- *Corresponding Author:
- S. K. Shrivastava
Pharmaceutical Chemistry Research Laboratory, Department of Pharmaceutics, Indian Institute of Technology, Banaras Hindu University, Varanasi-221 005
E-mail: skshrivastava.phe@itbhu.ac.in
| Date of Submission | 04 June 2012 |
| Date of Revision | 03 March 2013 |
| Date of Acceptance | 13 March 2013 |
| Indian J Pharm Sci 2013;75(3):277-283 |
Abstract
Present manuscript describes the sustained and targeted delivery of 5-aminosalicylic acid to the distal ileum and proximal colon, using dextran (40 kDa) as a carrier for targeting 5-aminosalicylic acid at the colonic site by attaching p-aminobenzoic acid and benzoic acid as linkers. Prepared conjugate were characterized by UV, HPLC, FT-IR, and 1 H NMR. The degree of substitution was estimated by complete hydrolysis of conjugates in borate buffer and in vitro hydrolysis study of conjugates was performed in different biological media. It was observed that 5-aminosalicylic acid alone have produced high incidence of gastric ulcer with high ulcer index whereas lower ulcer index was found for the dextran conjugates of 5-aminosalicylic acid. The release pattern of conjugates in 3% w/v rat caecal content was confirmed the colon specificity of 5-aminosalicylic acid conjugates.
Keywords
5‑ASA, dextran, colon-specific, ulcerogenic study
Five-aminosalicylic acid (5‑ASA) is the drug of choice in the treatment of active inflammatory bowel disease, ulcerative colitis, and Crohn’s disease. It is readily absorbed from the upper gastrointestinal tract (GIT) as soon as it passes through the stomach leading to significant gastrointestinal toxicity. Polymer‑drug conjugates (PDCs) act as carrier for drug targeting at desired site of body. The carrier then undergoes enzymic hydrolysis, where active drug is released out. The major attributes of PDCs include the following: capacity to be stored in depots, unique pharmacokinetic profiles, potential body distribution, and pharmacological efficacy [1]. Dextrans may be used as model polysaccharides for conjugation because it has excellent water solubility, low toxicity, and immunogenicity and are available in a wide molecular weight range with low polydispersity. The dextran conjugates of naproxen [2], nalidixic acid [3], and ketoprofen [4] were demonstrated for their colon specificity and found chemically stable during the transit through the gastrointestinal tract. In previous study we have synthesized dextran conjugate of lamotrigine [5], valproic acid [6] to minimize its hepatotoxicity and dextran conjugate of celecoxib [7], aceclofenac [8] for targeting at the colonic site to reduce ulceroginic activity.
The 5-ASA conjugates of dextrans were developed using sodium periodate method for the treatment of Crohn’s disease [9]. The dextran conjugates of corticosteroids like dexamethasone and methyl prednisolon were synthesized with different spacer succinic acid and glutaric acid and evaluated for colon specificity [10,11]. Sulfasalazine (an azo prodrug of 5‑ASA and sulfapyridine) is successfully utilized for targeting 5‑ASA to colon [12]. In sulfasalazine therapy, the 5‑ASA acts as a topical antiinflammatory agent in the colon whereas sulfapyridine absorbed well in colon and produces majority of side effects like hepatotoxicity, hypospermia, and severe blood disorders [13,14]. Even though few prodrugs of 5‑ASA like balsalazide, ipsalazine, and olsalazine have been reported but a small portion of intact prodrug gets absorbed from the upper GI tract [15‑17]. Recently nitric oxide and hydrogen sulfide conjugates of aspirin (NOSH‑aspirin) have been synthesized to reduce internal bleeding and stomach ulcers. In these type of conjugates aspirin used as a scaffold to support two molecules where one arm of the hybrid aspirin releases nitric oxide (NO), which helps in protecting the stomach lining and second arm releases hydrogen sulfide (H2S), which enhances aspirin’s cancer fighting ability [18]. The 5‑ASA molecule is amphoteric, and its solubility as well as ionization characteristics depend upon pH and the pKa values of the carboxylic and amino groups [19]. It is very soluble above pH 5.5 and thus it is readily absorbed from the gastrointestinal tract as soon as it passes through the stomach. Systemic absorption of 5‑ASA produces a significant antiinflammatory action but severe gastrointestinal irritation restrict its clinical use [20]. On the basis of the above facts our study has been focused on local (topical) delivery of 5‑ASA at the diseased site (distal ileum and proximal colon) by linking the moiety with para‑amino benzoic acid (PABA) and benzoic acid (BA). These linkers provide functionality for the attachment between the drug and polymer as it contains free amino and carboxyl group, which can be used for diazotization and attachment with polymer, respectively.
