- *Corresponding Author:
- R. K. Goyal
Executive Adviser (Research and Strategies), V ClinBio Labs, Sri Ramachandra Medical Center and Research Institute, SRU, Porur, Chennai-600 116, India
E-mail: goyalrk@gmail.com
| Date of Submission | 09 July 2014 |
| Date of Revision | 29 January 2015 |
| Date of Acceptance | 11 September 2015 |
| Indian J Pharm Sci 2015;77(5): 522-529 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Tephrosia purpurea has been reported to possess antidiabetic activity, however, its effects on cardiovascular complications and cataract associated with diabetes have not been studied. The objective of the present study was to investigate the effects of aqueous extract of Tephrosia purpurea on cardiovascular complications and cataract associated with streptozotocin-induced diabetes in rats. Sprague Dawley rats of either sex were made diabetic with streptozotocin (45 mg/kg, i.v.). Treatment of aqueous extract of Tephrosia purpurea was given in the dose of 300 and 500 mg/kg/day, p.o for 8 weeks. Various hemodynamic (blood pressure, heart rate, +dp/dt, -dp/dt) and biochemical (serum glucose, cholesterol, triglycerides, creatinine, urea, lactate dehydrogenase and creatinine kinase) parameters were recorded after 8 weeks of the treatment. To evaluate cataract, various biochemical estimations were done in eye lens. Streptozotocin produced hyperglycemia; hypoinsulinemia; hyperlipidemia; increased blood pressure; increased creatinine, cardiac enzymes, reduction in heart rate and cardiac hypertrophy in rats and all these changes were prevented by the treatment with aqueous extract of Tephrosia purpurea in both the doses. Streptozotocin also produced decrease in soluble protein and reduced glutathione in lens of rats that was prevented by aqueous extract of Tephrosia purpurea. Our data suggest that aqueous extract of Tephrosia purpurea prevents not only the streptozotocin-induced metabolic abnormalities but also cardiovascular complications as well as reduce the risk of development of cataract.
Keywords
Tephrosia purpurea, cardiovascular complications, cataract, antioxidant, rutin
Diabetes mellitus is a disease of metabolic dysregulation, most notably being abnormal glucose metabolism, accompanied by characteristic long-term complications such as cardiovascular and microvascular disorders. Moreover, it is one of the diseases for which a satisfactory treatment is not available in modern allopathic system of medicine. Thus, there is need to search for a drug which not only produces effective glycemic control but also prevent long-term complications of diabetes. Recently, the search for appropriate hypoglycemic agents has been focused on plants used in traditional medicine partly because of leads provided by traditional medicine which may prove to be better therapy than the currently used drugs [1]. There is tremendous interest worldwide for the use of herbs as an alternative system of medicine.
Tephrosia purpurea (Linn.) Pers. (Leguminosae) (T. purpurea), commonly known in Sanskrit as Sharapunkha is a highly branched, sub-erect, herbaceous perennial. According to Ayurveda, T. purpurea is used as digestible, anthelmintic, alexiteric, antipyretic, astringent, thermogenic, acrid and also used to cure diseases of liver, spleen, heart, blood, tumors, ulcers, leprosy and asthma [2]. Various parts of T. purpurea have been reported to produce antihyperglycemic activity in various animal models. The aqueous extract of seeds has shown a significant in vivo hypoglycemic activity in diabetic rabbits [3]. The aqueous and ethanol seed extracts have shown to produce antihyperglycemic activity in streptozotocin (STZ) diabetic rats [4,5]. T. purpurea leaf extract also produces antihyperglycemic activity [6]. Hypoglycemic activity of the root extracts has been reported in normal and alloxan diabetic rats [7]. However, effect of the plant on the long term diabetic complications has not been studied. The aim of this study was to investigate the effect of the aqueous extract of the whole plant on STZ-induced diabetes and associated cardiovascular complications and cataract.
Materials and Methods
Preparation and standardization of plant extract
The whole plant of T. purpurea was collected from the medicinal plant garden of Institute of Pharmacy, Nirma University, Ahmedabad, Gujarat, India and botanically authenticated. Voucher specimen (No. PL08SVBRKGtp001) was deposited in herbarium of the Institute of Pharmacy, Nirma University. It was shade-dried and powdered. The 50 g of coarse powder was suspended in 250 ml of water for 2 h and then heated at 60-65° for 30 min. The extract was collected and preserved. The process was repeated with the residual powder three times and the extracts thus collected were pooled and passed through fine cotton cloth. The total filtrate upon evaporation yielded 14% w/w extract. This was stored at 0-4° until used and was designated as AQTP (aqueous extract of Tephrosia purpurea). Each time fresh extract was prepared by dissolving the dried powdered extract in known volume of water.
