- *Corresponding Author:
- M. N. Saraf
Department of Pharmacology, Bombay College of Pharmacy, Kalina, Santacruz (E), Mumbai-400 098, India.
E-mail: saraf@bcpindia.org
| Date of Submission | 21 March 2005 |
| Date of Revision | 08 November 2005 |
| Date of Acceptance | 22 July 2006 |
| Indian J Pharm Sci,2006, 68 (4): 475-478 |
Abstract
A simple, rapid, selective and sensitive reversed phase HPLC method was developed and validated for the determination of rebamipide from plasma. The drug was extracted with a mixture of chloroform and isopropyl alcohol. Rebamipide was measured in plasma using a validated HPLC method with UV detection at 280 nm. Chromatographic peaks were separated on a 5 µm C-18 silica column using a mixture of acetonitrile, water, methanol and acetic acid as a mobile phase. The chromatograms showed good resolution and no interference from plasma.The retention time of rebamipide and internal standard were approximately 4.9±0.3 min and 7.6±0.3 min, respectively. The mean recovery from human plasma was found to be above 91%. The method was linear over the concentration range of 10 to 500 ng/ml with coefficient of correlation (r2) 0.991. Both intra-day and inter-day accuracy and precision data showed good reproducibility. This method can be successfully applied to pharmacokinetic studies.
Introduction
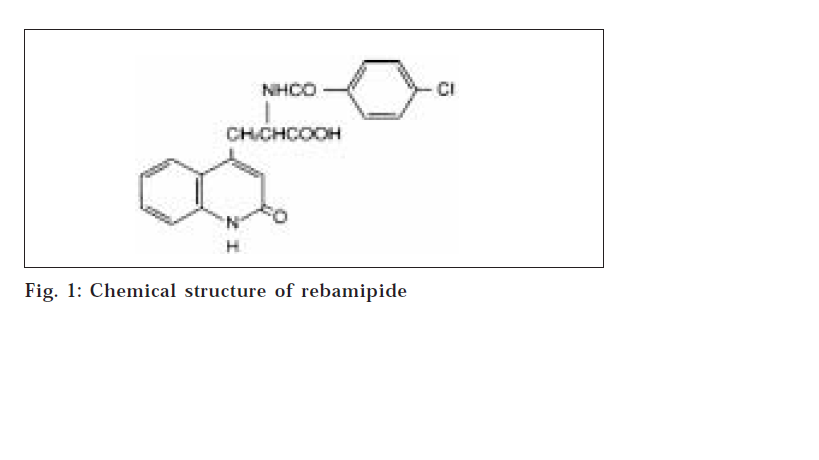
Rebamipide {2-(4-chlorobenzolylamino)-3-[2(1H)-quinolinon-4-yl] propionic acid} (fig. 1) is one of the mucosa-defensive agents that is known to prevent various acute experimental gastric mucosal lesions and to accelerate the healing of chronic gastric ulcer [1,2]. The mechanism of its antiulcer action has been reported to involve increased mucous secretion [3], enhanced generation of endogenous prostaglandins [4], suppression of neutrophil function [5] and inhibition of inflammatory cytokines [6].
Two methods have been reported for the determination of rebamipide from plasma. The first method described by Shimizu and coworkers was based on use of HPLC with UV detection [7]. The method was tedious and time consuming as it requires a two-step sample preparation procedure while the second method reported by Akamatsu and coworkers was based on HPLC with fluorimetric detection [8] which is a modification of first method and employed a six port switching valve, a precolumn and was complex to use. Hence, an attempt precolumn and was complex to use. Hence, an attempt rapid and sensitive HPLC method for the estimation of rebamipide from plasma which can be applied to pharmacokinetic study.