Materials and Methods
The reference standard 5-ASA was a generous gift from Lupin Research Park, Pune, India. The 1,1’‑carbonyldiimidazole (CDI) and dialysis membrane‑150 (10MT) were procured from High Media Chemicals, Mumbai, India. Dextran (Mr~40,000) was purchased from Biochemika, Fluka, Sigma‑Aldrich, Buchs. The pepsin extra pure, eosin, carrageenan and haematoxylin stain were obtained from High Media Chemicals, Mumbai, India. The PABA and benzoic acid were obtained from High Media Chemicals, Mumbai, India. HPLC grade solvents (methanol and acetonitrile) were purchased from Merck India and used for HPLC analysis. Thin layer chromatography (TLC) was performed on TLC silica gel 60 F254 aluminium sheets (Merck, India). The methanol:water (2:8) was used as mobile phase. UV spectra of synthesized conjugates were obtained using a Jasco (Model 7800) UV/Vis spectrophotometer. Fourier transformed infrared (FT-IR) spectra were recorded on a Shimadzu FT‑IR 8400S spectrophotometer at the scanning range of 400-4000 cm−1. 1H NMR spectra were recorded on a Jeol AL 300 FT‑NMR spectrophotometer in DMSO‑d6. The student’s paired t‑test (two tailed) was used to analyze significant differences between the control and experimental groups by using the GraphPad Prism version 5.0 for Windows (GraphPad Software, Inc., 2009). Data are expressed as mean±SD.
Preparation of benzoic acid‑dextran conjugate
BA (0.366 g, 3 mmol) and CDI (0.486 g, 3 mmol) was dissolved in 5.0 ml DMSO. The reaction mixture was stirred at 25° for 60 min to get benzoic acid imidazolide intermediate product. The solution of dextran (40 kDa) (equivalent to one glucose unit) was prepared in 3.0 ml of DMSO and TEA was added for pH adjustment with constant stirring. The solution was stirred at 25° for 24 h. The dextran‑ester conjugate (BA-DT) was precipitated by using a mixture of ethanol and diethyl ether (50:50). The liquid part of this solution was discarded and precipitated compound was redissolved in DMSO and precipitation step was repeated. The dispersed compound was washed with diethyl ether to remove sulfate formed during the reaction and dried in desiccator.
Preparation of 5‑ASA-benzoic acid‑dextran azo conjugate
5‑ASA (0.459 g, 3 mmol) was dissolved in 35% conc. HCl solution and the solution of 5‑ASA was cooled in ice bath for 5 min at the temperature 3‑5°. The (0.7 g in 10.0 ml) sodium nitrite (NaNO2) cold solution in water was added and diazotization reaction was monitored by starch iodide paper. After the completion of reaction highly soluble diazonium salt was formed. The BA‑DT ester conjugate (0.474 g, 1 mmol) was dissolved in 0.5 M NaOH and drop‑wise diazonium salt solution was added into the solution with continuous stirring. The pH of clear solution was maintained up to 10 using 1 N NaOH solution. After 5‑10 min, stirring solution was turned to dark brown color but stirring was continued up to 30 min. The 5-ASA-benzoic acid-dextran azo conjugate (5-ASA-BA-DT) conjugate solution was poured in presoaked dialysis sack and dipped in triple distilled water solution for 24 h. The distilled water solution was replaced after 6 h interval. After dialysis, solution was freeze dried at temperature −45° to remove water completely. The brown amorphous conjugate was formed after lyophilization [21,22].
Preparation of p‑aminobenzoic acid‑dextran conjugate
PABA (0.411 g, 3 mmol) and 0.486 g or 3 mmol of CDI was dissolved in 5.0 ml DMSO. Further reaction mixture was treated as BA‑DT to obtain p‑aminobenzoic acid‑dextran conjugate (PABA-DT).
Preparation of salicylic acid p‑aminobenzoic acid‑dextran azo conjugate
The molar concentration of SA (0.414 g, 3 mmol) and PABA‑DT (0.519 g, 3 mmol) was used for the preparation of salicylic acid p-aminobenzoic acid-dextran azo conjugate (SA-PABA-DT). The temperature of 3‑5° was extremely maintained during the reaction and remaining reaction procedure is followed as above. The orange color SA‑PABA‑DT azo conjugate was formed after complete lyophilization [21,22].