Total phenolic content of the AQTP was determined through the method previously described by Singleton and Rossi [8]. Total phenolic content was calculated from the calibration curve of gallic acid standard solution. Results were expressed as % w/w of gallic acid equivalent in dry extract. Total flavonoid content was measured using a previously described method [9]. Quercetin (M.P. Biomedicals LLC, France.) was used as the standard for the calibration curve. Results were expressed as % w/w of quercetin equivalent in dry extract.
The aqueous extract was analyzed by thin layer chromatography for the presence of rutin using ethyl acetate:n-butanol:formic acid:water (5:3:1:1 v/v) as solvent system. The resulting chromatogram was scanned and quantified using CAMAG TLC scanner III at 254 nm.
Animals and treatment
The protocol of the experiment was approved by institutional animal ethics committee as per the guidance of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Social Justice and Empowerment, Government of India (Protocol no. IPS/PCOL/PHD08/001 dated 4th March, 2008).
Healthy Sprague Dawley rats of either sex weighing 200–250 g were made diabetic by single tail vein injection of STZ (45 mg/kg) dissolved in 0.1 M citrate buffer. Control rats were injected with 0.1 M citrate buffer alone. The induction of diabetes was checked 48 h after the STZ injection by measuring the extent of glycosuria with Diastix (Bayer Diagnostics, Ltd). The further confirmation for the status of diabetes was done by estimation of blood glucose and animals showing serum glucose above 300 mg/dl were considered as diabetic. The rats were then randomly divided into 6 groups having 6 rats each: CON–control, COT3-control rats treated with AQTP (300 mg/kg/day, p.o); COT5–control rats treated with AQTP (500 mg/kg/day, p.o); DIA-diabetic control rats; DIT3-diabetic rats treated with AQTP (300 mg/kg/day, p.o); DIT5-diabetic rats treated with AQTP (500 mg/kg/day, p.o). The animals were provided with the standard lab chow diet (Pranav Agro Industries Ltd, Vadodara, Gujarat, India) and purified water ad libitum.
Blood sample collection and serum analysis
At the end of 8 weeks of treatment, body weight, food intake and water intake were estimated. Thereafter, animals were fasted for 12 h and blood samples were collected from the retro-orbital plexuses of each rat under light ether anesthesia. The serum was separated and analyzed for glucose, cholesterol, triglycerides, creatinine, urea, lactate dehydrogenase (LDH), and creatinine kinase (CK) spectrophotometrically (Shimadzu, Japan) using biochemical diagnostic kits (Accucare Diagnostics, Ltd, India). Serum insulin was estimated by radioimmunoassay using kits obtained from Board of Radiation and Isotope Technology, Mumbai, India, and gamma counter (Packard, Minnesota, USA).
Measurement of cardiovascular parameters
At the end of 8 weeks, blood pressure, heart rate, rate of pressure development (dp/dtmax) and decay (dp/dtmin) were recorded by carotid artery cannulation. Briefly, the animals were anaesthetized by ketamine (100 mg/kg, i.p.)+xylazine (7 mg/kg, i.m.). The body temperature was maintained at 37±1° during the experiment. The carotid artery behind the trachea was exposed and cannulated for the measurement of hemodynamic parameters using a transducer (BP 100) and Labscribe Systems (I-worx, USA). The hemodynamic parameters observed were mean arterial blood pressure (MAP), dp/dtmax and dp/dtmin. All the data were analyzed using Labscribe software (Version 2.0). After withdrawal of blood samples from retro-orbital plexus and recording of hemodynamic parameters, animals were sacrificed, hearts were excised, extraneous tissues were separated and wet weight of the entire heart and left ventricle (LV) was noted down to calculate the index of cardiac hypertrophy (wet heart weight to femur length ratio) and LV hypertrophy index (LV weight to heart weight ratio).
Histological study
For histological study, hearts were isolated and fixed in Bouin’s fixative and dehydrated with different grades of alcohol. The blocks were then prepared in paraffin wax and sectioned with a microtome. The sections were stained with hematoxylin and eosin and the slides were examined by light microscope. Several sections were taken and sections of uniform size and thickness were selected for microscopic examination.
Evaluation of oxidative stress in lens
At the end of 8 weeks, after the measurement of cardiovascular parameters, the animals were sacrificed and the lenses were dissected out by posterior approach. The homogenate was prepared in 50 mM phosphate buffer. The oxidative stress inflicted to the lens was assessed by measurement of reduced glutathione (GSH) [10], superoxide dismustase [11] and the level of lipid peroxidation [12]. The soluble proteins were assessed in the lens homogenate by the method described by Lowry et al [13].
