- Corresponding Author:
- S. Sathaye
Department of Pharmaceutical Sciences and Technology, Institute of Chemical Technology, Nathalal Parikh Marg, Matunga, Mumbai-400 019, India
E-mail: sadhanasathaye@hotmail.com
| Date of Submission | 30 January 2013 |
| Date of Revision | 03 May 2013 |
| Date of Acceptance | 05 May 2013 |
| Indian J Pharm Sci 2013;75(3):380-384 |
Abstract
In the present study, anticonvulsant activity of methanol extract of Eclipta alba (10-200 mg/kg) was studied using pentylenetetrazole- and picrotoxin-induced seizure models. Mechanism of effect of methanol extract of Eclipta alba was further elucidated by studying its GABA A receptor modulatory activity and its effect on levels of GABA in mice brain. Methanol extract of Eclipta alba exhibited potent anticonvulsant activity but has saturation of its pharmacological activity at 50 mg/kg. At the concentration of 10 mg/ml, contractions induced in guinea pig ileum was blocked by picrotoxin, but it didn't not show any increase in GABA levels in mice brain after treatment. Hence, it can be concluded that methanol extract of Eclipta alba possesses potent anticonvulsant activity because of its positive modulatory effect on GABA A receptors.
Keywords
Epilepsy, pentylenetetrazole, picrotoxin, GABA, wedelolactone, luteolin
Epilepsy is the third most common neurological disorder after stroke and Alzheimer’s disease with most of the newly identified cases occurring among children and adults [1]. Globally, there are nearly 50 million people suffering from epilepsy, 80% of which are in the developing countries and 90% of these do not receive appropriate treatment. India alone has approximately 8-10 million epileptics. Epilepsy affects not only the individual, but also has consequences for the family and the rest of society [2,3].
Traditional pharmacological strategies for the treatment of epilepsy using drugs like phenytoin, carbamazepine, valproate and phenobarbital have been associated side-effects like cognitive impairment, idiosyncratic reaction, weight gain, teratogenicity, and severe drug interactions. In spite of the newer antiepileptic drugs with better tolerability profiles, low interaction potential and significantly less enzyme inducing or inhibiting properties, 30-35% patient still continue to have uncontrolled seizures [4]. Furthermore, there is increasing evidence that despite early treatment and suppression of seizures, anticonvulsant drugs do not affect the progression or underlying natural history of epilepsy [5]. In order to achieve improved therapy for epilepsy, the real challenge in future is to create novel broad-acting anticonvulsant drugs with multiple mechanisms of action with decreased adverse effects in comparison to currently used medical therapies.
Eclipta alba Hassk (Asteraceae) has been used in traditional system of medicine and also by traditional healers, especially in Southern region of India for the treatment of epilepsy since ancient times. The pounded leaves of Eclipta alba with garlic juice and peppers are administered orally to treat epilepsy by the traditional healers [6]. Published data from our lab showed anticonvulsant potential of methanol extract of Eclipta alba (MEEA) in the electroshock model [7]. In continuation of the same work, we had tried to explore the mechanism of action of MEEA.
Eclipta alba was collected from Palghar area, near Mumbai, during rainy season of 2009. The whole plant and leaves of Eclipta alba were authenticated from the botonist of Khalsa College, King’s Circle, Mumbai (Voucher specimen no. DBKC-54). The dried and powered leaves were defatted using petroleum ether and then extracted with 95% methanol in a Soxhlet extractor at 60-80º for 24 h. The extract was further concentrated in vacuo at 50º to get the thick paste and was denoted as methanol extract of Eclipta alba (MEEA).
Pentylenetetrazol (PTZ) and picrotoxin (PIC) were procured from Sigma-Aldrich, St. Louis, MO, USA, and their solutions were prepared using fresh sterile saline (0.9% w/v). Solutions of MEEA were prepared using distilled water and were administered per orally (p.o.), whereas PTZ and PCT solutions were administered intraperitoneally (i.p.).