Azo dextran polymeric conjugates of 5‑ASA were synthesized using PABA and BA linker. Free amino group of PABA was used for diazotization and free carboxyl group was activated by CDI and used for conjugation with dextran. In another method benzoic acid was used as linker, in which the free carboxyl group was activated using CDI than further attached with dextran, while amino group of 5‑ASA was used for the formation of azo compound. Dextran was attached with the azo compound by ester linkage using CDI as activating agent. Diazotization on the aromatic amino group of PABA‑DT and 5‑ASA was performed under acidic condition with NaNO2. The highly soluble diazonium salts of PABA‑DT and 5‑ASA were reacted under basic environment with SA and BA‑DT respectively, to form dextran azo conjugates.
HPLC analysis
HPLC analysis of 5‑ASA was performed in mobile phase methanol:100 mM potassium dihydrogen phosphate buffer in the ratio (5:75, v/v) and pH 4.4 of the buffer solution adjusted using orthophosphoric acid. 5‑ASA showed retention time of 3.5±0.05 min with flow rate 1.0 ml/min at λmax 240.0 nm. Fresh sample solutions of standard drug were prepared and subjected to a system suitability test using standard chromatograms. Various parameters such as 10% plate efficiency, 50% plate efficiency, peak asymmetry, and RSD of the peak area were determined. Observed peak area was plotted against standard peak area and final concentration of the drug in the conjugate was obtained. The HPLC method used for the estimation of 5‑ASA was extensively validated for linearity; a series of dilutions were prepared and response ratios were determined, respectively. Accuracy of the method was determined by recovery studies in which known amount of standard drug were added to the previously analyzed sample and the mixture was analyzed by the proposed method (Table 1).
| Parameter | Mean* | SD | RSD | %RSD | Acceptance limits |
|---|---|---|---|---|---|
| Peak area (50 µg/ml) | 1739.89 | 5.01 | 0.003 | 0.29 | % RSD less than 2 |
| Retention time | 3.54 | 0.03 | 0.01 | 0.96 | SD±0.05 |
| 10% plate efficiency | 5368.67 | 91.48 | 0.017 | 1.7 | More than 5000 |
| 50% plate efficiency | 7064.32 | 140.4 | 0.02 | 1.99 | More than 7000 |
| Peak asymmetry | 0.73 | 0.01 | 0.019 | 1.86 | % RSD less than 2 |
* Mean of six replicates, 5-ASA=5-aminosalicylic acid, SD=standard deviation RSD=relative standard deviation.
Table 1: Hplc system suitability parameters for 5 asa analysis.
Degree of substitution
Degree of substitution of the dextran conjugate was determined using HPLC. Accurately weighed 5‑ASA‑BA‑DT and SA‑PABA‑DT conjugates (20.0 mg) were dissolved in 20 ml of 0.1 M borate buffer at pH 9.0. The solution was stirred at 70.0±0.5º for 1 h and left aside for 24 h for complete hydrolysis, and then neutralized with 1 M HCl. The amount of 2 ml of methanol was added to 2.0 ml of the neutralized sample solution and tubes were centrifuged at high speed. The clear supernatant was filtered using a 0.45‑μm HPLC syringe and injected into the HPLC system. The amount of 5‑ASA hydrolyzed was determined by the HPLC method.
In vitro hydrolysis
In vitro hydrolysis study of 5‑ASA‑BA‑DT and SA‑PABA‑DT conjugates was performed in different biological media, e.g. simulated gastric fluid (SGF) pH 1.2, simulated intestinal fluid (SIF) of pH 7.4, and 3% w/v rat caecal content media (removed caecal contents from rats were individually weighed, pooled and suspended in phosphate buffer solution (PBS) pH 6.8 to produce final caecal concentration of 3% w/v under nitrogen atmosphere). Samples of both conjugates, equivalent to 10 mg of standard drugs, were dissolved in respective medium and packed in the presoaked dialysis membrane bag. Dialysis bag was dipped into simulated fluids (SGF, SIF and 3% w/v rat caecal content), to get final concentration of solution 500 µg/ml. The temperature was maintained at 37±1.0° and hydrolysis was observed by continuous stirring. Hydrolysis study in SGF (pH 1.2), SIF (pH 7.4) and 3% w/v rat caecal content (pH 6.8) was performed up to 2, 12, and 24 h, respectively. The sample solutions were withdrawn into centrifuge tubes at predetermined time intervals and volume of the aliquot was made up with methanol and tubes were centrifuged at high speed for 10 min. The clear supernatant liquid solution separated out from each tube and filtered through a 0.45 μm HPLC filter and then analyzed by HPLC method. The percentage of 5‑ASA released from the conjugates was calculated as the cumulative amount of the drug released. The rate of hydrolysis and half‑life of the prepared conjugate were calculated by the following equations. k=(2.303/t)×log (a/(a‑x))…(1) and t1/2=0.693/k…(2), where k is the rate constant, t is the time in h, a is the initial concentration of conjugate, x is the amount of the conjugate hydrolyzed into free parent drug, a‑x is the amount of parent drug remained in conjugate form and t1/2 is the half life of conjugate.