Statistical analysis
Results are presented as mean±standard error of the mean (SEM). Statistical differences between the means of the various groups were evaluated using one-way analysis of variance (ANOVA) followed by Tukey’s test. Data were considered statistically significant at a P-value <0.05.
Results
Phytochemical studies
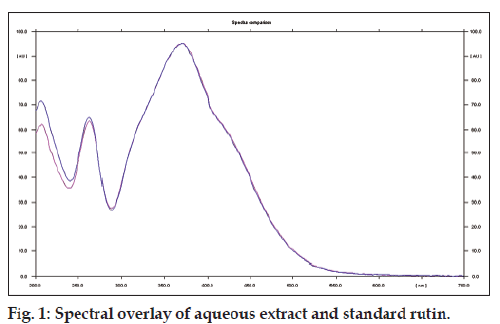
The preliminary studies showed the presence of flavonoids and phenolics. The level of total phenolic content and flavonoids in the aqueous extract was found to be 10.83 and 10.09 w/w, respectively. On performing HPTLC analysis of the AQTP, rutin (3.14 w/w) was detected as major flavonoid (fig. 1).
Pharmacological studies
Injection of STZ (45 mg/kg) into rats produced glycosuria and blood glucose levels were found to be more than 300 mg/dl in all the animals. No glucose was detectable in the urine of control animals. Diabetic rats showed a loss of body weight, polyphagia, and polydipsia. Chronic treatment with AQTP (300 and 500 mg/kg/day) failed to prevent loss of body weight and there was no significant effect on the food intake at the dose of 500 mg/kg/day while a minor significant effect was observed at a dose of 300 mg/kg/day. A significant effect was observed on water intakes of treated rats with AQTP (300 and 500 mg/kg/day, Table 1).
| Parameter | CON | COT3 | COT5 | DIA | DIT3 | DIT5 |
|---|---|---|---|---|---|---|
| Change in body weight after treatment (g) | 8.41 ± 1.91 | 12.91 ± 2.51 | 10.57 ± 2.55 | −18.05 ± 6.08* | −14.13 ± 5.23 | −19.98 ± 6.96 |
| Food intake (g/animal/day) | 22.50 ± 0.94 | 22.50 ± 1.1.2 | 20.84 ± 0.37 | 37.19 ± 0.84* | 27.50 ± 1.42 | 35.63 ± 3.20 |
| Water intake (ml/animal/day) | 32.50 ± 0.94 | 27.50 ± 2.61 | 35.00 ± 0.75 | 158.75 ± 3.31* | 143.75 ± 2.36 | 123.75 ± 3.63 |
Table 1: Effect of chronic treatment of aqueous extract of T. purpurea on various general parameters
Biochemical parameters
STZ-diabetic rats were found to exhibit significant hyperglycemia and hypoinsulinemia as compared to control rats. Treatment with AQTP (300 and 500 mg/kg/day) produced significant decrease in elevated serum glucose levels and significant increase in insulin levels (Table 2).
| Parameter | CON | COT3 | COT5 | DIA | DIT3 | DIT5 |
|---|---|---|---|---|---|---|
| Serum glucose (mg/dl) | 89.54 ± 2.54 | 114.54 ± 15.41 | 115.19 ± 16.15 | 532.07 ± 27.95* | 376.61 ± 34.59# | 362.94 ± 33.22# |
| Serum insulin (µU/ml) | 43 ± 2.43 | 38.6 ± 1.99 | 38.33 ± 3.27 | 19.0 ± 0.89* | 30.50 ± 1.52# | 28.5 ± 2.18# |
| Serum creatinine (mg/dl) | 0.8 ± 0.075 | 0.83 ± 0.072 | 0.73 ± 0.084 | 2.98 ± 0.303* | 1.66 ± 0.162# | 2.01 ± 0.054# |
| Serum urea (mg/dl) | 33.71 ± 1.39 | 31.41 ± 3.91 | 32.03 ± 3.23 | 98.83 ± 17.44* | 77.6 ± 10.66# | 72.5 ± 9.32# |
Table 2: Effect of chronic treatment of aqueous extract of T. purpurea on biochemical Parameters
There was significant increase in total cholesterol, very low-density lipoprotein (VLDL), low-density lipoprotein (LDL)-cholesterol and triglyceride levels and significant decrease in high-density lipoprotein (HDL)-cholesterol levels in STZ-diabetic rats as compared to control rats. Treatment with AQTP (300 and 500 mg/kg/day) significantly reduced the elevated total cholesterol, VLDL, LDL-cholesterol and triglyceride levels in diabetic rats and increased the serum HDL-cholesterol levels (Table 3).