Adult Swiss albino mice (20-25 g) were procured from Haffkins Institute, Mumbai, India and were acclimatized in the animal house of the Institute of Chemical Technology (ICT), Matunga, Mumbai. Mice were grouped, housed, and maintained at 23±2º under 12:12 h light/dark cycle with free access to rodent chow and tap water. The animal study was approved by the Institutional Animal Ethics Committee (UICT/ PH/IAEC/0213/07 and UICT/PH/IAEC/0213/08), ICT, Mumbai.
For PTZ-induced seizure model, mice were divided into 7 groups containing 6 animals each and treated once a day for 7 days. First group was vehicle control and were given saline, next five groups were test groups and received 10, 25, 50, 100, and 200 mg/kg of MEEA, respectively, and last group was positive control and received diazepam (2 mg/kg). One hour after the last dose on the seventh day, animals were given PTZ (100 mg/kg). Animals were observed for a period of 30 min post-PTZ administration. The parameter noted were onset of seizure and % protection from death [8]. A similar procedure was followed for the PIC-induced epileptic model.
GABAA receptor assays were conducted as prescribed by Arulmozhia et al [9]. Briefly, guinea pigs were euthanized (thiopental sodium 100 mg) followed by exsanguination, whole segments of terminal ileum (2 cm) was rapidly excised and placed in tyrode solution with the following composition: NaCl 137 mM; KCl 2.7 mM; CaCl2 1.8 mM; MgCl21.0 mM; NaHCO3 11.9 mM; NaH2PO4 0.4 mM; dextrose 5.55 mM. The preparations were suspended in 10 ml organ baths containing tyrode solution (pH 7.4) aerated with carbogen.
The tissues were maintained at a resting tension of 1 g for 45-60 min for equilibration with intervening washings prior to the recording of isotonic contractile responses. A response of KCl (25 mM) was obtained to check the integrity of the tissue. Picrotoxin is used as an antagonist for the GABAA receptor as this can give a clear idea about the involvement of GABAA receptor in the activity. The response of GABA (1 µM) alone was compared with the response of GABA (1 µM) in presence of PIC (1 µM), where picrotoxin was administered 10 min before GABA. Similarly, responses of MEEA (10 mg/ml) were obtained in the presence and absence of picrotoxin (1 µM). In order to confirm the blocking effect and to validate the results, same procedure was repeated thrice [10].
Estimation of GABA was carried out on normal animals treated with 200 mg/kg for 7 days (highest dose given to the animals in PTZ and PIC model). It was studied by TLC with the original method developed by Sadasivudu and Murthy [10] and was adapted with some modifications as described by Shankaranarayana Rao et al. [11]. Whole brain was homogenized in 80% double-distilled ethanol (for 100 mg of the brain tissue, 2 ml of 80% alcohol was used). Homogenates were transferred to polypropylene tubes and centrifuged at 1200 rpm for 10 min. The supernatant (1 ml) was then transferred into small test tubes and evaporated to dryness at 70º in an oven. The residue was reconstituted in 100 µl distilled water, and 2 µl was used for spotting on silica TLC plates. The plate was placed in a chromatography chamber containing butanol:acetic acid:water (65: 15: 25 v/v) as solvent. When the solvent front reached the top of the plate, the plate was removed and dried. After drying, plate was sprayed with ninhydrin reagent and placed in an oven at 100º for 4 min. The spots were scanned at 520 nm using Camag scanner and were analyzed using winCATS planar chromatography software ver 1.1.2 [11,12].
All the values are expressed as mean±SEM. Statistical analysis of in vivo experiments was done with one-way ANOVA followed by Bonferroni test, whereas results of in vitro experiment on guinea pig ileum and effect of neurotransmitter levels were evaluated with the help of Student’s t-test, and P<0.05 was considered as significant.