Biological evaluation
Pharmacological experiments were performed according to the Institutional Animal Ethical Committee Guidelines on Wistar rats weighing 120-150 g obtained from central animal house, Institute of Medical Science, Banaras Hindu University, Varanasi (Registration No. 542/02/ab/ CPCSEA). Ulcerogenic studies were determined by the cold stress model [23]. The best model to observe the maximum ulcer effects of compounds. This is an acute study model used to determine ulcerogenic potency of a drug at a 10 times higher dose. Wistar rats of either sex weighing between 120 g and 150 g were randomly distributed into control and experimental groups, each group containing six rats. The rats used in this study starved for 24 h and were given water ad libitum before the experiment. Suspensions of the standard drug and its conjugates were prepared in 1% gum acacia and administered orally. The rats were stressed by the exposure to cold (–15º for 45 min). Equally cold exposures to the rats were done to minimize the study error and after 2 h of the drug administration, the rats were sacrificed. The abdomen was opened by a midline incision. The stomach and the first 3 cm of the duodenum were removed. The stomach was opened along the greater curvature and washed with saline water. The mucus was wiped off and observed for ulcer in the glandular portion of the stomach. The number of ulcers was noted and the severity of ulcers was scored by means of a magnifying lens (×10). The average of six readings was calculated and was expressed as (mean±SE).
The lesion index (LI) of these compounds was also determined by the following equation: LI=number of ulcers+ulcer score+incidence percent/10. Tissue segments of 1 cm in length were fixed in 10% buffered formalin solution for histopathological studies. In the histopathological studies of the stomach, the serial sections of each paraffin block were cut and stained, using hematoxylin and eosin. The colored microscopical images of the stomach sections were taken on a Nikon Eclipse 50i Fi1 optical microscope with the resolution of ×10 and ×40 attached to a camera. All the biological data evaluated were expressed as (mean±SE) for six rats in each group. Statistical analysis was performed using the one‑way ANOVA using the Prism GraphPad, Prism version 5.0 for Windows, GraphPad Software (San Diego, CA, USA). All differences were considered significant at P<0.05 with respect to the control group.
Results and Discussion
Azo dextran polymeric conjugates of 5‑ASA were synthesized using PABA and BA linker. Physical properties of 5‑ASA and its dextran conjugates were cited in Table 2. All conjugates were characterized by UV, FT-IR and 1H NMR in order to check their purity and structure. Ester characteristic absorption peak C=O stretching for PABA-DT and BA‑DT was observed at 1718.63 and 1693.56 cm−1, respectively. The ‑OH stretching for polymeric ‑OH of dextran was observed in the range of 3250‑3500 cm−1, characteristic stretching vibrational bands of the ‑N=N‑ group was observed in the range of 1456.30‑1487.17 cm−1 (Table 3). 1H NMR spectrum of synthesized SA-PABA azo compound was shown the chemical shift of aromatic ring proton in the range of 7.24‑8.32 ppm. The broad signal for the carboxyl proton was found at 13.05 ppm after conjugation of PABA with SA confirmed the availability of carboxyl group for further conjugation with polymer. The phenolic hydroxyl proton present in azo compound showed NMR signal at 3.75 ppm. Dextran azo conjugates showed signals for aromatic ring proton in the range of 8.32‑7.67 ppm. The 1H NMR spectra of dextran conjugates showed a characteristic shifting of glucosidic ring anomeric proton signals from 4.5 to 4.9 ppm indicated the formation of ester linkage at C‑2 position of glucosidic ring. The signals of NH2 protons observed in case of PABA‑DT at 5.0 ppm, which was disappeared in NMR spectra of conjugates, confirmed the involvement of –NH2 protons in the formation of azo bond (Table 3).