| Parameter | CON | COT3 | COT5 | DIA | DIT3 | DIT5 |
|---|---|---|---|---|---|---|
| Total cholesterol (mg/dl) | 71.16 ± 4.12 | 75.2 ± 3.356 | 70.64 ± 4.311 | 99.84 ± 4.021* | 75.74 ± 6.243# | 72.5 ± 5.147# |
| LDL-cholesterol (mg/dl) | 27.88 ± 1.98 | 34.39 ± 2.96 | 27.72 ± 2.49 | 50.40 ± 4.41* | 40.28 ± 3.32# | 35.72 ± 2.3# |
| VLDL-cholesterol (mg/dl) | 21.47 ± 2.67 | 21.65 ± 3.84 | 20.79 ± 3.96 | 40.62 ± 6.63* | 20.60 ± 3.61# | 19.44 ± 5.04# |
| HDL-cholesterol (mg/dl) | 21.81 ± 2.73 | 19.16 ± 1.11 | 22.13 ± 280 | 8.82 ± 0.59* | 14.86 ± 0.96# | 17.33 ± 1.93# |
| Triglyceride (mg/dl) | 107.36 ± 8.5 | 108.23 ± 5.8 | 103.96 ± 6.9 | 203.12 ± 16.1* | 102.98 ± 10.25# | 97.22 ± 6.6# |
Table 3: Effect of chronic treatment of aqueous extract of T. purpurea on lipid profile
STZ also produced significant increase in serum creatinine and urea levels as compared to control rats. Chronic treatment with AQTP (300 and 500 mg/kg/day) significantly reduced the elevated creatinine and urea levels of diabetic rats (Table 2).
Cardiac parameters
STZ produced a significant increase in serum LDH and CK levels as compared to control rats. Chronic treatment with AQTP (300 and 500 mg/kg/day) significantly reduced the elevated serum LDH levels. However, CK levels of diabetic rats were found to be decreased by the chronic treatment with AQTP (500 mg/kg/day) whereas the dose of 300 mg/kg/day was not able to show any effect (Table 4).
| Parameter | CON | COT3 | COT5 | DIA | DIT3 | DIT5 |
|---|---|---|---|---|---|---|
| Creatine kinase (U/L) | 19.92 ± 1.64 | 18.84 ± 2.33 | 22.51 ± 1.36 | 60.21 ± 5.76* | 57.67 ± 6.62 | 37.14 ± 9.04# |
| LDH (U/L) | 89.54 ± 2.54 | 114.54 ± 15.41 | 115.19 ± 16.15 | 532.07 ± 27.95* | 376.61 ± 34.59# | 362.94 ± 33.22# |
| Cardiac hypertrophy (mg/cm) | 288.83 ± 20.91 | 304.04 ± 27.35 | 312.19 ± 23.19 | 382.88 ± 26.17* | 246.14 ± 5.33# | 245.48 ± 11.85# |
| Left ventricular hypertrophy (mg/mg) | 0.53 ± 0.035 | 0.56 ± 0.026 | 0.59 ± 0.034 | 0.81 ± 0.055* | 0.63 ± 0.009# | 0.62 ± 0.036# |
| Blood pressure (mmHg) | 174.15 ± 3.90 | 163.36 ± 9.02 | 166.75 ± 4.85 | 248.68 ± 10.70* | 257.51 ± 1.61 | 248.45 ± 2.74 |
| Heart rate (bpm) | 342.17 ± 8.53 | 350.83 ± 12.79 | 364.5 ± 4.09 | 198.75 ± 25.00* | 283.17 ± 16.55# | 275 ± 9.40# |
Table 4: Effect of chronic treatment of aqueous extract of T. purpurea on cardiac and Hemodynamic parameters
There was significant increase in LV weight to heart weight ratio and wet heart weight to femur length ratio in diabetic control animals as compared to nondiabetic control animals. Chronic treatment with AQTP (300 and 500 mg/kg/day) significantly reduced the elevated LV hypertrophy index as well as cardiac hypertrophy index of diabetic rats (Table 4).
