- *Corresponding Author:
- Archana P. Raina
Germplasm Evaluation Division, National Bureau of Plant Genetic Resources, Pusa Campus, New Delhi-110 012
E-mail: aprraina@yahoo.co.in
| Date of Submission | 02 May 2014 |
| Date of Revision | 27 October 2014 |
| Date of Acceptance | 13 March 2015 |
| Indian J Pharm Sci 2015;77(2):218-222 |
Abstract
Valeriana jatamansi Jones germplasm collected from sub-temperate Himalayan region of Uttarakhand and North-East state of Meghalaya, India was evaluated under identical conditions at National Bureau of Plant Genetic Resources, Bhowali, India, to study germplasm diversity based on essential oil composition. Twenty one compounds were identified in V. jatamansi root oil by GC and GC-MS. The major compounds identified were patchouli alcohol (0.4-63.7%), maaliol (2.9-53.8%), seychellene (4.1-27.4%), calarene/ί-gurjunene (3.0-20.8%), α-santalene (0.6-12.0%). Other compounds present were bornyl acetate (0.6-1.5%), α-guaiene (0.7-2.3%), α-bulnesene/d-guaiene (0.7-6.3%), 7-epi-α-selinene (0.4-1.4%), kessane (2.1-3.3%), spathulenol (0.7-3.4%), viridiflorol (0.9-7.1%), α-patchoulene (0.8-6.6%), ί-patchoulene (0.4-0.8%). Two superior chemotypes identified in V. jatamansi oil from Uttarakhand were: patchouli alcohol rich (IC573221, 63.7%) and maaliol rich (IC573222, 53.8%; IC589096, 51.7%), while accession from north-east was patchouli alcohol rich chemotype (IC574522, 57.2%). These superior chemotypes with higher amounts of patchouli alcohol and maaliol could be used for promoting cultivation as well as for meeting need of pharmaceutical industries.
Keywords
Valeriana jatamansi Jones (Syn. V. wallichii DC.), Indian Valerian, chemotypes, essential oil composition, Valerianaceae
The genus Valeriana (Family-Valerianaceae) comprises over 250 species, distributed throughout the world [1] and about 12 species are found in India [2]. The genus is known for its popular name ‘Valerian’. Valeriana jatamansi Jones (Syn. V. wallichii DC.) is commonly known as Indian Valerian, Muskbala, Sugandhbala (Hindi) and Tagar (Sanskrit). Indian valerian is a wild herb distributed in subtropical and temperate Himalaya up to an altitude of 3000 meters and between 1500 to 1800 meters in Khasi and Jaintia hills. It has been used an ingredient of herbal medicines in Indian systems of medicine and is used as a substitute of European Valeriana officinalis in India. It is an erect pubescent herb, having horizontal, thick rootstock/ rhizomes, with thick descending fibrous roots. The root of the plant yields 0.3-2.1% (v/w) essential oil that gives a musky, woody, sweet and balsamic odor. V. jatamansi has been used since long in Ayurvedic and Unani systems of medicine. In traditional medicines, the roots of the plant are used for ulcers, convulsions, jaundice, cardiac debility, dry cough, asthma, seminal weakness, skin diseases, leprosy, general debility and for sleep enhancement [3,4], while its oil finds use in perfumery and in insect repellent formulations. Essential oil and extract of the species is used in flavour, pharmaceutical and fragrance Industries and about 30 products are commercially available [2]. The species is reported to be used in several Ayurvedic preparations and is used as carminative. The valerian roots are prescribed for hysteria, hypochondriasis, nervous unrest [5]. The pharmacological activity of valerian is believed to be due to two major groups of constituents-the valepotriates and sesquiterpenoids. According to the European Pharmacopoeia, the crude drug Valerianae radix must contain not less than 0.5% (v/w) of essential oil. The crude drugs from the roots/ rhizomes and Valerian derived phytomedicines are used as mild sedatives in pharmaceutical industry. The root/rhizome parts are highly aromatic and as a result Valerian oil in great demand. Some studies in the past have reported variation in essential oil composition of valerians [6-13]. V. jatamansi root oil has reported major terpenoids like sesquiterpene hydrocarbons (ar-curcumene, ß-farnesene, α-and ß-patchoulenes and sesquifenchene), valeranone, cryptomeridiol, maaliol, xanthorrhizzol, patchouli alcohol, and others. Survey and collection of V. jatamansi from the northwestern Himalayan region showed morphologically similar but chemically distinct two chemotypes rich in maaliol and patchouli alcohol [11]. Chemical evaluation of Valeriana populations from Himachal Pradesh showed presence of three different groups based on chemical composition of essential oil [12]. However, there is no detailed information available on V. jatamansi oil composition from North-East, India except one by [13] to the best of our knowledge. Considering the use of V. jatamansi in various neurodegenerative diseases and in view of growing demand for its raw material in the industries, the present study was done to assess the variation in chemical composition of essential oil of V. jatamansi germplasm, collected from different geographical locations of Uttarakhand and Meghalaya in North-East, India.