Materials and Methods
Rebamipide and carbamazepine were obtained from Macleods Pharmaceuticals, Mumbai and Dr. M. K. R.Drug Testing and Training Laboratory, Mumbai,respectively. HPLC grade chloroform, isopropyl alcohol, diethyl ether, methanol and acetonitrile were purchased from Qualigens, Mumbai, Analytical grade potassium dihydrogen phosphate and acetic acid were purchased from S. D. Fine Chemicals, Mumbai. Pooled drug free human plasma was purchased from K. E. M. blood fractionation centre, Parel, Mumbai.
Chromatographic conditions
The HPLC system consisted of a Jasco–PU980 intelligent pump (Jasco Ltd., Japan), manual injector port with 50 μl loop (Rheodyne, USA), Jasco UV–Vis 975 intelligent detector (Jasco Ltd, Japan). The wavelength of the detector was set at 280 nm. Detector output was quantified on Jasco–Borwin (Version 1.50) chromatography software with Hercules 2000 chromatography interface (Version 2.0). Separation was carried out on a Lichrospher® 100 C-18 250×4.6 mm i.d, 5 μm (Merck, Germany) using acetonitrile: water: methanol: acetic acid in the proportion of 37:61:1:1, v/v respectively as a mobile phase, at a flow rate of 1 ml/min. The mobile phase was filtered through nylon membrane filter (0.45 μm pore size, Pall Life Sciences, Mumbai) and ultrasonically degassed prior to use. Chromatography was carried out at room temperature (temperature was maintained at 20-24°).
Preparation of standard solutions
Stock solutions of rebamipide (0.5 mg/ml) and carbamazepine (internal standard) (1 mg/ml) were prepared in methanol. Further dilutions were carried out in methanol. Calibration standards were prepared freshly by spiking drug free plasma with rebamipide stock solution to give the concentrations of 1, 10, 25, 50, 100, 250, 500, 1000 and 2000 ng/ml.
Quality control standards
Lowest quality control standards, median quality control standards and highest quality control standards were prepared by spiking drug free plasma with rebamipide to give solutions containing 10 ng/ml, 100 ng/ml and 500 ng/ ml, respectively. They were stored at -20° till analysed.
Sample preparation
To 1 ml of calibration standard, internal standard (100 μl of 1 μg/ml) was added and vortexed for 1 min, followed by addition of 0.4 ml of phosphate buffer pH 7 and vortexed. The drug was extracted with 7 ml mixture of chloroform: isopropyl alcohol [95:5, v/v] by vortexing for 3 min followed by centrifugation at 2500 rpm/min on a cooling centrifuge for 15 min at 4°. The organic phase was withdrawn and dried using nitrogen evaporator. To the residue 0.4 ml of diethyl ether was added and shaken for 5 to 10 s and the ether layer was discarded. The residue was reconstituted with 200 μl of mobile phase and 50 μl was injected onto the column.
Specificity
A solution containing 100 ng/ml was injected onto the column under the optimized chromatographic conditions to demonstrate the separation of rebamipide from the impurities of plasma. The specificity of the method was checked for the interference from plasma.
Linearity range
Quantitative analytical results are highly influenced by the quality of the calibration curve. Spiked concentrations were plotted against the peak area ratios of rebamipide to internal standards and the best fit line was obtained by linear regression analysis of the resultant curve. The linearity equation (y=mx+c) and the regression coefficient was calculated. Wide range calibration was initially investigated by injecting solutions containing 1.0-2000 ng/ml.
Recovery studies
The recovery of rebamipide was determined by comparing the peak obtained from the area of the extracted calibration standards with the peak area of working standards of the respective concentration.
Limit of quantitation
In order to estimate limit of quantitation, a drug free blank plasma sample was extracted and injected six times and analysed as described under optimized chromatographic conditions. The noise level was then determined. The limit of quantitation for rebamipide was determined [signal-tonoise ratio=10].
Precision and accuracy
Intra-day precision and accuracy was determined by analyzing quality control standards (10, 100 and 500 ng/ml) five times a day randomly, inter-day precision and accuracy was determined from the analysis of each quality control standards (10, 100 and 500 ng/ml) once on each of five different days.
