- *Corresponding Author:
- Smita patil
Department of Pharmaceutical Chemistry
Government College of Pharmacy, Karad, 415 124, India
E-mail: smit1000@rediffmail.com
| Date of Submission | 28 October 2007 |
| Date of Revision | 01 July 2008 |
| Date of Acceptance | 24 January, 2009 |
| Indian J Pharm Sci, 2009, 71 (1): 58-61 |
Abstract
An accurate, specific and precise assay level gradient reverse-phase high-performance liquid chromatographic method was developed for simultaneous determination of montelukast sodium and bambuterol hydrochloride in tablet dosage form. An inertsil ODS C-18, 5 µm column having 250×4.6 mm I.D. in gradient mode, with mobile phase A, containing 0.025 M sodium phosphate buffer: methanol (85:15) and mobile phase B, containing acetonitrile:methanol (85:15) was used at different time intervals. The flow rate was 1.5 ml/min and effluent was monitored at 218 nm. The retention times of montelukast sodium and bambuterol hydrochloride were 21.2 min and 5.8 min respectively. The linearity for both the drugs was in the range of 0.25-0.75 mg/ml with correlation coefficients of 0.9999 and 0.9996 for montelukast sodium and bambuterol hydrochloride, respectively.
Keywords
Montelukast sodium, bambuterol hydrochloride, HPLC, simultaneous, dosage form
Montelukast sodium (MTK), 1-[({(R)-m-[(E)-2- (7-chloro-2-quinolyl) vinyl]-α-[o-(1-hydroxyl-1- me thyl e thyl )phene thyl ]benzyl}thio)me thyl ] cyclopropaneacetate sodium is a leukotriene receptor antagonist, used in the treatment of asthma [1-3]. It is not official in IP and BP. Various analytical methods, such as liquid chromatography with fluorescence detection [4-6], stereoselective HPLC for MTK and its S-enantiomer [7], simultaneous HPLC and derivative spectroscopic method with loratadine [8], stability indicating HPLC method for MTK in tablets and human plasma [9] have been already reported.
Bambuterol hydrochloride (BBL), (RS)-5-(2- tert-butylamino-1-hydroxyethyl)-m-phenylene bis(dimethylcarbamate) hydrochloride is a direct acting sympathomimetic with predominantly -adrenergic activity (β2-agonist) [10]. It is an ester prodrug of β2 adrenergic agonist terbutaline [11]. Bambuterol hydrochloride is official in BP [12]. Different HPLC methods have been reported for the estimation of BBL in pharmaceutical dosage form [13-15]. The drug has been also estimated by solid-state NMR spectroscopy [16]. The combination dosage forms of MTK and BBL are available in the market for the prophylaxis and treatment of chronic asthma and chronic bronchitis in pediatrics. Present study involves development and validation of RP-HPLC method for the estimation of MTK and BBL in combination dosage form.
Combination tablet formulation containing montelukast sodium equivalent to montelukast 10 mg and bambuterol hydrochloride 10 mg (Montair Plus, Okasa Pharma, Satara, India) was procured from the local pharmacy. HPLC grade acetonitrile, methanol (Rankem, India) and HPLC grade water (Milli-Q) were used in this method. NaH2PO4 was of analytical grade obtained from Qualigens (India). Mobile phase A was prepared by mixing 850 ml of 0.025M NaH2PO4 buffer with 150 ml of methanol and mobile phase B was prepared by mixing 850 ml of acetonitrile with 150 ml of methanol. The solution was sonicated for 10 min and filtered using Whatman filter paper (No.41).
A Shimadzu HPLC LC-2010 AHK unit and Agilent 1100 system with variable wavelength programmable UV/Vis detector, an inertsil ODS C-18, 5 μm column of dimensions 250×4.6 mm was used. A Rheodyne injector with a 10 μl loop was used for the injection of sample.