| Compound | Intrinsic viscosity | Mw | Physical state | Solubility | |||||
|---|---|---|---|---|---|---|---|---|---|
| Water | DMSO | CHCl3 | Ether | 0.1 N HCl | MeOH | ||||
| 5‑ASA | ‑ | ‑ | Yellowish white | ++ | +++ | + | ‑ | ++++ | ++ |
| 5‑ASA‑BA‑DT | 0.228 | 46265 | Reddish brown | +++ | +++ | ‑ | ‑ | + | + |
| SA‑PABA‑DT | 0.226 | 45457 | Orange | +++ | +++ | ‑ | ‑ | + | + |
SA-PABA-DT=Salicylic acid para-amino benzoic acid dextran, 5-ASA=5-Aminosalicylic acid, BA-DT=Benzoic acid-dextran, Mw=Molecular weight
Table 2: Physical properties of 5 asa and its dextran conjugates
| Compound | Spectral data |
|---|---|
| 5‑ASA | IR, ṽ/cm−1: 1654 (C=O str.), 3477 (‑OH str.), 2956 (aromatic ring C‑H str.), 763.8 (aromatic C‑H bend.), 3608.93 (phenolic OH str.), 3300‑3446 (N‑H str.), 1581.68 (N‑H bend.) cm−1; 1H NMR (DMSO‑d6), ä: 7.2‑6.2 (m, 3H, Ar‑H), 2.5 (s, 2H, ‑NH2) |
| SA-PABA | IR, ṽ/cm−1: 1707 (C=O str.), 3441.12 (‑OH str.), 3090 (aromatic ring C‑H str.), 786.89 (aromatic C‑H bend.), 1477.52 (‑N=N‑ str.) cm−1 1H NMR (DMSO‑d6), ä: 13.05 (s, 2H, ‑COOH), 7.17‑8‑37 (m, 7H, Ar‑H), 3.87 (d, 1H, ‑OH) |
| PABA‑DT | IR, ṽ/cm−1: 1718.63 (C=O str.), 2928 (aromatic ring C‑H str.), 765.77 (aromatic C‑H bend.), 3318‑3448.8 (‑OH str. polymeric ‑OH dextran). cm−1 1H NMR (DMSO‑d6), ä: 7.8‑6.5 (m, 4H, Ar‑H), 5.8 (d, 2H, Ar‑NH2), 4.92 (d, dextran anomeric proton), 3.2‑4.7 (m, H, protons of glucosidic unit) |
| BA‑DT | IR, ṽ/cm−1: 1710.92 (C=O str.), 2929.97 (aromatic ring C‑H str.), 769.62 (aromatic C‑H bend.), 3250‑3500 (‑OH str. polymeric ‑OH dextran). cm−1 1H NMR (DMSO‑d6), ä: 8.0‑7.0 (m, 4H, Ar‑H), 4.91 (d, dextran anomeric proton), 3.2‑4.6 (m, H, protons of glucosidic unit) |
| 5‑ASA‑BA‑DT | IR, ṽ/cm−1: 1718.63 (C=O str.), 2926 (C‑H aromatic str.), 769.62 (C‑H aromatic bend.), 1405 (carboxylate anion str.), 1487.17 (‑N=N‑ str.), 3230‑3425 (‑OH str. polymeric ‑OH dextran). cm−1 1H NMR (DMSO‑d6), ä: 8.32‑7.69 (m, 7H, Ar‑H), 6.60 (s, 1H, Ar‑OH), 4.95 (d, dextran anomeric proton), 3.41‑4.68 (m, H, protons of glucosidic unit) |
| SA‑PABA‑DT | IR, ṽ/cm−1: 1693.56 (C=O str.), 2926 (C‑H aromatic str.), 767.69 (C‑H aromatic bend.), 1383 (carboxylate anion str.), 3745 (phenolic OH str.), 1456.30 (‑N=N‑ str.), 3389‑3446.91 (‑OH str. polymeric ‑OH dextran). cm−1 1H NMR (DMSO‑d6), ä: 8.29‑7.85 (m, 7H, Ar‑H), 6.74 (s, 1H, Ar‑OH), 4.94 (d, dextran anomeric proton), 3.37‑4.89 (m, H, protons of glucosidic unit) |
Table 3: Spectral data of 5‑asa and its dextran conjugates
The degree of substitution was determined by complete hydrolysis of conjugates in borate buffer and found to be 7.5±0.25%. Cumulative hydrolytic release patterns of the conjugates were determined through in vitro hydrolysis studies. The hydrolysis of conjugates in SGF pH 1.2 and SIF pH 7.4 was observed for 2 and 12 h, respectively [6,24], where a negligible amount of 5‑ASA was hydrolyzed. No degradation of conjugates at pH 1.2 and 7.4 showed that the conjugates were stable and remained intact at the gastric pH. The azo linkage present in conjugates hindered the release of 5‑ASA at pH 1.2 and 7.4, because degradation of azo bond required bacterial environment.