In the present study, methanol extract of Eclipta alba was evaluated for its antiepileptic potentials using the suitable animal models. The yield of the MEEA was found to be 14.8% w/w of dried leave powder. In the PTZ-induced seizure model, delay in onset of seizure was observed at all evaluated doses of MEEA and diazepam (2 mg/kg). Significant delay in onset of seizers was observed in diazepam-treated animals when compared with control (Table 1). Although a significant difference in the onset of seizers were observed at 50 mg/kg of MEEA with respect to vehicle control, the effect was not equivalent to that of diazepam (P<0.05). Delay in onsets of seizures were observed at 100 and 200 mg/kg, showing saturation of pharmacological activity at 50 mg/kg. Diazepam at the dose of 2 mg/kg and MEEA at the dose of 50, 100, and 200 mg/kg exhibited 100% protection from death of animals.
| Treatment | Pentylenetetrazole model | Picrotoxin model | ||
|---|---|---|---|---|
| Onset of seizure (sec) | % Protection | Onset of seizure (min) | % Protection | |
| Control | 87.82 ± 3.69 | 0 | 6.16 ± 0.29 | 0 |
| Diazepam 2 mg/kg | 600 ± 0.00* | 100 | 13.57 ± 0.25* | 66.66 |
| MEEA 10 mg/kg | 123.17 ± 3.44 | 0 | 8.64 ± 0.48 | 0 |
| MEEA 25 mg/kg | 172.17 ± 26.66* | 0 | 9.80 ± 0.93 | 0 |
| MEEA 50 mg/kg | 257.5 ± 3.44* | 100 | 14.34 ± 1.03** | 33.33 |
| MEEA 100 mg/kg | 218 ± 18.78* | 100 | 16.75 ± 3.88** | 66.66 |
| MEEA 200 mg/kg | 186.17 ± 24.77* | 100 | 12.35 ± 0.73 | 50 |
MEEA=Methanol extract Eclipta alba leaves; All the values are expressed as mean±SEM; Statistical analysis was done with one-way ANOVA followed by Bonferroni test; *P<0.05 was considered Vs control group.
Table 1: Anticonvulsant activity of methanol extract of eclipta alba leaves
Diazepam (2 mg/kg) showed a statistical significant delay in the onset of seizure in PIC-induced seizure model (P<0.05), with respect to the vehicle control (Table 1). A significant difference was found in the onset of seizers at 50 and 100 mg/ kg dose of MEEA, with respect to the control but no significant difference observed, with respect to the diazepam (positive control); this supports the antiepileptic potential of MEEA. Dose-dependent increase was observed in the onset of action of the MEEA extract, except for 200 mg/kg dose where there was a decrease, indicating saturation of the pharmacological activity. In the PIC-induced seizure model, the % protection by the 100 mg/kg dose of MEEA was 66.66%, which was equivalent to that of diazepam (66.66%).
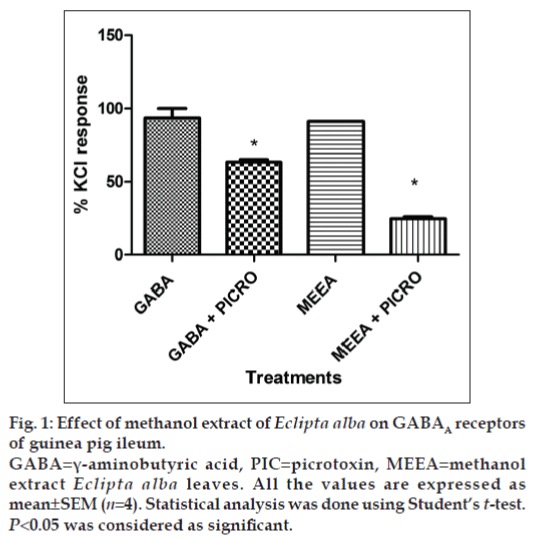
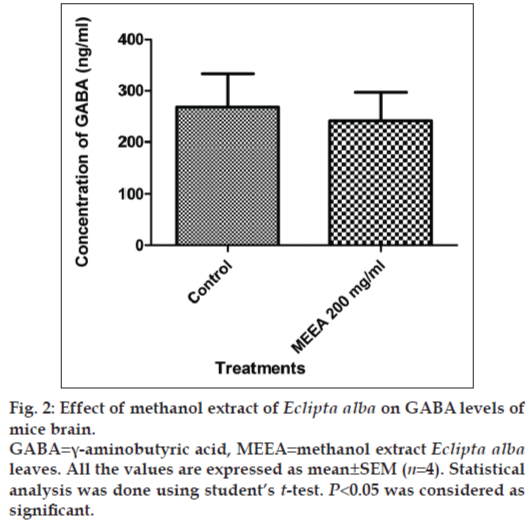
In vitro GABAA receptor bioassay showed that Picrotoxin (1 µM) inhibited the contraction in guinea pig ileum induced by GABA (1 µM) significantly (P<0.05). A similar effect was observed when 10 mg/ml of MEEA was used for the contraction of the ileum (fig. 1). MEEA, when administered to normal mice with 200 mg/kg dose for 7 days, did not alter the levels of GABA in brains, indicating that it is neither facilitating nor inhibiting the release of GABA in the brain (fig. 2).