V. jatamansi accessions were collected from five districts of Uttarakhand viz; Uttarkashi (1), Bageshwar (2), Nainital (1), Almora (1) and Deharadun (1) situated at different geographical locations and one accession was collected from Shillong district of Meghalaya during the years 2008-2010 (Table 1). The botanical identification of specimen was done at National Bureau of Plant Genetic Resources Regional Station, Bhowali, Uttarakhand. These germplasm collections were grown for evaluation at identical sub-temperate conditions of NBPGR Regional Station, Bhowali (1600 m altitude, 79˚30’ N latitude and 29˚20’ E longitude) during the years 2011 and 2012. The roots of V. jatamansi accessions were harvested in the month of April-May and then air-dried in the shade at room temperature. The essential oil was extracted by hydro distillation using a Clevenger-type apparatus. The essential oil obtained was dried over anhydrous sodium sulphate and stored in a glass vial in the dark at 4° till further chemical analysis. The gas chromatography (GC) analyses of the volatile constituents in oil samples was performed using Agilent GC model 7890 A, equipped with flame ionization detector (FID) and a HP-5MS capillary column; 5% diphenyl 95% dimethyl polydimethylsiloxane (30 m length×0.32 mm internal diameter×0.25 μm film thickness). Helium was used as carrier gas at the flow rate of 1 ml/min. The column oven temperature was programmed from 60 to 240° with an increase in rate of 4˚/min with an initial hold time of 10 min at 60˚, followed by final hold time of 10 min at 240°. Detector temperature was maintained at 250°. The oil sample (0.2 μl) was injected in split ratio (1:40) at 220°. The gas chromatography-mass spectrometry (GC-MS) analyses were performed on Agilent 6890 gas chromatograph interfaced a MSD detector 5975C and a HP-5MS fused silica capillary column as mentioned above. The operating conditions were as follows: Injection and detector temperatures, 220° and 280°, respectively; Oven temperatures programme: 10 min initial hold at 60°, subsequently raised to 240° at 4°/min with a final hold time of 10 min at 240˚. Other MS operating parameters were: Interface temperature 250°; electron impact ionization at 70 eV with scan mass range of 40-400 m/z at a sampling rate of 1.0 scan/s. The identity of each compound was assigned by comparison of their retention index (RI), relative to a standard mixture of n-alkanes, as well as by comparison of their spectra with those available from MS libraries (NIST/Wiley/Adams) and with the literature values [14]. Relative amounts of individual components were calculated based on GC peak area (FID response) without using any correction factor.
| Accessions | Collection site | Essential oil content* (%) | ||
|---|---|---|---|---|
| Village | district | state | ||
| IC 582516 | Ginoti | Uttarkashi | Uttarakhand | 1.67 |
| IC 573221 | Kanda | Bageshwar | Uttarakhand | 1.71 |
| IC 573222 | Kanda | Bageshwar | Uttarakhand | 2.00 |
| IC 573210 | Niglat | Nainital | Uttarakhand | 1.06 |
| IC 573212 | Dunagiri | Almora | Uttarakhand | 1.20 |
| IC 589096 | Mussorrie | Deharadun | Uttarakhand | 0.89 |
| IC 574522 | Shillong | Shillong | Meghalaya | 0.64 |
*Essential oil (v/w) extracted from roots of the plant and expressed on dry weight basis
Table 1: Valeriana jatamansi from uttarakhand and meghalaya.