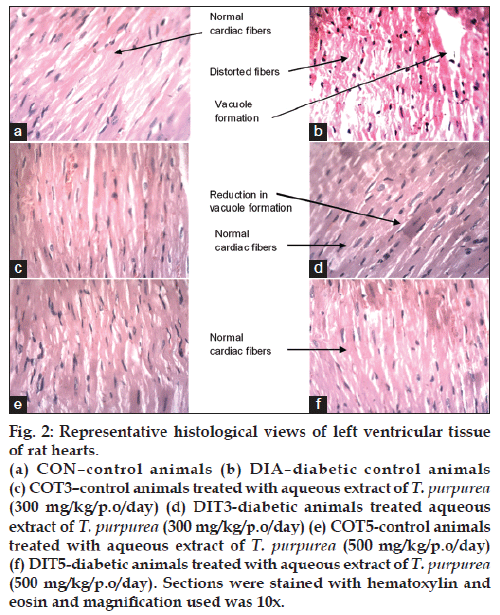
Histological examination of left ventricular tissues from diabetic animals showed degenerative changes in cardiac muscle fibers such as disarrayed fibers and vacuole formation. Treatment with AQTP at both the doses normalized the damage to the cardiac fibers showing less vacuole formation (fig. 2).
Figure 2: Representative histological views of left ventricular tissue
of rat hearts.
(a) CON–control animals (b) DIA–diabetic control animals
(c) COT3–control animals treated with aqueous extract of T. purpurea (300 mg/kg/p.o/day) (d) DIT3-diabetic animals treated aqueous
extract of T. purpurea (300 mg/kg/p.o/day) (e) COT5-control animals
treated with aqueous extract of T. purpurea (500 mg/kg/p.o/day)
(f) DIT5-diabetic animals treated with aqueous extract of T. purpurea (500 mg/kg/p.o/day). Sections were stained with hematoxylin and
eosin and magnification used was 10x.
Hemodynamic parameters
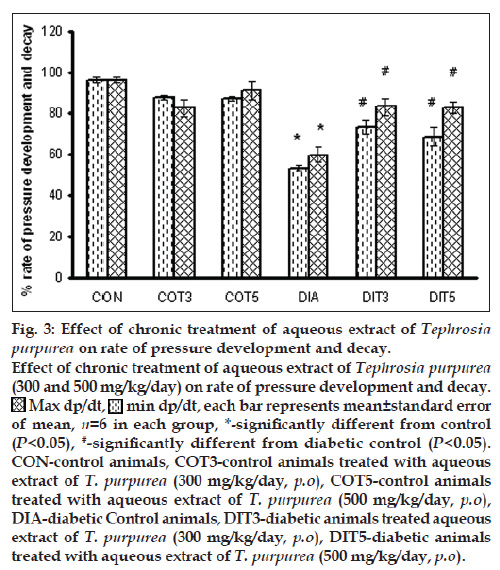
Blood pressure was found to be significantly elevated in the diabetic control rats, which was not affected by the treatment with the AQTP (300 and 500 mg/kg/day). Heart rate was found to be significantly lower in diabetic rats as compared to controls. Chronic treatment with AQTP (300 and 500 mg/kg/day) in diabetic rats exhibited significant increase in heart rate as compared to diabetic control animals (Table 4). The rates of pressure development (dp/dtmax) as well as decay (dp/dtmin) were severely reduced in the diabetic control rats. Chronic treatment with AQTP (300 and 500 mg/kg/day) showed a significant improvement in them (fig. 3).
Cataract parameters in lens
No visual cataract was seen in the lens of diabetic animals. However, there was significant decrease in soluble protein and reduced glutathione in lens of diabetic animals as compared to controls. Treatment with AQTP (300 and 500 mg/kg/day) significantly increased soluble protein and reduced glutathione in lens of diabetic rats (Table 5). Lipid peroxidation was found to be significantly elevated in the diabetic control rats. Chronic treatment with AQTP (300 and 500 mg/kg/day) showed a significant reduction in the lipid peroxidation levels (Table 5).
| Parameter | CON | COT3 | COT5 | DIA | DIT3 | DIT5 |
|---|---|---|---|---|---|---|
| Soluble protein (µg/mg wet tissue) | 65.30 ± 4.93 | 58.27 ± 3.94 | 59.43 ± 4.42 | 29.65 ± 2.22* | 67.25 ± 6.62# | 54.53 ± 1.86# |
| Glutathione (µg/mg protein) | 21.07 ± 2.40 | 20.96 ± 1.80 | 23.51 ± 1.30 | 9.89 ± 0.95* | 21.78 ± 1.33# | 21.03 ± 3.84# |
| Superoxide dismutase (U/mg protein) | 4.11 ± 0.05 | 4.03 ± 0.04 | 3.98 ± 0.05 | 2.53 ± 0.01* | 2.98 ± 0.04# | 2.77 ± 0.02# |
| Lipid peroxidation (nmol MDA/mg protein) | 1.44 ± 0.17 | 1.47 ± 0.38 | 1.14 ± 0.20 | 4.74 ± 0.62 | 2.36 ± 0.48 | 2.75 ± 0.26 |
Table 5: Effect of chronic treatment of aqueous extract of T. purpurea on cataract Parameters in lens
Discussion
In the present study, STZ produced cardinal signs and characteristics of diabetes, i.e., polyphagia and polydipsia, which are consistent with those reported earlier [14]. Chronic treatment with AQTP (300 and 500 mg/kg/day) did not prevent the loss of body weight, polyphagia, or polydipsia in STZ-diabetic rats.