Figure 1: Effect of methanol extract of Eclipta alba on GABAA receptors of guinea pig ileum.
GABA=γ-aminobutyric acid, PIC=picrotoxin, MEEA=methanol extract Eclipta alba leaves. All the values are expressed as mean±SEM (n=4). Statistical analysis was done using Student’s t-test. P<0.05 was considered as significant.
PTZ and PIC are two convulsion-inducing agents [13,14]. PTZ exerts its convulsive effect by inhibiting the activity of GABA at GABAA receptors, which is the major inhibitory neurotransmitter in the brain [15-17]. So, the enhancement of the neurotransmission of GABA will attenuate the convulsive effect of PTZ. Results of the present study indicated the onset of seizure produced by PTZ was significantly delayed by MEEA and diazepam, indicating that the anticonvulsant effect of MEEA might be mediated by facilitating the GABA-mediated opening of chloride channel.
According to Rang et al. [13], and Nicoll [14], picrotoxin exerts its convulsive effect by blocking the GABAA receptor-linked chloride ion channel that normally opens to allow increased chloride ion conductance into the brain cells following the activation of GABAA receptors by GABA. Results of the present study showed that MEEA and diazepam attenuated the convulsive effect of picrotoxin. These results further support the hypothesis that MEEA has ability to modulate the GABA-induced neurotransmission.
GABA at the concentration of 1 µM produces contraction in guinea pig ileum, which is further inhibited by picrotoxin (1 µM) confirming presence of GABAA receptors on guinea pig ileum. MEEA at the dose concentration of 10 mg/ml also induces contraction on guinea pig ileum that was significantly inhibited by picrotoxin (1 µM). This confirms the involvement of GABAA receptors in MEEA-induced contraction in guinea pig ileum. These results again support the hypothesis that anticonvulsant effect of MEEA in PTZ and PIC model may be because of its modulatory effect on GABA receptors.
MEEA at the given dose (200 mg/kg) failed to modulate the GABA levels in the brain of treated mice. This unchanged level of GABA in the brain suggested that the anticonvulsant effect is independent of release of GABA.
Our previous study showed that MEEA contains phytoconstituents like wedelolactone and luteolin and β-amyrin [7]. It has been reported that wedelolactone has selectivity and affinity towards BZD (benzodiazepine) binding site on GABAA receptors [18]. Also, luteolin has unique antiinflammatory and neuroprotective activity and has affinity towards BZD binding site on the GABAA receptors [19]. So, the anticonvulsant activity of MEEA in this study may be because of presence of these phytoconstituents in methanol extract.
In conclusion, the present study proves that methanol extract of Eclipta alba possesses anticonvulsant activity and provides the scientific basis for its traditional use in epilepsy. This study also provides the evidence that the anticonvulsant activity of MEEA is because of its positive modulatory effect on GABAA receptor and not by release of GABA in the synapse. The phytoconstituents like wedelolactone or luteolin in MEEA could be responsible for its anticonvulsant activity. Further studies are required to explore the phytoconstituents responsible for its activity, and their therapeutic applications in the treatment of epilepsy are ongoing in our laboratory.
Acknowledgments
Authors would like to acknowledge Indian Council of Medical Research, New Delhi, India for providing Senior Research Fellowship (File no. 45/61/09/PHA‑BMS) to carry out the research work. Authors also wish to thank by Dr. Harshad Pandit, Botany Department, Khalsa College, Mumbai, India for authentication of the plant materials.