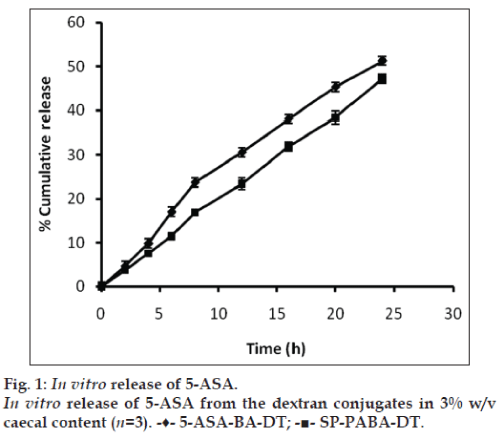
To check the colon specificity of azo polymeric conjugates of 5‑ASA, the release pattern in 3% w/v rat caecal content was observed for 24 h because the usual colonic transit time is 20‑30 h [25]. Percent amount of 5‑ASA regenerated from 5‑ASA‑BA‑DT and SA‑PABA‑DT dextran conjugates in 3% w/v rat caecal content was found to be 51.25±1.48 and 47.15±1.03, respectively (fig. 1). Regeneration of drug from dextran azo conjugates was depends on the azo linkage as well as ester bond formed between polymeric carrier and linker, cleavage of ester linkage between dextran macromolecule and PABA/BA linker was responsible for the release of 5‑ASA. The breakdown of dextran unit depends on the dextranases enzyme present in the colonic microflora. Dextranases are the enzymes which hydrolyze the glycosidic linkages between spacer and dextran and cleaved the dextran chain randomly [26]. The presence of azo reductase enzyme play pivotal role in the release of drug from azo bond of polymeric conjugate [27]. The release of 5‑ASA from dextran conjugates take place after cleavage of the azo bond by azo reductase enzyme present in the colonic microflora. The half‑life (t1/2) of the dextran conjugates 5‑ASA‑BA‑DT and SA‑PABA‑DT was found to be 25.93 and 33.78 h, respectively, in 3% w/v rat caecal content media.
Ulcerogenic studies were done by the cold stress model. Gastrointestinal mucosa of rats was observed for the presence of lesions using single high dose of 5‑ASA and conjugates. Incidence of gastric ulcers was noted with the 5‑ASA as 100% and lesion index found to be 35.67. Numbers of deep ulcers were observed after oral administration of 5‑ASA, which were spread throughout the stomach and duodenal mucosa. In case of dextran conjugates lesion index was found to be 7.92 and 6.08, respectively. Gastrointestinal toxicity of dextran conjugates of 5‑ASA was found very less as compared to parent drug and it showed less mucosal ulcer with edema and inflammation of the gastric mucosa. It might be due to the dextran conjugates protected the absorption and degradation of 5‑ASA in the upper GIT (Table 4).