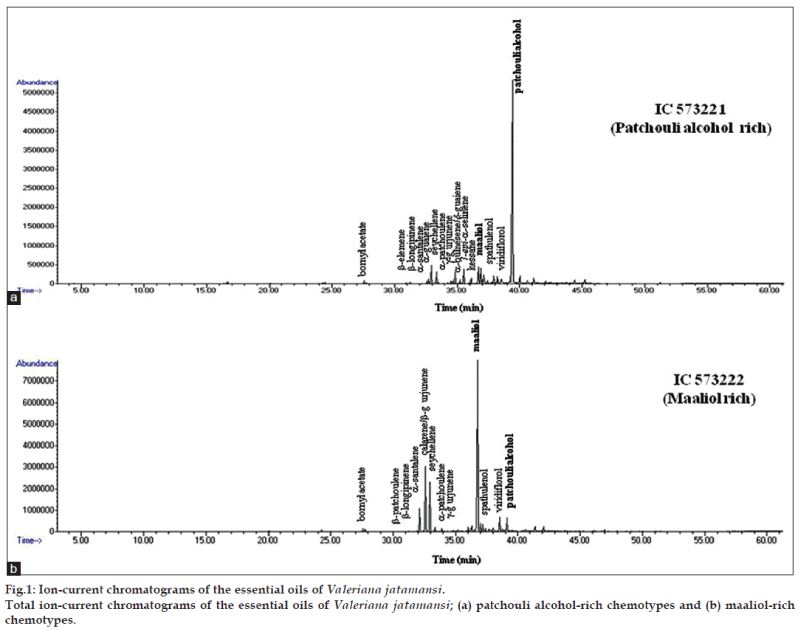
Present study revealed that V. jatamansi germplasm from different geographical regions contain essential oil content from 0.6 to 2.0% (v/w) in roots on dry weight basis (Table 1). The moisture content present in fresh roots (0.5 to 1.2%) was taken into consideration while determining oil content on dry weight basis. Twenty one compounds were identified in the oil by GC and GC-MS which represented 91.8 to 98.9% of the total oil (Table 2). Essential oil composition was predominated by oxygenated sesquiterpenes (33.2-84.7%), followed by sesquiterpene hydrocarbons (13.5-61.6%), whereas monoterpene hydrocarbons and oxygenated monoterpenes were less than 2.0% in all the seven accessions. The major compounds identified were patchouli alcohol (0.4-63.7%), maaliol (2.9-53.8%), seychellene (4.1-27.4%), calarene/ ß-gurjunene (3.0-20.8%), α-santalene (0.6-12.0%). Other compounds were bornyl acetate (0.6-1.5%), α-guaiene (0.7-2.3%), α-bulnesene/δ-guaiene (0.7-6.3%), 7-epi-α-selinene (0.4-1.4%), kessane (2.1-3.3%), spathulenol (0.7-3.4%), viridiflorol (0.9-7.1%), α-patchoulene (0.8-6.6%), ß-patchoulene (0.4-0.8%). The GC and GC-MS comparison of oils from different natural habitats of V. jatamansi showed two distinct chemotypes (fig. 1). Chemotype-I was characterized by prevalence of patchouli alcohol and three accessions identified in this group were: IC573221 (63.7%, Bageshwar); IC574522 (57.2%, Shillong) and IC573210 (43.1%, Nainital). Patchouli alcohol is known for its wide use in perfumery. The second chemotype was characterized by the prevalence of maaliol and three accessions identified in this group were IC573222 (53.8%, Bageshwar), IC589096 (51.7%, Tehri) and IC582516 (42.1%, Uttarkashi). Compounds such as kessane were present in patchouli alcohol rich chemotype only while ß-gurjunene, α-santalene was present in higher amounts in maaliol rich chemotypes. Maaliol rich collections also showed low amounts of patchouli alcohol (<3%). Collection from Almora showed different relative distribution of compounds, like seychellene, maaliol, patchouli alcohol and α-santalene. Compounds like xanthorrhizol, 8-acetoxy patchouli alcohol, 3-methyl valeric acid, carvacrol methyl ether, pogostol, juniper camphor, azulene were not detected in oil from all these collections of V. jatamansi as compared to earlier report [9,15]. Medicinal plants are known to exhibit considerable diversity of chemical constituents and their quantities define the chemotypes within the species. They have shown chemotypic diversity over localities and altitude despite inherent properties of the species.