References
- Vezzani M, French J, Bartfai T, Baram T. The role of inlammation in epilepsy. Nat Rev Neurol 2011;7:31 40.
- WHO (World Health Organization). Global Campaign against Epilepsy “Out of the Shadows”. Geneva: WHO; 2003. p. 22-8. Available from: http://www.who.int/mental_health/management/en/GcaeBroEn.pdf [Last accessed on 2013 Jan 30].
- Scott RA, Lhatoo SD, Sander JW. The treatment of epilepsy in developing countries: Where we go from here? Bull World Health Org 2011;79:344 51.
- Schmidt D. Drug treatment of epilepsy: Options and limitations. Epilepsy Behav 2009;15:56 65.
- Shinner S, Berg AT. Does antiepileptic drug therapy prevent the development of chronic epilepsy? Epilepsia 1996;37:701 8.
- Reddy MB, Reddy KR, Reddy MN. A Survey of plant crude drugs of Anantapur District, Andhra Pradesh, India. Int J Crude Drug Res 1989;27:145 55.
- Shaikh MF, Sancheti J, Sathaye S. Phytochemical and pharmacological investigations of EcliptaalbaHassk leaves for antiepileptic activity. Int J Pharm PharmSci 2012;4:319 23.
- Mahendran S, Thippeswamy BS, Veerapur VP, Badami S. Anticonvulsant activity of embelin isolated from Embeliaribes. Phytomedicine 2011;18:186 8.
- Quitans LJ, Souza TT, Leite BS, Lessa NM, Bonjardim LR, Santos MR, et al. Phytochemical screening and anticonvulsant activity of CymbopogonwinterianusJowitt (Poaceae) leaf essential oil in rodents. Phytomedicine 2008;15:619 24.
- Arulmozhia DK, Sridhar N, Bodhankar SL, Veeranjaneyulua A, Arora SK. In vitro pharmacological investigations of Sapindustrifoliatus in various migraine targets. J Ethnopharmacol 2004;95:239-45.
- Sadasivudu B, Murthy RK. Effects of ammonia on monoamine oxidase and enzymes of GABA metabolism in mouse brain. Arch IntPhysiolBiochim 1978;86:67-82.
- ShankaranarayanaRao BS, Raju TR, Meti BL. Self-stimulation of lateral hypothalamus and ventral tegmentum increases the levels of noradrenaline, dopamine, glutamate and AChE activity, but not 5-hydroxytryptamine and GABA levels in hippocampus and motor cortex. Neurochem Res 1998;23:1053-9.
- Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 5th ed. Edinburgh: Churchill Livingstone; 2003. p. 585-7.
- Nicoll RA. Introduction to the pharmacology of CNS drugs. In: Katzung BG, editor. Basic and Clinical Pharmacology. 8th ed. New York: Lange Medical Books/McGraw-Hill press; 2001. p. 351-63.
- De Sarro A, Cecchetti V, Fravolini V, Naccari F, Tabarrini O, De Sarro G. Effects of novel 6-desfluoroquinolones and classic quinolones on pentylenetetrazole-induced seizures in mice. AntimicrobAgents Chemother 1999;43:1729-36.
- Meldrum BS. GABA agonists as antiepileptic agents. AdvBiochemPsychopharmacol 1981;26:207-17.
- Gale K. GABA and epilepsy: Basic concepts from preclinical research. Epilepsia 1992;33:S3-12.
- Pocas ES, Lopez DV, Da Silva AJ, Pimenta PH, Leitao FB, Netto CD, et al. Structure-activity relationship of wedelolactone analogues: Structural requirements for inhibition of Na+, K+-ATPase and binding to the central benzodiazepine receptor. Bioorg Med Chem 2006;14:7962-6.
- Dirscherl K, Karlstetter M, Ebert S, Kraus D, Hlawatsch J, Walczak Y, et al. Luteolin triggers global changes in the microglial transcriptome leading to a unique antiinlammatory and neuroprotective phenotype. J Neuroinlamm 2010;7:1-16.