Stability studies
The stability of rebamipide was determined by measuring concentration change in control samples over time. The plasma control samples were stored in polypropylene plasma tubes at -20°. Stability was tested by subjecting the plasma controls to three freeze-thaw cycles and storage for 24 h at room temperature.
Results and Discussion
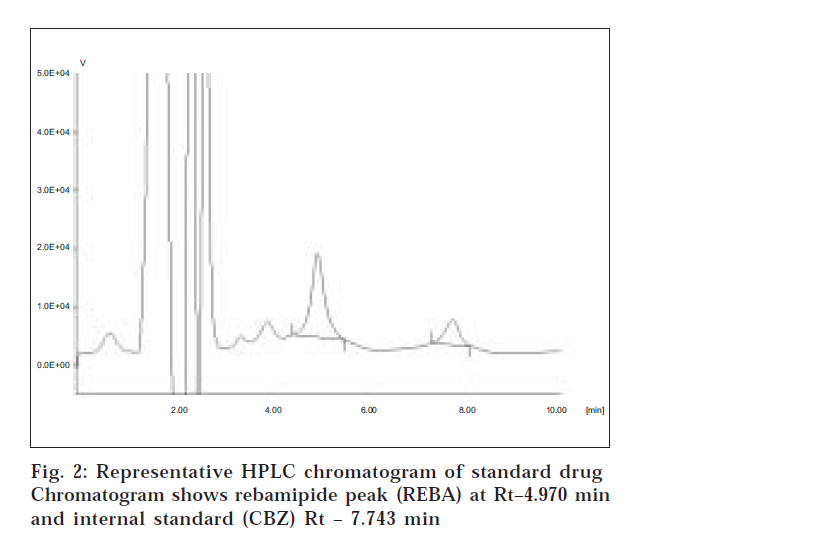
Under the chromatographic conditions employed, the sample showed sharp peaks of drug and internal standard with good resolution. The retention time of the drug was found to be 4.9±0.2 min and the retention time of the internal standard was 7.5±0.3 min (fig. 2). The method developed was validated for specificity, intra-day and inter-day accuracy and precision, linearity and stability as per USFDA guidelines (http:// www.fda.gov/cder/guidance/ index.html accessed on Dec 2003). The results of validation parameters are given below.
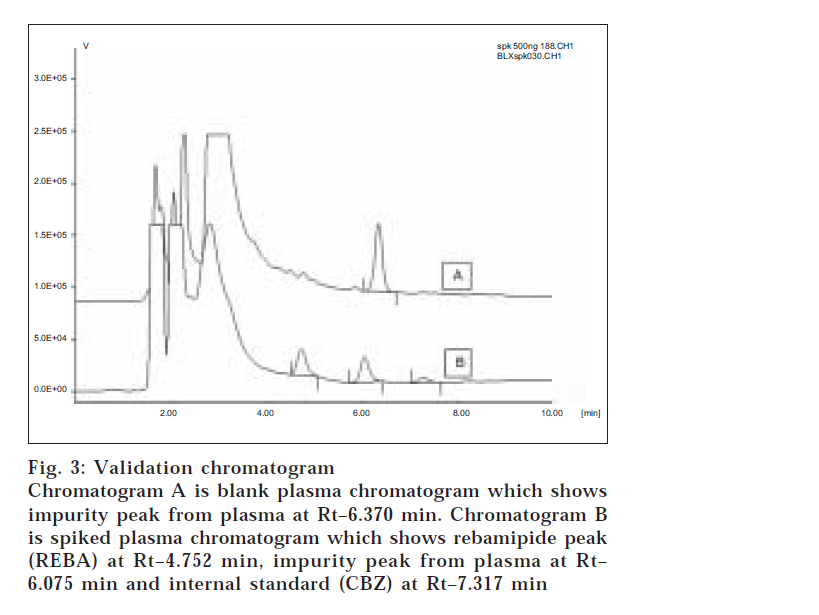
Specificity of the method was proven by the absence of interfering peaks near the retention time of the drug as well as the internal standard (fig. 3). The calibration function (Peak area ratio Vs concentration) was linear over working range of 10-500 ng/ml with six point calibration used for quantitation by linear regression. The regression equation for the analysis was Y=0.0047X0.1028; with coefficient of correlation (r2) 0.991.