| Collection sites | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | RI | Bageshwar (2) | Uttarkashi C | Nainital D | Dehradun E | Almora F | Shillong G | Range (%) | |
| A | B | ||||||||
| Thymol methyl ether | 1232 | - | - | 0.3 | - | - | 0.5 | - | 0.3-0.5 |
| Bornyl acetate | 1277 | 0.7 | 0.6 | 1.2 | 0.7 | 0.8 | 1.5 | - | 0.6-1.5 |
| b-Patchoulene | 1377 | - | 0.8 | 0.4 | 0.8 | 0.4 | 0.6 | 0.7 | 0.4-0.8 |
| b-Elemene | 1392 | 0.1 | - | - | 0.4 | 0.5 | 0.1-0.5 | ||
| b-Longipinene | 1398 | 0.7 | 1.8 | - | - | - | 1.2 | - | 0.7-1.8 |
| a-Santalene | 1414 | 0.6 | 4.5 | 8.7 | 1.5 | 6.0 | 12.0 | 1.0 | 0.6-12.0 |
| Calarene/b-Gurjunene | 1433 | - | 14.2 | 20.8 | 3.0 | 13.2 | 13.6 | - | 3.0-20.8 |
| a-Guaiene | 1442 | 1.0 | - | - | 2.3 | - | 0.7 | - | 0.7-2.3 |
| Seychellene | 1448 | 4.1 | 9.8 | 17.6 | 8.0 | 13.7 | 27.4 | 10.8 | 4.1-27.4 |
| a-Humulene | 1453 | - | - | 0.5 | 1.1 | - | - | - | 0.5-1.1 |
| a-Patchoulene | 1454 | 2.7 | 0.8 | 2.6 | 5.7 | 1.5 | 2.4 | 6.6 | 0.8-6.6 |
| g-Gurjunene | 1473 | 0.8 | 0.8 | 0.7 | 1.5 | - | 0.5 | - | 0.5-1.5 |
| ar-Curcumene | 1510 | - | - | 0.2 | - | - | 0.4 | - | 0.2-0.4 |
| a-Bulnesene/d-Guaiene | 1516 | 2.6 | - | 0.7 | 6.3 | 0.7 | 1.7 | 5.2 | 0.7-6.3 |
| 7-epi-a-Selinene | 1518 | 0.9 | - | 0.4 | 1.4 | 1.0 | 0.6 | - | 0.4-1.4 |
| Kessane | 1528 | 3.3 | - | - | 2.1 | - | - | - | 2.1-3.3 |
| Maaliol | 1566 | 13.3 | 53.8 | 42.1 | 5.8 | 51.7 | 15.5 | 2.9 | 2.9-53.8 |
| Spathulenol | 1576 | 3.4 | 1.7 | 1.2 | 2.1 | 1.9 | 0.7 | 1.1 | 0.7-3.4 |
| Viridiflorol | 1594 | 1.1 | 0.9 | - | 7.1 | 3.9 | 3.2 | 6.0 | 0.9-7.1 |
| Aromadendrene | 1639 | - | 2.8 | 1.0 | 4.4 | - | 1.6 | - | 1.0-4.4 |
| Patchouli alcohol | 1665 | 63.7 | 0.7 | 0.4 | 43.1 | 2.7 | 12.2 | 57.2 | 0.4-63.7 |
| Class composition | |||||||||
| Oxygenated monoterpenes | 0.7 | 0.6 | 1.5 | 0.7 | 0.8 | 2.0 | - | 0.6-2.0 | |
| Sesquiterpene hydrocarbons | 13.5 | 32.7 | 52.5 | 31.6 | 36.5 | 61.6 | 24.7 | 13.5-61.6 | |
| Oxygenated sesquiterpenes | 84.7 | 59.8 | 44.6 | 64.5 | 60.3 | 33.2 | 67.1 | 33.2-84.7 | |
| Total identified (%) | 98.9 | 93.1 | 98.6 | 96.9 | 97.6 | 96.8 | 91.8 | 91.8-98.9 | |
RI is retention indices on HP-5 MS capillary column. Percentage of components (average of three replicates), A- IC573221, B- IC573222, C- IC582516, D-IC573210, E-IC 589096, F- IC573212 and G- IC574522
Table 2: Essential oil composition of valeriana jatamansi jones accessions from uttarakhand and meghalaya.