Treatment with AQTP (300 and 500 mg/kg/day) significantly reduced the serum glucose levels and produced elevation in the serum insulin levels of STZ-diabetic rats. Phytochemical investigations of T. purpurea have revealed the presence of glycosides, rotenoids, isoflavones, flavanones, chalcones, flavanols, flavones and sterols [15]. Due to the rich flavonoid content, these components may be suggested to be the main antidiabetic principles of the plant. The flavonoids are group of compounds possessing potential antioxidant properties that may protect pancreatic islets and help in regeneration of β-cells which in turn may lead to increase in insulin levels. In addition, flavonoids exert their effect either by promoting the entry of glucose into cells, thus stimulating glycolytic enzymes and glycogenic enzymes [16] or by inhibiting the glucose-6-phosphatase in the liver, consequently reducing the release of glucose in the blood [17]. Rutin is a flavonol type of flavonoid present in about 3.14% in AQTP. Increased insulin levels could be due to the stimulatory effect of rutin thereby potentiating the existing β-cells of the islets of langerhans in diabetic rats [18]. Moreover, the aglycone of rutin i.e. quercetin is reported to bring about the regeneration of the pancreatic islets and probably increase the insulin levels in STZ-induced diabetic rats [19].
Insulin deficiency is also reported to be associated with hypercholesterolemia and hypertriglyceridemia [14]. Abnormal lipid levels lead to the development of coronary artery disease in diabetic patients. In the present investigation, the rise in serum triglycerides, cholesterol and LDL and VLDL-cholesterol levels indicate derangement of lipid metabolism and increased incidence of cardiac dysfunction in diabetic rats. Elevation of serum lipids indicates either the defective removal or overproduction (or both) of one or more lipoproteins [20]. Pavana et al. [6] have reported that AQTP have antihyperlipidemic effect in STZ diabetic rats which may be due to stimulation of activities of lipid metabolizing enzymes such as LCAT and LPL. Flavonoids are reported to be the major constituents of T. purpurea that are shown to decrease blood levels of triglycerides and total cholesterol. Flavonoids may suppress LDL oxidation and inflammatory progression in the artery wall. Moreover, rutin is a potent inhibitor of HMG-CoA reductase, an enzyme responsible for cholesterol synthesis, and also beneficial for lowering serum cholesterol levels [21]. This may suggest the mechanism of cholesterol lowering effect of the extracts. Besides this, the extract was also able to significantly augment serum HDL cholesterol in rats with STZ-induced diabetes. This finding is advantageous since HDLcholesterol is responsible for the transportation of cholesterol from peripheral tissues to the liver for metabolization. Further, rutin has been reported to prevent HDL-C from oxidative modification in vitro [22].
There was a significant elevation in serum creatinine and urea levels indicating impaired renal function in diabetic animals. Treatment with AQTP at both doses produced significant decrease in elevated serum creatinine and urea levels in diabetic animals. The ethanol extract of T. purpurea leaves has also shown marked nephroprotective and curative effect against gentamicin-induced acute renal injury in albino rats [23]. Also, hydroalcoholic extract of T. purpurea has shown protective effect in animals from arsenic-induced nephrotoxicity [24]. They have proposed antioxidant activity and inhibition of overproduction of NO and Cox-2 expression as the mechanisms involved in nephroprotection. These activities may be attributed to phenolic and flavonoidal compounds suggesting that T. purpurea extract may be beneficial in providing protection against diabetic nephropathy. However, further studies are required to prove the efficacy of the T. purpurea extract in diabetic nephropathy.
Within 12–24 h after a myocardial infarction, LDH and CK levels are increased which indicate cardiac muscular damage. In our study also, we found significant rise in LDH and CK levels in STZ-diabetic rats as compared to control rats. Treatment with AQTP normalized the LDH activity in the diabetic rats. CK levels were however reduced only with the treatment at the dose of 500 mg/kg/day. Normal LDH activity is indicative of improved channeling of (pyruvate) glucose by mitochondrial oxidation. Rutin has been reported to normalize the LDH and CK levels in isoproterenol-induced myocardial infarction in rats, isoproterenol-induced cardio toxic rats and in doxorubicin-induced cardiotoxicity [25] indicating its cardioprotective effect which may be due to antioxidant property, free radical scavenging or membrane stabilizing properties. Lowering the CK levels with the treatment may help prevent the progression of diabetic cardiomyopathy [26].