Fig. 3: Validation chromatogram Chromatogram A is blank plasma chromatogram which shows impurity peak from plasma at Rt–6.370 min. Chromatogram B is spiked plasma chromatogram which shows rebamipide peak (REBA) at Rt–4.752 min, impurity peak from plasma at Rt– 6.075 min and internal standard (CBZ) at Rt–7.317 min
The recovery (n=5) was found to be above 91% at all concentration (Table 1). The intra-day and inter-day precision of the method showed % C.V. in between 0.88 and 5.21 and the intra-day and inter-day accuracy of the method was found to be in between 97 to 105% for the quality control samples (Table 2). The limit of quantitation was found to be 10 ng/ml. At such concentration the inter-day precision was 1.3 and the accuracy was 95.93%. Plasma control samples of rebamipide were found to be stable for at least one month (Table 3).
| Concentration of | Mean percent recovery±S.D. | ||
|---|---|---|---|
| rebamipide (ng/ml) | (n = 5) | ||
| Rebamipide | Internal standard | ||
| (100 ng/ml) | |||
| 10 | 91.24±1.52 | 96.13±3.60 | |
| 100 | 93.42±3.20 | 95.83±3.26 | |
| 250 | 92.89±2.86 | 96.62±2.89 | |
| 500 | 93.46±2.00 | 96.12±2.65 | |
The tables gives mean percent recovery, calculated from five samples at each of the concentrations mentioned (n=5) along with their standard deviation (±SD).
Table 1: Mean Percent Recoveries Of Rebamipide And Internal Standard From Plasma
| Spiked | Intra–day | Inter–day | ||||
|---|---|---|---|---|---|---|
| concentration | >Mean concentration | >C. V. (%) | Accuracy (%) | >Mean concentration | >C. V. (%) | Accuracy (%) |
| (ng/ml) | >(n = 5) (ng/ml) | >(n = 5) (ng/ml) | ||||
| 10 | >10.25 | >3.57 | >102.50 | >10.56 | >5.21 | >105.60 |
| 100 | >96.88 | >1.47 | >96.88 | >96.56 | >1.94 | >95.56 |
| 500 | >485.17 | >0.88 | >97.03 | >488.50 | >0.98 | >97.70 |
The tables gives mean percent recovery, calculated from five samples at each of the concentrations mentioned (n=5) along with their coefficient of variation (% C.V).% Accuracy = [Measured concentration / spiked concentration] *100
Table 2: Precision And Accuracy Of Quality Control Standards Of Rebamipide
| Number of | LQC (10 ng/ml) | MQC (250 ng/ml) | HQC (500 ng/ml) | |||
|---|---|---|---|---|---|---|
| days stored | Mean±S.D. (n = 2) | % C.V. | Mean±S.D. (n = 2) | % C.V. | Mean±S.D. (n = 2) | % C.V. |
| 5 days | 9.96±0.09 | 0.90 | 248±1.41 | 0.57 | 500±2.82 | 0.56 |
| 15 days | 10.00±0.00 | 0.00 | 249.5±2.12 | 0.85 | 498.5±3.54 | 0.71 |
| 30 days | 9.98±0.11 | 1.10 | 251.5±4.95 | 1.97 | 501.5±3.54 | 0.71 |
The table gives stability data of rebamipide in human plasma at 3 different concentrations i.e. lowest quality control, median quality control. Highest quality control (HQC) standards along with their respective standard deviations (±SD) and coefficient of variation (% C.V). Each sample was analysed in replicates (n=2).
