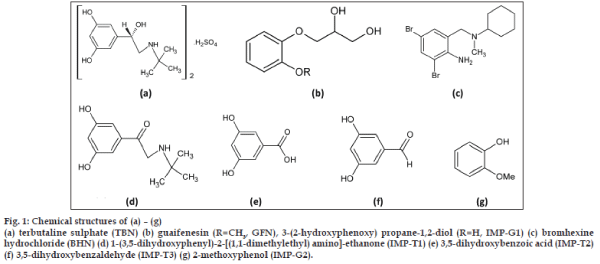
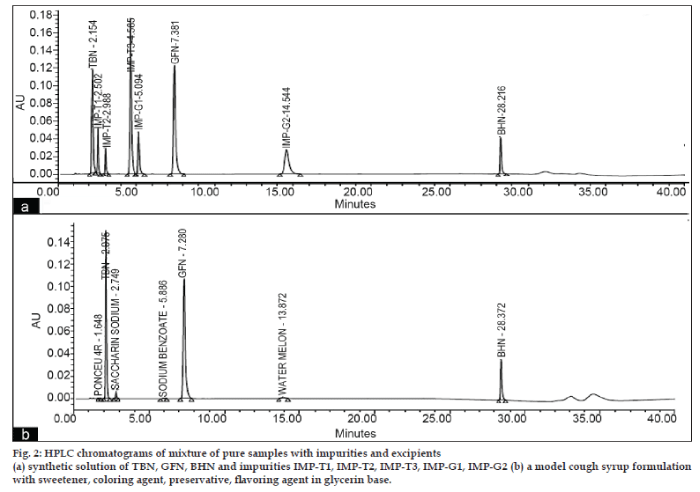
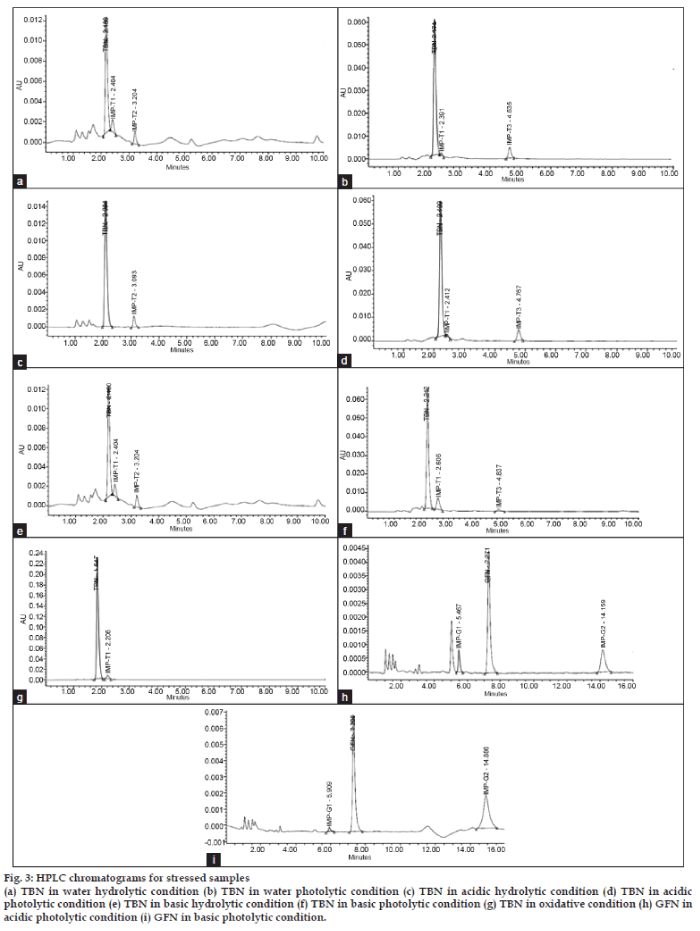
- *Corresponding Author:
- S. C. Shin
College of Pharmacy,Chonnam National University, Gwangju-500 757, Korea
E-mail: shinsc@chonnam.ac.kr
| Date of Submission | May 01, 2011 |
| Date of Revision | March 18, 2012 |
| Date of Acceptance | March 24, 2012 |
| Indian J Pharm Sci, 2012, 74 (2): 127-132 |
Abstract
To avoid the systemic adverse effects that might occur after oral administration, transdermal delivery of ambroxol was studied as a method for maintaining proper blood levels for an extended period. Release of ambroxol according to concentration and temperature was determined, and permeation of drug through rat skin was studied using two chamber-diffusion cells. The solubility according to PEG 400 volume fraction was highest at 40% PEG 400. The rate of drug release from the EVA matrix increased with increased temperature and drug loading doses. A linear relationship existed between the release rate and the square root of loading rate. The activation energy (Ea) was measured from the slope of the plot of log P versus 1000/T and was found to be 10.71, 10.39, 10.33 and 9.87 kcal/mol for 2, 3, 4 and 5% loading dose from the EVA matrix, respectively. To increase the permeation rate of ambroxol across rat skin from the EVA matrix, various penetration enhancers such as fatty acids (saturated, unsaturated), propylene glycols, glycerides, pyrrolidones, and non-ionic surfactants were used. The enhancing effects of the incorporated enhancers on the skin permeation of ambroxol were evaluated using Franz diffusion cells fitted with intact excised rat skin at 37° using 40% PEG 400 solution as a receptor medium. Among the enhancers used, polyoxyethylene-2-oleyl ether increased the permeation rate by 4.25-fold. In conclusion, EVA matrix containing plasticizer and permeation enhancer could be developed for enhanced transdermal delivery of ambroxol.
Keywords
Ambroxol, EVA matrix, plasticizer, penetration enhancer, transdermal delivery
Ambroxol is an active metabolite of the mucolytic drug bromhexine and is used in the treatment of bronchitis to improve expectoration [1-4]. However, due to transient high blood concentrations, oral administration of ambroxol may cause side effects such as headache, drowsiness, dizziness, insomnia and languor. To overcome those adverse effects, ambroxol could be administered using a transdermal drug delivery system. The skin is an attractive route for drug administration since the first-pass hepatic metabolism of drugs intended for systemic action may be avoided, thus offering potentially decreased drug dosages and reduced side effects [5]. Skin is a complex, dynamic, layered organ, the functions of which go far beyond its role as a barrier to the environment. The highly organized structure of the stratum corneum (SC) forms an effective barrier to