 |
| 16b | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 |
| 17a | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 |
| 17b | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 |
| 18 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 |
| 19 | 200 | 200 | 200 | 200 | 50 | 100 | 100 | 100 |
| Chloromycin | 3.1 | 6.2 | 3.1 | 1.6 | 6.2 | 3.1 | 3.1 | 1.6 |
| Enonxacin | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 |
Table 3: Antibacterial Activity of Compounds 14–19
The results of preliminary cytotoxic activity showed that the tested compounds exhibited weak growth inhibition against PC-3 cell line at the concentration of 100 μM (Figure 2). Among the tested piperazine derivatives, compounds 15b and 15e showed inhibitory activity against PC-3 cell line at the concentration of 100 μM with the cell survive rate only of 67 and 60 %, respectively. It was thought that the presence of nitroimidazole and benzimidazole ring would afford beneficial effect to antiproliferative properties. Meanwhile, the antiproliferative results indicated that all the compounds showed dose-dependent antiproliferative activity in the tested cell lines. Further studies are in progress to define the important mechanisms of action of the above mentioned compounds.
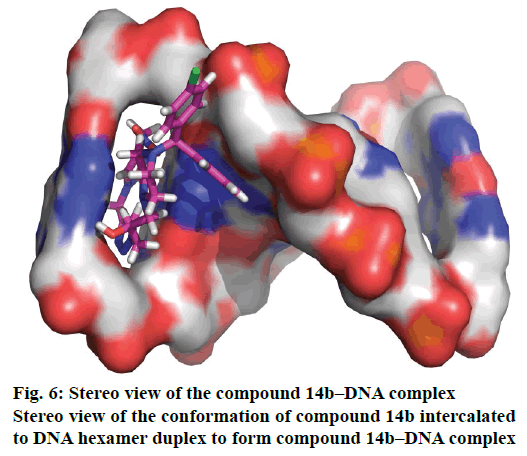
Molecular docking study as a useful strategy is extensively employed to research the binding modes of small molecules to DNA [20]. In our research, molecular docking study was performed between compound 14b and DNA hexamer duplex (PDB code: 3FT6) to understand the binding model. The docking mode with lowest binding free energy (−27.8 kJ·mol−1) is shown in Figures 5 and 6. The results showed that the nitro and hydroxyl groups of compound 14b could form hydrogen bonds with the guanine of DNA, and thus prevent the formation of hydrogen bond between cytosine in DNA. This kind of interaction resulted in decreased stability of DNA, thus inhibiting its physiological function.
In conclusion, a novel series of diphenylpiperazine 1,2,3-triazole derivatives have been successfully prepared starting from commercially available 4-chlorophenyl)(phenyl)methanone. The new compounds were confirmed by 1H NMR, 13C NMR, IR and MS spectra. The assessment of in vitro antibacterial and antifungal activities against eight bacterial strains and three fungal strains showed that some compounds exhibited moderate to good antibacterial and antifungal activities, especially nitroimidazole piperazine 14b gave good activity against bacteria B. subtilis, M. luteus, B. proteus, E. coli and B. typhi with MIC values of 25 μg/ml, respectively, and it showed moderate activity against C. albicans and S. cerevisiae (MIC=50 μg/ml). Moreover, the 1,2,3-triazole-linked nitroimidazole diphenylpiperazine compound 15b and benzimidazole contained one 15e were found to be effective in vitro against the PC-3 cell line, and the cell survive rates only of 67 and 60 %, respectively at concentration of 100 μM in a dose-dependent manner. Furthermore, molecular docking experiments suggested that the nitro and hydroxyl groups of compound 14b could insert into base-pairs of DNA hexamer duplex by the formation of hydrogen bonds with guanine of DNA. All these results opened up a promising starting point to optimize the structures of diphenylpiperazine 1,2,3-triazole derivatives as potent biological agents.
Acknowledgements
This work was partially supported by National Natural Science Foundation of China (21672173), Fundamental Research Funds for the Central Universities (XDJK2016E058), the Shandong Provincial Natural Science Foundation (ZR2017PB001), the Program of Chongqing Medical and Pharmaceutical College (ygz2013108), the Chongqing Research Program of Basic Research and Frontier Technology (cstc2016jcyjA0275), and Doctoral Scientific Research Foundation of Linyi University (No. LYDX2016BS030).
Conflict of interest
There are no conflicts of interest.
References
- Chellat MF, Raguž L, Riedl R. Targeting antibiotic resistance. Angew Chem Int Ed Engl 2016;55(23):2-30.
- Shingade SG, Bari SB. Synthesis and antimicrobial screening of 4-thiazolidinone and 2-azetidinone derivatives of piperazine. Med Chem Res2013;22:699-706.
- Wang XL, Gan LL, Yan CY, Zhou CH. Synthesis and their evaluation for their antimicrobial activity of diphenylpiperazine-based sulfanilamides. Sci Sin Chim2011;41:451-60.
- Boxer MB, Jiang JK, Vander Heiden MG, Shen M, Skoumbourdis AP, Southall N, et al. Evaluation of substituted N,N’-diarylsulfonamides as activators of the tumor cell specific M2 isoform of pyruvate kinase.J Med Chem2010;53: 1048-55.