Table 3: Stability Studies On Rebamipide
Rebamipide is soluble in methanol and hence, standard solutions were prepared in methanol. To develop suitable chromatographic conditions, initially pure sample of rebamipide was used. Chromatograms of four different concentrations of rebamipide were studied using Lichrosphere and ODS extrasil column at maximum absorption i.e., 280 nm.
The proportion of acetonitrile in the mobile phase was optimized to 37%. A slight increase or decrease in concentration of acetonitrile resulted in the drug peak merger with the plasma peak or, increased the retention time from 4.9 min to 7.2 min, respectively. The ODS extrasil column showed tailing. However, in case of Lichrosphere a small peak base broadening was seen which was overcome by the addition of 1% of methanol to mobile phase. Hence, Lichrosphere column was selected for analysis. The use of 1% glacial acetic acid in non–buffered mobile phase improved the peak shape and detection down to 10 ng/ml. Hence the mobile phase finally optimized to the mixture of acetonitrile: water: methanol: acetic acid in the proportion of 37:61:1:1, v/v, respectively.
The extraction of rebamipide was based on liquid- liquid extraction technique. Various solvents systems were tried for recovery studies. The maximum recovery was obtained with a mixture of chloroform: isopropyl alcohol (95:5, v/v) at pH 7. Hence, chloroform:isopropyl alcohol mixture was used for the extraction of drug from plasma. The extraction was carried out in one simple step, which was followed by diethyl ether cleanup step, which removed interferences due to plasma and yielded cleaner chromatograms.
Five drugs were attempted for selection as internal standard. Carbamazepine showed good resolution and extractability under optimized chromatographic conditions; hence, carbamazepine was selected as the internal standard. The others drugs tried were found to be overlapping with retention time of rebamipide under the optimised chromatographic conditions.
Once the method was developed and optimized,validation for specificity, linearity, limit of quantitation,accuracy and precision were carried out. The recovery and stability of rebamipide from plasma were determined. Thus the analytical method developed for the quantitative determination of rebamipide from plasma was simple, rapid, specific sensitive, accurate and precise. Hence, the method is quite suitable to detect the drug from plasma samples of human volunteers.
Acknowledgements
The authors thank M/s Macleods Pharmaceuticals, Mumbai and Dr. M. K. R. Drug testing and training Laboratory, Mumbai for providing gift sample of rebamipide and carbamazepine, respectively.
References
- Yamasaki, K., Ishiyama, H., Imaizumi, T., Kanbe, T. and Yabuuchi, Y., Jpn. J. Pharmacol., 1989, 49, 441.
- Takemoto, T., J. Adult Dis., 1989, 19, 127.
- Ishihara, K., Komure, Y., Nishiyama, N., Yamasaki, K. and Hotta, K., Arzneim-Forsch/Drug Res., 1992, 42, 1462.
- Yamasaki, K., Kanbe, T., Ishiyama, H. and Moria, S., Eur. J. Pharmacol., 1987, 142, 23.
- Murakami, K., Okajima, K., Uchiba, M., Harada, N., Johno, M., Okabe, H. and Takatsuki, K., Dig. Dis. Sci., 1997, 42, 319.
- Aihara, M., Azuma, A., Takizawa, H., Tsuchimoto, D., Funakoshi, Y., Shindo, Y., Ohmoto, Y., Imagawa, K., Kikuchi, M., Mukaida, N. and Matsushita, K., Dig. Dis. Sci., 1998, 43, 74.
- Shioya, Y. and Shimizu, T., J. Chromatogr., 1988, 434, 283.
- Akamatsu, T., Nakamura, N., Furuya, N., Shimizu, T., Gotou, A., Kiyosawa, K., Katsuyama, T., Osumi, T., Hirao, Y. and Miyamoto, G., Dig. Dis. Sci., 2002, 47, 1399.