the penetration of a diverse range of substances that must be administered. However, a major problem encountered using this route of administration is the low permeability of the skin. One way to reduce this problem and improve the bioavailability following transdermal administration of drugs is to include a penetration enhancer in the formulation. Several technologies have been successively developed to control the release and transdermal permeation of drugs. The use of a polymer membrane is one such method that regulates drug release. The use of drugs dispersed in an inert polymer matrix to achieve controlled release by diffusion has garnered considerable attention. Among many available polymers, ethylene vinyl acetate (EVA) copolymer is a heat processible, flexible, and inexpensive material [6].
The aim of this study was to assess the feasibility of transdermal delivery of ambroxol by studying in vitro release and to develop an ambroxol-EVA matrix containing a permeation enhancer for the transdermal delivery of ambroxol. We studied the feasibility of transdermal delivery of ambroxol by studying its in vitro release characteristics. To confirm the controlled release of drug, release studies were performed according to loading dose. The effects of temperature and plasticizer on the release were evaluated. To increase the drug permeation rate of ambroxol through rat skin, various enhancers such as non-ionic surfactants, glycerides, propylene glycol derivatives, fatty acids (saturated and unsaturated), and pyrrolidones, were incorporated in the EVA matrix and the level of ambroxol permeation through rat skin was evaluated.
Materials and Methods
Ambroxol was supplied by Handok Pharm. Co., Ltd., Korea. Ethylene vinyl acetate (EVA, 40%) was purchased from Aldrich Chemical Co., Inc., USA, and PEG 400, methylene chloride, and diethyl ether were from Daejung Chemical and Metals Co., Ltd., Korea. Acetyl tributyl citrate (ATBC), tributyl citrate (TBC), acetyl triethyl citrate (ATEC), and triethyl citrate (TEC) were purchased from Morflex, Inc., USA. Diethyl phthalate (DEP) and di-n-butyl phthalate (DBP) were from Junsei Chemical Co., Ltd., Japan. Myristic acid, linoleic acid, lauric acid, oleic acid, caprylic acid, polyoxyethylene-23-lauryl ether (Brij 35), polyoxyethylene-2-oleyl ether (Brij 92), polyethylene-2-stearyl ether (Brij 72), tetraethylene glycol (TEG), and diethylene glycol (DEG) were purchased from Sigma-Aldrich Co., USA. Oleyl macrogol-6 glycerides, caprylocaproyl macrogol-8 glycerides, propylene glycol monocaprylate, propylene glycol laurate, and propylene glycol monolaurate were gifts from Gattefose, France. 1-Methyl-2-pyrrolidone and 2-pyrrolidone were purchased from Acros Organics, USA. Acetonitrile and methanol were HPLC grade from J. T. Baker Inc., USA. All reagents were of analytical grade and were used without further purification. Distilled water was used for all studies.