Standard stock solution was prepared by weighing pure MTK and BBL (25 mg each) and dissolving in 30 ml of diluent in 50 ml volumetric flask. The solution was sonicated for 15 min, cooled and volume was made up to the mark with diluent to obtain final concentration of 500 μg/ml each. The solution was filtered. Calibration curves were prepared by taking appropriate aliquots of standard MTK and BBL stock solution in 10 ml volumetric flask and diluted up to the mark with diluent to obtain final concentrations of 250, 300, 400, 500, 600, 700, 750 μg/ml of each. Standard solutions (n=6) were injected through 10 μl loop system, and chromatograms were obtained using 1.5 ml/min flow rate. The time programme was set for gradient elution. Different compositions of mobile phases at different time intervals (mobile phase A:mobile phase B, 85:15 at 0 min, 15:85 after 15 min, 15:85 after 22 min, 85:15 after 28 min and 85:15 after 33 min) were run to obtain the satisfactory resolution. The effluent was monitored at 218 nm. Calibration curve was constructed by plotting average peak area against concentration, and regression equations were computed.
Five intact tablets (0.9380 g) containing MTK and BBL, each of 10 mg, were weighed accurately and transferred to 100 ml volumetric flask, sonicated for 15 min and the volume made up to the mark with diluent (water:acetonitrile:methanol, 1:1:1) to obtain final concentration of 500 μg/ml of each drug. The solution was filtered. Sample solutions were chromatographed (n=6), and concentrations of MTK and BBL in tablet samples were found using regression equations.
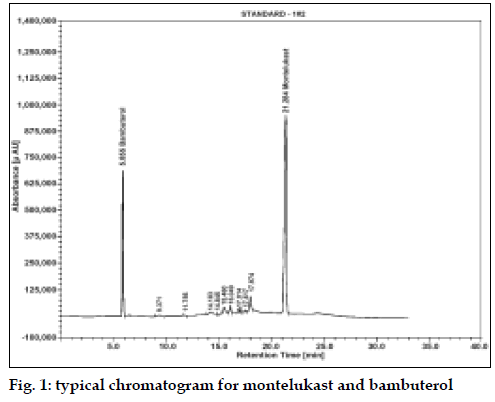
The average retention time for MTK and BBL was found to be 21.2 min (% RSD, 0.28) and 5.8 min (% RSD, 0.15), respectively (fig. 1). The linearity of the assay was checked at 50-150% of the assay level concentration of MTK and BBL. The calibration was linear in the range of 0.25-0.75 mg/ml for both the drugs with regression coefficient 0.9999 and 0.9996, intercept −24564.35 and 69825.13 and slope 22166620.23 and 8402793.74 for MTK and BBL, respectively. The low % RSD value of peak area, 0.32 (MTK) and 0.19 (BBL) indicated that the method is precise and accurate (Table 1).
| Parameter | MTK | BBL |
|---|---|---|
| Correlation coefficient | 0.9999 | 0.9996 |
| Slope | 22166620.23 | 8402793.74 |
| Intercept | - 24564.35 | 69825.13 |
| % RSD of area of standard | 0.32a | 0.19a |
| % RSD of retention time (min) of standard |
0.21a | 0.09a |
aValues of % RSD of six estimations; RSD: Relative standard deviation
Table 1: Linearity And Precision of Hplc Method
The content of MTK and BBL were determined using regression equation of standards. The % drug content was found to be 101.3±0.66 for MTK and 98.56±0.82 for BBL. Recovery studies were carried out at 50%, 100% and 150% level. The mean recoveries (n=3) were found to be 99.45-99.97% (% RSD 0.56-1.92) for MTK and 99.76-100.3% (% RSD 0.76-1.87) for BBL. The low % RSD values obtained for repeatability (n=6), intra-day (n=3), inter-day variation (n=3) and robustness (n=3) indicated that the method was precise.