Diabetic cardiomyopathy is characterized by cardiac hypertrophy and diastolic and/or systolic contractile dysfunction. Treatment with the AQTP prevented significantly both the cardiac hypertrophy as well as LV hypertrophy. These results are further substantiated by histopathological findings. Diabetic animals show decrease in dp/dtmax, dp/dtmin and bradycardia even in the absence of atherosclerosis or frank abnormalities of the microcirculation [27]. Our results are consistent with these reports. Chronic treatment with AQTP prevented STZ-induced bradycardia in the diabetic animals and also produced an improvement in cardiac hemodynamic parameters such as dp/dtmax and dp/dtmin in the diabetic rats but the hypertension was not improved. Studies have reported that the sorbitol pathway is significantly activated in the hearts of the STZ-induced diabetic rats, with marked cardiac accumulation of fructose. Increased fructose levels cause the non-enzymatic glycation of various intracellular components in the diabetic myocardium, resulting in formation of advanced glycation end-products. These advanced glycation end-products impair the intracellular Ca2+ homeostasis which thereby affects the cardiac contractility [28]. Flavonoids are reported to be effective aldose reductase inhibitors [29]. Also, T. purpurea has shown potent antioxidant activity [30]. Dodda and Ciddi [31] suggest that plants with both the AR inhibiting and antioxidant activity may be more effective in diabetic complications. Thus, it is possible that the flavonoids present in T. purpurea extract may inhibit the sorbitol pathway which alongwith the antioxidant activity of the plant can provide cardioprotection. Rutin, a flavonoid in T. purpurea is proven to be effective in providing cardioprotection by improving left ventricular dysfunction in STZ-diabetic rats.
In cardiomyocytes, reactive oxygen species have been found to mediate cardiac hypertrophy induced by several stimuli, such as mechanical stretch, angiotensin II, and tumor necrosis factor-α (TNF-α). T. purpurea has been known to have antioxidant activity [30]. It is thus possible that the antioxidant potential of T. purpurea may be one of the mechanisms in the prevention of cardiac hypertrophy in T. purpurea treated diabetic rats.
The role of increased oxidative stress is widely accepted in the development and progression of diabetes and its complications. Reports indicate that diabetic complications are associated with overproduction of free radicals and accumulation of lipid peroxidation by-products. Many nonenzymatic antioxidants like glutathione and enzymatic antioxidants like superoxide dismutase are also involved in the protection of free radical induced oxidative damage. Oxygen free radicals are formed disproportionately in diabetes by glucose oxidation, nonenzymatic glycation of proteins and the subsequent oxidative degradation of glycated proteins. Increased glycation also causes generation of oxygen free radicals by increasing lipid peroxidation and decrease in superoxide dismutase as well as reduced glutathione. All these changes bring an increased level of insoluble proteins. Treatment with AQTP significantly increased the level of soluble proteins. The possible mechanism of this activity may be the prevention of deposition of sorbitol in lens by inhibition of enzyme aldose reductase, or prevention of formation of advanced glycosylation end products. Treatment with AQTP was also found to decrease oxidative stress by decreasing lipid peroxidation and increasing the levels of reduced glutathione and superoxide dismutase in diabetic lens. It can also be hypothesized that the antioxidant property may be because of the presence of quercetin or rutin in T. purpurea extract.
Our data suggest that aqueous extract of T. purpurea possesses potential antidiabetic activity at both doses. Further, T. purpurea extract may be beneficial in either preventing or delaying the cardiac complications, nephropathy as well as cataract development associated with diabetes mellitus. Further studies are required to prove its efficacy and the mechanism of action.
Financial support and sponsorship
The authors would like to acknowledge Nirma University, Ahmedabad for its financial support in completion of this work.
Conflicts of interest
There are no conflicts of interest.
References
- Rates SM. Plants as source of drugs. Toxicon 2001;39:603-13.
- Warrier PK, Nambiar VP, Ramankutty C. Indian Medicinal Plants: A Compendium of 500 Species. Vol. 5. Hyderabad: Orient Longman Private Ltd.; 2004.
- Rahman H, Kashifudduja M, Syed M, Saleemuddin M. Hypoglycemic activity of Tephrosi apurpurea seeds. Indian J Med Res 1985;81:418-21.
- Pavana P, Sethupathy S, Manoharan S. Antihyperglycemic and antilipidperoxidative effects of Tephrosi apurpurea seed extract in streptozotocin induced diabetic rats. Indian J Clin Biochem 2007;22:77-83.