Determination of drug solubility
Excess amounts of ambroxol were equilibrated with saline containing various concentrations of PEG 400. Each solution was shaken at 37° for 24 h in a shaking incubator. The solutions were then filtered through filter papers. The concentration of ambroxol was determined by HPLC after proper dilution.
Drug-loaded EVA matrix preparation
Drug-loaded EVA matrix was prepared by casting process. The weighed amount of EVA copolymer beads was dissolved in 20 ml of methylene chloride in a beaker and the drug solution was added. This mixture was poured onto a glass plate and the solvent was allowed to evaporate off at room temperature overnight. The membrane was removed from the plate. A piece of matrix was then cut properly, and the thickness was measured before the experiment. The drug content was calculated from the weight ratio of drug and copolymer used.
HPLC determination of ambroxol
Ambroxol was assayed by HPLC. The HPLC system consisted of a pump (Waters 501, USA), an ultraviolet detector (Waters 484, USA), RESTEK C18 column (250×4.6 mm, 5 μm), degasser, and an integrator (D520A, Youngin Scientific Co., Ltd., Korea). The mobile phase was composed of a mixture of acetonitrile and 0.01 M diammonium phosphate buffer, adjusted to pH 6 with H3PO4 (70:30, v/v). A flow rate of 1.5 ml/min yielded an operation pressure of ~1000 psi. The UV detector was operated at a wavelength of 252 nm. Under these conditions, the ambroxol peak appeared at a retention time of 4.6 min.
In vitro release studies
The in vitro release of ambroxol from the EVA matrix was examined in a modified Keshary-Chien cell. A unit of the EVA matrix was clamped between the cell cap and receptor cell. The diameter of the cell was 2 cm, providing 3.14 cm2 effective surface areas between the matrix and the bulk solution of 20 ml. 40% PEG 400 solution was used as the receptor solution. At predetermined time intervals, the whole solution from the receptor cell was taken and replaced with fresh solution. The cumulative amount of ambroxol released from the matrix was determined at 252 nm by HPLC. The effects of drug concentration on its release from the EVA matrix were studied according to drug concentration of 2, 3, 4 and 5% (w/w), and the effects of temperature on drug release were studied at 27, 32, 37 and 42º. Each data point represents the average of three determinations.
Preparation of plasticizer or enhancer containing EVA matrix
A plasticizer reduces the brittleness, improves flow, imparts flexibility, and increases toughness, strength, tear resistance, and impact resistance of the polymer. Increasing the amount of plasticizer could lead to an increase in free film elongation and a decrease in tensile strength and Young’s modulus. Plasticizer was added into drug-containing EVA solution and mixed for 1 h. This method was chosen in order to produce large unharmed pieces of membrane with no orientation of the molecules. Alkyl citrates, such as ATBC, TBC, ATEC and TEC, and phthalates, such as DEP and DBP, were used.
An enhancer might affect the fluidity of the stratum corneum structure such that drugs could better permeate through rat skin. Three different types of enhancer were used and the effects were compared. As penetration enhancers, fatty acids such as caprylic acid, myristic acid, and lauric acid, glycols such as diethylene glycol and tetraethylene glycol, and non-ionic surfactants such as Brij 92, Brij 72, and Brij 35 were used. The enhancer-containing EVA matrix was prepared by casting process. The weighed EVA copolymer beads were dissolved in 20 ml of methylene-chloride in a beaker; drug solution and plasticizer or enhancer was then added with vigorous stirring. This mixture was poured onto a glass plate and the solvent was allowed to evaporate off at room temperature overnight. The matrix was removed from the plate. A piece of matrix was then cut properly, and the thickness was measured before the experiment. The drug content was calculated from the weight ratio of drug and copolymer.