| Compound | Normal dose (mg/kg)a | Number of lesion (mean±SE) | Average severity | % incidence/10 | Lesion index |
|---|---|---|---|---|---|
| Control | ‑ | 0.33±0.21 | 0 | 0.17±0.17 | 0.5 |
| 5‑ASA | 300 | 19.7±1.06* | 6.00±0.52 | 10±0.0 | 35.67 |
| SA-PABA | 300b | 14.8±0.40* | 5.3±0.33 | 10 | 30.2 |
| 5‑ASA‑BA‑DT | 300b | 4.67±0.50* | 1.83±0.17 | 1.42±0.33 | 7.92 |
| SA‑PABA‑DT | 300b | 3.67±0.33* | 1.50±0.22 | 0.92±0.33 | 6.08 |
aTen times higher from normal dose, bDose calculated equivalent to standard drug according to degree of substitution by hydrolysis method, n=6, *P>0.05 compared with normal control, SA-PABA-DT=Salicylic acid para-amino benzoic acid dextran, 5-ASA=5-Aminosalicylic acid, BA-DT=Benzoic acid-dextran
Table 4: Ulcerogenic effect of 5 asa and its dextran conjugates on wistar rats
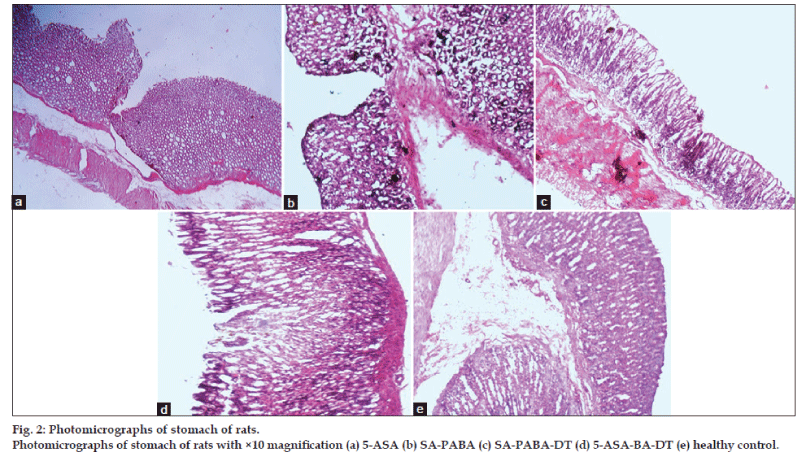
The ulcer involved the entire depth of the epithelial lining reaching to the muscularis mucosa (fig. 2a). The margins were raised and ill defined marked hemorrhagic stains were observed in the 5‑ASA derivative (SA_PABA) (fig. 2b). Dextran conjugate with PABA linker exhibited few scattered superficial ulcers. It showed superficial ulcers penetrating the epithelial lining with marked congestion (fig. 2c), while conjugates prepared using BA linker showed larger number of ulcers. The ulcers were small in diameter and superficial scattered throughout the mucosa of the stomach (fig. 2d). The gastric gland altered morphology seen in the 5‑ASA‑treated group compared to this polymer‑treated rats exhibited normal morphology. The morphology of control (fig. 2e) mucosa showed the normal mucosal layer, sub‑mucosa, and muscular layer. The capillaries, veins, and nerves distributed in the sub‑mucosa can be well visualized. Moreover, the gastric glands along with the mucus secreting cells are well organized within the epithelial layer of the mucosa.
The gross photographs of rat’s stomach cut open to expose the mucosa in order to show the severity of ulcer in drug as well as conjugate‑treated group, shown in fig. 2 treated group showed deep ulceration, swelling in gastric mucosal folds, and necrotic changes in with severe perforation in the gastric mucosa (fig. 2a). The control group exhibited normal gastric mucosa with intact epithelium (fig. 2e).
Acknowledgements
The financial assistance received from the University Grant Commission, New Delhi, India, in the form of senior research fellowship is gratefully acknowledged.
References
- Ouchi T, Ohya Y. Macromolecular prodrugs. ProgPolymSci 1995;20:211‑57.
- Larsen C, Harboe E, Johansen M, Olesen HP. Macromolecular prodrugs. XVI. Colon targeted delivery‑comparison of the rate of release of naproxen from dextran ester prodrugs in homogenates of various segments of the pig gastrointestinal tract. Pharm Res 1989;6:995‑9.
- Lee JS, Jung YJ, Doh MJ, Kim YM. Synthesis and properties of dextran‑nalidixic acid ester as a colon‑specific prodrug of nalidixic acid. Drug DevInd Pharm 2001;27:331‑6.
- Larsen C, Jensen BH. Bioavailability of ketoprofen from orally administered ketoprofen-dextran ester prodrugs in the pig. Acta Pharm Nord 1991;3:71‑6.
- Pugazhendhy S, Shrivastava PK, Sinha SK, Shrivastava SK. Lamaotrigine‑dextran conjugates: Synthesis, characterization and biological evaluation. Med Chem Res 2011;20:595‑600.
- Shrivastava PK, Praveen B, Shrivastava SK. In‑vitro Release and pharmacological study of synthesized valproic acid‑dextran conjugate. Acta Pharm Sci 2009;51:69‑176.