The results of present study showed presence of superior chemotypes in our V. jatamansi germplasm with much higher percentage of patchouli alcohol and maaliol as compared to the earlier reports of two chemotypes from the North Western Himalaya [10,11,16]. Germplasm collection from Shillong was found to be patchouli alcohol rich chemotype (>57.2%) in contrast to earlier report of presence of maaliol (26.1%), patchouli alcohol (9.3%) and α-gurjunene (8.7%) in V. jatamansi collected from the Khasi hills of north-east India [13]. Chemotypes having patchouli alcohol >50% may be utilized commercially in perfumery. These two major compounds namely maaliol and patchouli alcohol can act as marker constituents and could be utilized as an important tool in oil authentications.
Thus present study has provided information on variation in chemical composition of V. jatamansi collections from Himalayan regions of India and availability of chemotypes in the germplasm. The quantitative composition and the relative proportions of the oil components are found to be widely influenced by the environmental factors and growing conditions. These may largely be responsible for the observed compositional variations between the studied oil samples and previous results published elsewhere. Considering the use of V. jatamansi in various neurological diseases and in view of growing demand for its raw material in the industries, these superior chemotypes with greater amounts of patchouli alcohol and maaliol could be used for commercial benefits and mass scale plantation of the species may be adopted for getting these compounds. Besides, the wide variation in chemical composition would suggest that the variation can be reduced by collecting the samples from the planted sources.
These findings would cater to the need of promoting cultivation for meeting need of pharmaceutical industries and also reducing pressure from wild populations.
References
- Bhattacharjee SK. Handbook of Aromatic Plants. Jaipur: Pointer Publishers; 2000. p. 458-9.
- Prakash V. Indian Valerianaceae. A Monograph on Medicinally Important Family. Vol. 1-2. Jodhpur, India: Scientific Publishers; 1999.
- Atal CK, Kapur BM. editors. Cultivation and Utilization of Medicinal and Aromatic Plants. Jammu-Tawi: Regional Research Laboratory; 1977. p. 393.
- Bos R, Woerdenbag HJ, Hendriks H, Smit HF, Wikstrom HV, Scheffer JJ. Composition of the essential oil from roots and rhizomes of ValerianawallichiiDC.FlavFragr J 1997;12:123-31.
- Chowdhury AR. GC/MS studies on the essential oil from the roots of ValerianawallichiiDC. Indian Perfumer 1999;43:147-9.
- Bos R, Woerdenbag HJ, Putten FM, Hendriks H, Scheffer JJ. Seasonal variations of the essential oil, valerenic acid and derivatives, and valepotriates in Valerianaofficinalisroots and rhizomes andthe selection of plants suitable for phytomedicines. PlantaMedica 1998;64:143-7.
- Mathela CS, Dev V. Chemical variation among natural and commercial Valerian (Valerianawallichii DC) samples. Indian Perfumer 2003;47:25-7.
- Mathela CS, Tiwari M, Sammal SS, Chanotiya CS. ValerianawallichiiDC, a new chemotype from north western Himalaya. J Essent Oil Res 2003;17:672-5.
- Mathela CS, Chanotiya CS, Sammal SS, Pant AK, Pandey S. Compositional diversity of terpenoids in the Himalayan Valeriana genera. ChemBiodivers 2005;2:1174-82.
- Sati S, Chanotiya CS, Mathela CS. Comparative investigations on the leaf and roots oils of Valerianawallichii DC. from North Western Himalayn. J Essent Oil Res 2005;17:408-9.
- Raal A, Orav A, Arak E, Kailas T, Muurisepp M. Variation in the composition of the essential oil of Valerianaofficinalis L. root from Estonia. Proc Estonian AcadSciChem 2007;56:67-74.
- Verma RS, Verma RK, Padalia RC, Chauhan A, Singh A, Singh HP. Chemical diversity in the essential oil of Indian valerian (ValerianajatamansiJones). ChemBiodivers 2011;8:1921-9.
- Verma RS, Padalia RC, Chauhan A. Assessment of similarities and dissimilarities in the essential oils of patchouli and Indian Valerian. J Essent Oil Res 2012;24:487-91.
- Singh SK, Katoch R, Kapila RK. Chemotypic variation for essential oils in ValerianajatamansiJones populations from Himachal Pradesh.J Essent Oil Res 2013;25:154-9.
- Das J, Mao AA, Handique PJ. Terpenoid compositions and antioxidant activities of two Indian valerian oils from the Khasi hills of north-east India. Nat Prod Commun 2011;6:129-32.
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometery. Carol Stream IL, USA: Allured Publishing Corp; 2007.