Permeation of ambroxol through rat skin
Permeation of ambroxol from the EVA matrix and through the rat skin was examined in a modified Keshary-Chien cell. A male rat (Sprague Dawley rat strain) was sacrificed by snapping the spinal cord at the neck. The hair of the abdominal area was carefully removed with an electric clipper. A square section of the abdominal skin was excised. After excision, the adhering fat and other visceral debris in the skin were carefully removed from the undersurface with tweezers [7]. The excised skin was used immediately. The freshly excised full-thickness skin sample was mounted on the receptor site of the diffusion cell with the stratum corneum side facing upwards into the donor compartment and the dermal side facing downwards into the receptor compartment. An appropriately sized matrix sample was placed on the stratum corneum side, covered with a round glass plate and clamped. To achieve sink conditions, the receptor medium was 40% PEG 400 solution and was maintained at 37° in a circulating water bath. Total samples were withdrawn at predetermined time intervals and immediately replaced by an equal volume of fresh medium. The amount of permeated drug was analyzed by HPLC at 252 nm. Each data point represents the average of four determinations. The enhancement factor (EF) was calculated using the following equation: EF = (flux of EVA matrix containing enhancers)/(flux of control sample)
Results and Discussion
The aqueous solubility of ambroxol is slightly low and can be improved by addition of a water-miscible hydrophilic polymer like PEG 400 into the aqueous solution as a solubilizer. PEG 400 has been reported to be an excellent solubilizer for many drugs [8]. In the present investigation, we observed that the solubility of ambroxol increased as the volume fraction of PEG 400 increased, and the solubility was the highest at 40% PEG 400 (fig. 1).
A characteristic drug release profile of matrix-type drug delivery systems can be represented by Higuchi’s equation [9]. The release from a system having dispersed drug in a homogeneous matrix should follow the relationship: Q = [D(2A―Cs)Cs t]1/2… Eqn. 1. Where, Q is the amount of drug released after time t per unit exposed area, D is the diffusivity of the drug in the matrix, A is the initial drug loading dose dispersed in the polymer matrix, and Cs is the drug solubility in the matrix.
A similar relationship for release from a granular matrix system in which diffusion occurs through channels was later derived [10]: Q = [(D ε/τ) × (2A―ε Cs) Cs t]1/2 ... Eqn. 2, where D and Cs refer to diffusivity and solubility in the permeability field, respectively; τ is the tortuosity of the matrix and ε is the porosity of the matrix. Although the two equations are for different mechanisms, they both describe drug release as being linear with the square root of time [11-12]. Q = KH · t1/2 ...Eqn. 3, where for the homogeneous matrix system: KH = [D (2A―Cs) Cs]1/2 ...Eqn. 4 and for the granular matrix system. KH = [(D ε/τ) × (2A―ε Cs) Cs]1/2...Eqn. 5. The validity of the relationships has been confirmed experimentally using various systems [13].
The release profiles of ambroxol from the EVA matrices of different drug loading over a 24 h period are shown in fig. 2. The plot of cumulative amount of ambroxol released (Q) versus the square root of time (t½) shows good linearity for all four concentrations. As expected from Equation 3, a plot of Q/t½ versus square root of loading dose (A) yields a straight line (fig. 2).
The dependency of the drug release profile on temperature is illustrated in fig. 3. The cumulative amount of drug released (Q) is plotted versus the square root of time (t½). After an initial period of drug release, the release was approximately linear with respect to t½. The steady-state rate of drug release (Q/ t½) was estimated from the slope of the linear Q-t½ profile from 4 to 24 h. The higher the temperature, the greater the drug released. In the EVA matrix containing 2% ambroxol, the Q/t½ values at 28, 32, 37 and 42º were 81.75, 85.17, 98.14 and 112.78 μg/ cm2/ h½, respectively. It should be noted that the rate of drug release increased about 1.4-fold when the temperature of the drug release system was raised from 27 to 42°. But, for practical use, the temperature of 37° was chosen to reflect the temperature of the stratum corneum [14]. This observation clearly indicates that the release of ambroxol from the EVA matrix is an energy-linked process [6].