- Shrivastava PK, Shrivastava SK. Dextran carrier macromolecule for colon specific delivery of celecoxib. Curr Drug Deliv 2010;7:144‑51.
- Shrivastava PK, Singh R, Shrivastava SK. Polyamido amine dendrimer and dextran conjugate: Preparation, characterization and in vitro‑in vivo evaluation. Chem Paper 2010;64:592‑601.
- Ahmad S, Tester RF, Corbett A, Karkalas J. Dextran and 5‑aminosalicylic acid (5‑ASA) conjugates: Synthesis, characterization and enzymic hydrolysis. Carbohydr Res 2006;341:2694‑701.
- McLeod AD, Friend DR, Tozer TN. Synthesis and chemical stability of glucocorticoid-dextran esters: Potential prodrugs for colon‑specific delivery. Int J Pharm 1993;92:105‑14.
- Pang YN, Zhang Y, Zhang ZR. Synthesis of an enzyme‑dependent prodrug and evaluation of its potential for colon targeting. World J Gastroenterol 2006;8:913‑7.
- Sands BE. Therapy of inflammatory bowel disease. Gastroenterology 2000;118:S68‑82.
- Peppercorn MA. Sulfasalazine: Pharmacology, clinical use, toxicity and related new drug development. Ann Intern Med 1984;3:377‑86.
- Sutherland LR, Daniel E, Roth PL. Alternatives to sulfasalazine: A meta‑analysis of 5‑ASA in the treatment of ulcerative colitis. Inflamm Bowel Dis 2006;3:65‑78.
- Laursen L, Stokholm M, Bukhave K, Rask‑Madsen J, Lauritsen K. Disposition of 5‑aminosalicylic acid by olsalazine and three mesalazine preparations in patients with ulcerative colitis: Comparison of intraluminal colonic concentrations, serum values, and urinary excretion. Gut 1990;31:1271‑6.
- Mahmud N, Kamm MA, Dupas JL, Jewell DP, Moraine CA, Weira DG, et al. Olsalazine is not superior to placebo in maintaining remissionof inactive Crohn’s colitis and ileocolitis: A double blind, parallel, randomised, multicentre study. Gut 2001;49:552‑6.
- Rao SS, Read NW, Holdsworth CD. Influence of olsalazine on gastrointestinal transit in ulcerative colitis. Gut 1987;28:1474‑7.
- Available from: http://epaper.timesofindia.com/Default/Scripting/ ArticleWin.asp?From=ArchiveandSource=PageandSkin=TOINEWandBaseHref=TOIL/2012/03/10andPageLabel=1andEntityId=Ar00103andVie wMode=HTML [Last accessed on 2013 Mar 03].
- French DL, Mauger JW. Evaluation of the physicochemical properties and dissolution characteristics of mesalaminesrelevance to controlled intestinal drug‑delivery. Pharm Res 1993;10:1285‑90.
- Bourikas LA, Kolios G, Valatas V, Drygiannakis I, Notas G, Manousou P, et al. A differential effect of 5‑ASA and NSAIDs on colonic epithelial cell proliferation. Ann Gastroenterol 2009;22:97‑101.
- Vogel AI, Tatchell AR, Furnis BS, Hannaford AJ, Smith PW. Vogel’s Textbook of Practical Organic Chemistry. 5th ed. England: Longman Scientific and Technical; 1989. p. 399‑412.
- Mehvar R. Dextrans for targeted and sustained delivery of therapeutic and imaging agents. J Control Release 2000;69:1‑25.
- Rainsford KD. A synergistic interaction between aspirin, or other non‑steroidal antiinflammatory drugs, and stress which produces severe gastric mucosal damage in rats and pigs. Agents Actions 1975;5:553‑8.
- Khan Z, Zeljko P, Nevenka K. A pH‑dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation of drug release using Eudragit® L100‑55 and Eudragit® S100 combinations. J Control Release 1999;58:215‑22.
- Gang C, Feng A, Mei‑Juan Z, Jin S, Xiu‑Hua H, Yun‑Xia H. Time and pH‑dependent colon‑specific drug delivery for orally administered diclofenac sodium and 5‑amino salicylic acid. World J Gastroenterol 2004;10:1769‑74.
- Sinha VR, Kumria R. Colon drug delivery: Prodrug approach. Pharm Res 2001;53:41‑7.
- Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm PharmaceutSci 2003;6:33‑66.