- Narendra Sharath Chandra JN, Sadashiva CT, Kavitha CV, Rangappa KS. Synthesis and in vitro antimicrobial studies of medicinally important novel N-alkyl and N-sulfonyl derivatives of 1-[bis(4-fluorophenyl)-methyl]piperazine. Bioorg Med Chem2006;14: 6621-7.
- Jiang JR, Xu F, Ke ZL. Pharmacological activities and development of diarylmethyl piperazine derivatives. China Med Herald 2016;13:32-5.
- Gan LL, Fang B, Zhou CH. Synthesis of azole-containing piperazine derivatives and evaluation of their antibacterial, antifungal and cytotoxic activities. Bull Korean Chem Soc2010;31:3684-92.
- Khedar P, Pericherla K, Singh RP, Jha PN, Kumar A. Click chemistry inspired synthesis of piperazine-triazole derivatives and evaluation of their antimicrobial activities.Med Chem Res2015;24:3117-26.
- Lv JS, Peng XM, Kishore B, Zhou CH. 1,2,3-Triazole-derived naphthalimides as a novel type of potential antimicrobial agents: Synthesis, antimicrobial activity, interaction with calf thymus DNA and human serum albumin. Bioorg Med Chem Lett2014;4: 308-13.
- Nagesh HN, Suresh N, Prakash GVSB, Gupta S, Rao JV, Sekhar KVGC. Synthesis and biological evaluation of novel phenanthridinyl piperazine triazoles via click chemistry as anti-proliferative agents. Med Chem Res2015;24:523-32.
- Zhang HZ, Wei JJ, Kumar KV, Rasheed S, Zhou CH. Synthesis and biological evaluation of novel D-glucose-derived 1,2,3-triazoles as potential antibacterial and antifungal agents. Med Chem Res 2015;24:182-96.
- Zhou CH, Wang Y. Recent researches in triazole compounds as medicinal drugs. Curr Med Chem2012;19:239-80.
- Zhang L, Peng XM, Damu GLV, Geng RX, Zhou CH. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med Res Rev2014;34:340-437.
- Zhang HZ, Damu GLV, Cai GX, Zhou CH. Design, synthesis and antimicrobial evaluation of novel benzimidazole type of Fluconazole analogues and their synergistic effects with Chloromycin, Norfloxacin and Fluconazole. Eur J Med Chem2013;64:329-44.
- Zhang HZ, Jeyakkumar P, Kumar KV, Zhou CH. Synthesis of novel sulfonamide azoles via C–N cleavage of sulfonamides by azole ring and relational antimicrobial study. New J Chem 2015;39:5776-96.
- Zhang HZ, Lin JM, Rasheed S, Zhou CH. Design, synthesis, and biological evaluation of novel benzimidazole derivatives and their interaction with calf thymus DNA and synergistic effects with clinical drugs. Sci China Chem2014;57:807-22.
- Zhang HZ, Cui SF, Nagarajan S, Rasheed S, Cai GX, Zhou CH. A unique one-pot reaction via C-C cleavage from aminomethylene benzimidazoles to access benzimidazolones with wide potentiality. Tetrahedron Lett2014;55:4105-9.
- Zhang HZ, He SC, Peng YJ, Zhang HJ, Gopala L, Tangadanchu VKR, et al. Design, synthesis and antimicrobial evaluation of novel benzimidazole-incorporated sulfonamide analogues. Eur J Med Chem2017;136:165-83.
- Zhang HZ, Gan LL, Wang H, Zhou CH. New progress in azole compounds as antimicrobial agents. Mini Rev Med Chem2017;17:122-66.
- Ren Y, Zhang HZ, Zhang SL, Luo YL, Zhang L, Zhou CH, et al. Synthesis and bioactive evaluations of novel benzotriazole compounds as potential antimicrobial agents and the interaction with calf thymus DNA. J Chem Sci2015;127:2251-60.
- Gao Y, Gao HX, Piekarski C, Shreeve JM. Synthesis of functionalized azolium salts and their dicyanamide, dinitramide, perchlorate and nitrate compounds. Eur J Inorg Chem2007;31:4965-72.
- Bhujanga RAKS, Gundu RC, Reckitt SBB. Studies on N-propargylation and N-allenylation of 4(5)-nitroimidazoles. J Chem Res 1993;12:506-7.
- Azaroual MF, El Hadrami EM, Bentama A, Hamdach A. Synthesis of 4- and 5-substituted ribofuranosyl triazoles. Phys Chem News2006;32:107-11.
- Lingam VSPR, Vinodkumar R, Mukkanti K, Thomas A, Gopalan B. A simple approach to highly functionalized benzo[b]furans from phenols and aryl iodides via aryl propargyl ethers. Tetrahedron Lett2008;49:4260-4.
- Mori T, Ke CF, Yang C, Yang ZX, Wu WJ, Inoue Y, et al. Synthesis of functionalized b-cyclodextrins by "click chemistry". Heterocycles 2008;76:155-60.
- Ananda Kumar CS, Benaka Prasad SB, Vinaya K, Chandrappa S, Thimmegowda NR, Kumar YC, et al. Synthesis and in vitro antiproliferative activity of novel 1-benzhydrylpiperazine derivatives against human cancer cell lines. Eur J Med Chem2009;44:1223-9.