The permeability coefficient is then defined by: P = Flux/Solubility ...Eqn. 6 P = P0×e―Ea/RT...Eqn. 7 by taking the logarithm on both sides equation becomes, Log P = log P0- [(Ea/R.2.303.1000)× (1000/T)...Eqn. 8 and Slope = - [(Ea/R.2.303.1000) × (1000/T)...Eqn. 9. Substituting Eqn. 9 in Eqn. 8 we get, Ea = -Slope×R× 2.303 × 1000 cal = -slope × 1.987 2.303 kcal... Eqn. 10.
As expected from Eqn. 10, a plot of log P versus 1000/T yielded a straight line (fig. 3). The activation energy (Ea), which was measured from the slope of log P versus 1000/T plot, was 10.71 kcal/mol for 2% loading dose, 10.39 kcal/mol for 3% loading dose, 10.33 kcal/mol for 4% loading dose, and 9.87 kcal/ mol for 5% loading dose from the EVA matrix.
Generally plasticizers increase the amount of drug release with the increased chain mobility of polymer. The plasticizer reduces the brittleness, improves flow, imparts flexibility, and increases toughness, strength, tear resistance, and impact resistance of the polymer. Increasing the amount of plasticizer can increase the free film elongation and decrease the tensile strength. A strong interaction between a drug and a polymer has been reported to significantly influence drug release through a polymeric film [15]. Among the plasticizers used, such as the citrates and the phthalates, TEC showed the highest effects (Table 1).
| Plasticizer | Flux mg/cm2/ h1/2 | E.F. |
|---|---|---|
| Citrate group | ||
| ATBC | 198.44±6.04 | 1.15 |
| ATEC | 220.46±8.54 | 1.31 |
| TBC | 216.24±7.55 | 1.61 |
| TEC | 209.16±9.93 | 1.88 |
| Phthalate group | ||
| DBP | 143.37±6.40 | 1.26 |
| DEP | 107.72±4.99 | 1.60 |
| Control | 100.73±3.48 | 1.00 |
Table 1: Effect of plasticizers on the flux of ambroxol from the eva matrix
The effects of enhancers on the permeation of ambroxol across rat skin were examined. Enhancers such as ethylene glycols, propylene glycols, glycerides, non-ionic surfactants and fatty acids, were used. Table 2 shows the permeation data of ambroxol according to enhancer. The cumulative amount of ambroxol through the rat skin was plotted against time (t). A linear profile was observed over 24 h and the slope of the linear portion of the curve was determined by linear regression. The effectiveness of penetration enhancers (enhancement factor) is shown in Table 2.
| Enhancer | Flux (mg/cm2/h) | EF |
|---|---|---|
| Control | 0.08±0.004 | 1 |
| Polyoxyethylene-23-lauryl ether | 0.20±0.011 | 2.5 |
| Polyoxyethylene-2-stearyl ether | 0.28±0.014 | 3.5 |
| Polyoxyethylene-2-oleyl ether | 0.34±0.014 | 4.25 |
| Oleic acid | 0.10±0.004 | 1.25 |
| Linoleic acid | 0.11±0.004 | 1.38 |
| Myristic acid | 0.19±0.004 | 2.38 |
| Lauric acid | 0.10±0.004 | 1.25 |
| Caprylic acid | 0.09±0.003 | 1.13 |
| Oleoyl macrogol-6 glycerides | 0.16±0.008 | 2 |
| Capryocaproyl macrogol-8 glycerides | 0.26±0.013 | 3.25 |
| Propylene glycol mono caprylate | 0.32±0.012 | 4.01 |
| Propylene glycol laurate | 0.21±0.014 | 2.63 |
| Propylene glycol monolaurate | 0.31±0.012 | 3.86 |
| NMP | 0.20±0.011 | 2.52 |
| 2-pyrrolidone | 0.18±0.014 | 2.25 |
Table 2: Emhamcenent factor according to various enhancers.
The permeation of ambroxol from the EVA matrix containing an enhancer was better than that without the enhancer. In previous studies [15-17] on the effects of a penetration enhancer, thermal analysis and histological examinations suggested that the incorporation of a penetration enhancer decreases the lipid order and has a fluidizing effect on the lipids of the stratum corneum. The role of the penetration enhancer might be explained as an interfacial saturation phenomenon. For the stratum corneum lipids to be dissolved, it is most likely that enhancers, such as a surfactant, accumulate at the lipid/liquid interface after penetrating into the tissue. The enhancer might affect the fluidity of the stratum corneum structure, which can allow drugs to permeate better through the skin. Among the enhancers used in this test, Brij 92 (polyoxyethylene-2-oleyl ether) increased the permeation rate by 4.25-fold (Table 2).