- Pavana P, Sethupathy S, Santha K, Manoharan S. Effects of Tephrosi apurpurea aqueous seed extract on blood glucose and antioxidantenzyme activities in streptozotocin induced diabetic rats. Afr J Tradit Complement Altern Med 2008;6:78-86.
- Pavana P, Manoharan S, Renju GL, Sethupathy S. Antihyperglycemic and antihyperlipidemic effects of Tephrosi apurpurea leaf extract in streptozotocin induced diabetic rats. J Environ Biol 2007;28:833-7.
- Joshi NC, Murugananthan G, Thabah P, Nandakumar K. Hypoglycemic and antidiabetic activity of Tephrosi apurpurea (linn) root extracts. Pharmacologyonline 2008;3:926-33.
- Singleton VL, Rossi JA Jr. Colorimetry of total phenolics with phosphomolybdic – Phosphotungstic acid reagents. Am J Enol Vitic 1965;16:144-58.
- Zhishen J, Mengcheng T, Jianming W. Research on antioxidant activity of flavonoids from natural materials. Food Chem 1999;64:555-9.
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882-8.
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247:3170-5.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265-75.
- Bhadada SV, Goyal RK. Comparative evaluation of atenolol and metoprolol on cardiovascular complications associated with streptozotocin-induced diabetic rats. Can J Physiol Pharmacol 2007;85:831-6.
- Pelter A, Ward RS, Rao EV, Raju NR. 8-substituted flavonoids and 3′-substituted 7-oxygenated chalcones from Tephrosi apurpurea. J ChemSoc Perkin Trans 1 1981;1:2491-8.
- Sarkhail P, Rahmanipour S, Fadyevatan S, Mohammadirad A, Dehghan G, Amin G, et al. Antidiabetic effect of Phlomis anisodonta: Effects on hepatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Pharmacol Res 2007;56:261-6.
- Naik SR, Barbosa Filho JM, Dhuley JN, Deshmukh V. Probable mechanism of hypoglycemic activity of bassic acid, a natural product isolated from Bumeliasartorum. J Ethnopharmacol 1991;33:37-44.
- Kamalakkannan N, Prince PS. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin Pharmacol Toxicol 2006;98:97-103.
- Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol 2003;135C:357-64.
- Akula A, Kota MK, Gopisetty SG, Chitrapu RV, Kalagara M, Kalagara S, et al.Biochemical, histological and echocardiographic changes during experimental cardiomyopathy in STZ-induced diabetic rats. Pharmacol Res 2003;48:429-35.
- Bok SH, Lee SH, Park YB, Bae KH, Son KH, Jeong TS, et al. Plasmaand hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: Cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J Nutr 1999;129:1182-5.
- Li QS, Lou GY, Qian MZ. Effect of hesperidin and rutin on oxidative modification of high density lipoprotein in vitro. Zhong Xi Yi Jie He Xue Bao 2004;2:115-6, 119.
- Jain A, Nahata A, Singhai AK. Effect of Tephrosi apurpurea (L.) pers. Leaves on gentamicin-induced nephrotoxicity in rats. Sci Pharm 2013;81:1071-87.
- Gora RH, Kerketta P, Baxla SL, Toppo R, Prasad R, Patra PH, et al.Ameliorative effect of Tephrosiapsurpurea in arsenic-induced nephrotoxicity in rats. Toxicol Int 2014;21:78-83.
- Hozayen WG, AbouSeif HS. Protective effects of rutin and hesperidin against doxorubicin-induced lipodystrophy and cardiotoxicity in albino rats. J Am Sci 2011;7:765-75.
- Matsumoto Y, Kaneko M, Kobayashi A, Fujise Y, Yamazaki N. Creatine kinase kinetics in diabetic cardiomyopathy. Am J Physiol 1995;268(6 Pt 1):E1070-6.
- Malone MA, Schocken DD, Hanna SK, Liang X, Malone JI. Diabetes-induced bradycardia is an intrinsic metabolic defect reversed by carnitine. Metabolism 2007;56:1118-23.
- Petrova R, Yamamoto Y, Muraki K, Yonekura H, Sakurai S, Watanabe T, et al. Advanced glycationend product-induced calcium handling impairment in mouse cardiac myocytes. J Mol Cell Cardiol 2002;34:1425-31.
- Varma SD, Mikuni I, Kinoshita JH. Flavonoids as inhibitors of lens aldose reductase. Science 1975;188:1215-6.
- Patel A, Patel A, Patel A, Patel NM. Determination of polyphenols and free radical scavenging activity of Tephrosi apurpurea linn leaves (Leguminosae). Pharmacognosy Res 2010;2:152-8.
- Dodda D, Ciddi V. Plants used in the management of diabetic complications. Indian J Pharm Sci 2014;76:97-106