The content of MTK and BBL were determined using regression equation of standards. The % drug content was found to be 101.3±0.66 for MTK and 98.56±0.82 for BBL. Recovery studies were carried out at 50%, 100% and 150% level. The mean recoveries (n=3) were found to be 99.45-99.97% (% RSD 0.56-1.92) for MTK and 99.76-100.3% (% RSD 0.76-1.87) for BBL. The low % RSD values obtained for repeatability (n=6), intra-day (n=3), inter-day variation (n=3) and robustness (n=3) indicated that the method was precise. chromatographic procedure for the simultaneous determination of MTK and BBL in tablets was developed in the present investigation. Satisfactory separation was obtained with the gradient system. The results obtained by the proposed method were close to the label claim of both the drugs. The low value of % RSD and recovery experiments indicates that the method is accurate.
Acknowledgements
The authors thank Okasa Pharma, Satara, India for providing drug samples. The authors also thank the Principal, Government College of Pharmacy, Karad, India for providing laboratory facilities.
References
- Sweetman SC. editors. Martindale: The Complete Drug Reference. 33rd ed. London: Pharmaceutical Press; 2002. p. 768.
- Budavari S. editors. In; The Merck Index, 12th ed. Whitehouse Station, NJ: Merck & Co. Inc.; 1996. p. 1070.
- Morrow JD, Roberts LJ. In; Hardman JG, Limbird LE, Gilman AG. editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 10th ed. New York: McGraw-Hill; 2001. p. 669.
- Al-Rawithi S, Al-Gazlan S, Al-Ahmadi W, Alshowaier I, Yusuf A, Raines D. Expedient liquid chromatographic method with fluorescence detection for montelukast sodium in microsamples of plasma. J Chromatogr B Biomed SciAppl 2001;754:527-31.
- Ochiai H, Uchiyama N, Takano T, Hara K, Kamei T. Determination of montelukast sodium in human plasma by column-switching high performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Appl 1998;713:409-14.
- Chauhan B, Shubha Rani, Nivsarkar M, Padh H. New liquid liquid extraction method for determination for montelukast in small volume human plasma samples using HPLC with fluorescence detector. Indian J Pharm Sci 2006;68:517-20.
- Liu L, Cheng H, Zhao JJ, Rogers JD. Determination of montelukast (MK-0476) and S-enatiomer in human plasma by stereoselective high performance liquid chromatography with column switching. J Pharm Biomed Anal 1997;15:631-8.
- Radhakrishna T, Narasaraju A, Ramakrishna M, Satyanarayana A. Simultaneous determination of montelukast and loratadine by HPLC and derivative Spectrophotometric methods. J Pharm Biomed Anal 2003;31:359-68.
- Alsarra I. Development of a stability-indicating HPLC method for the determination of montelukast in tablets and human plasma and its applications to pharmacokinetic and stability studies. Saudi Pharm J 2004;12:136-43.
- Sweetman SC. editors. Martindale: The Complete Drug Reference, 33rd ed. London: Pharmaceutical Press; 2002. p. 761.
- Budavari S. editors. In; The Merck Index, 12th ed. Whitehouse Station, NJ: Merck & Co. Inc.; 1996. p. 163.
- The British Pharmacopoeia, 3rd ed. Vol.1. London: Stationary Office Books; 2001. p. 174-5.
- Zhang Y. Determination bambuterol hydrochloride tablets by. Jiangsu Pharm Clin Res 2001;9:13-4.
- Bartolincic A, Druskovic V, Sporec A, Vinkovic V. Development and validation of HPLC methods for the enantioselective analysis of bambuterol and albuterol. J Pharm Biomed Anal 2005;36:1003-10.
- Wannerberg O, Persson B. Liquid chromatographic methods for the determination of bambuterol hydrochloride and relatedcompounds. J Chromatogr A 1988;435:199-203.
- Harris R, Hodgkinson P, Larsson T, Muruganatham A. Quantification of bambuterol hydrochloride in a formulated product using solid-state NMR. J Pharm Biomed Anal 2005;38:858-64.