An increase of drug concentration or temperature increased the drug release rate. A linear relationship existed between the release rate and the square root of the loading rate. The activation energy (Ea), which was measured from the slope of a plot of log P versus 1000/T, was 10.71 kcal/mol for 2% loading dose, 10.39 kcal/mol for 3% loading dose, 10.33 kcal/mol for 4% loading dose, and 9.87 kcal/mol for 5% loading dose from the EVA matrix. Among the various enhancers tested, polyoxyethylene-2-oleyl ether showed the highest effect, increasing the permeation rate by 4.25-fold. In conclusion, for the controlled transdermal delivery of ambroxol, an EVA matrix containing a permeation enhancer could be developed.
References
- SeverinaIS,Bussygina OG, Pyatakova NV, Khropov YV, Krasnoperov RA. Ambroxol as an inhibitor of nitric oxide-dependent activation of soluble guanylatecyclase. Eur J Pharmacol 2000;407:61-4.
- Dariusz ND, Antczak A, Król M. Antioxidant properties of ambroxol. Free RadicBiol Med 1994;16:517-22.
- Guyatt GH, Townsend M, Kazim, Newhouse MT. A controlled trial of ambroxol in chronic bronchitis. Chest 1987;92:618-20.
- Ericsson CH, Juhasz J, Johnson E, Mossberg B. Ambroxol and simple chronic bronchitis, Effects on subjective symptoms and ventillatoryfunction. Respiration 1987;51:33-6.
- Phipps B, Cormier M, Gale B, van Osdal B, Audett J, Padmanabhan R, et al. Transdermal drug delivery. In: Bowlin GL, Wnek G, editors.Encyclopedia of Biomaterials and Biomedical Engineering, Vol. 1. New York: Marcel Dekker; 2004. p. 1677-89.
- Miyazaki S, Ishii K, Takada M. Controlled release of anticancer agents through ethylene-vinyl acetate copolymer membrane. Yakuzaigaku1983;42:259.
- Durrhein H, Flynn GL, Higuchi WI, Behl CR. Permeation of hairless mouse skin I: Experimental methods and comparison with human epidermis permeation by alkanols. J Pharm Sci 1980;69:781-6.
- Chien YW, Lambert HJ. Solubilization of steroids by multiple co-solvent systems. Chem Pharm Bull 1975;23:1085-90.
- Higuchi T. Rate of release of medicaments from ointment bases containing drug in suspension. J Pharm Sci 1961;50:874-5.
- Higuchi T. Mechanism of sustained-action medication: Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 1963;52:1145-9.
- Desai SJ, Simonelli AP, Higuchi WI. Investigation of factors influencing release of solid drug dispersed in inert matrices. J Pharm Sci 1965;54:1459-64.
- Desai SJ, Singh P, Simonelli AP, Higuchi WI. Factors influencing release of solid drug dispersed in inert matrices II. Quantitation of procedures. J Pharm Sci 1966;55:1224-9.
- Farhadieh B, Boradkin S, Buddenhagen J. Drug release from methyl acrylate-methyl methacrylate copolymer matrix II: Control of release rate by exposure to acetone vapor. J Pharm Sci 1971;60:212-5.
- Chien YW, Lau EP. Controlled drug release from polymeric delivery devices IV: In vitro-in vivo correlation of subcutaneous release of norgestomet from hydrophilic implants. J Pharm Sci 1976; 65:488-92.
- Shin SC, Kim JY. Enhanced permeation of triamcinolone acetonidethrough the buccal mucosa. Eur J Pharm Biopharm 2000;50:217-20.
- Choi JS, Shin SC. Enhanced bioavailability of ambroxol by transdermal administration of the EVA matrix containing penetration enhancer in rats. BiomolTherap 2010;18:106-10.
- Cho CW, Choi JS, Shin SC. Enhanced local anesthetic efficacy of bioadhesiveropivacaine gels. BiomolTherap 2011;19:357-63.