- *Corresponding Author:
- K. Rungsihirunrat
College of Public Health Sciences, Chulalongkorn University, Bangkok 10330, Thailand
E-mail: kanchana.r@chula.ac.th
| Date of Submission | 15 November 2016 |
| Date of Revision | 28 July 2017 |
| Date of Acceptance | 25 February 2018 |
| Indian J Pharm Sci 2018;80(2):359-365 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Plants in genus Cassia contain anthraquinones that possess stimulant laxative property. Cassia grandis L.f. and Cassia garrettiana Craib have been widely distributed throughout Thailand as Thai herbal medicine and ornamental plant. The objective of this study was to develop and validate TLC densitometry and TLC image analysis for quantitative analysis of aloe-emodin content in Cassia grandis and Cassia garrettiana leaves. The fresh mature leaves of Cassia grandis and Cassia garrettiana, collected from 15 locations throughout Thailand were successively extracted with dichloromethane. Silica gel 60 GF254 was used as a stationary phase and hexane-ethyl acetate (1:1, v/v) was used as the mobile phase. Aloe-emodin contents estimated using TLC densitometry and TLC image analysis in Cassia grandis dried crude drug were 0.4122±0.0668 and 0.4130±0.0751 g % and Cassia garrettiana dried crude drug were 0.0346±0.0067 and 0.0351±0.0056 g %, respectively. The method was validated in terms of calibration range, accuracy, repeatability and intermediate precision, limit of detection, limit of quantitation, specificity and robustness. A statistical comparison of the quantitative analysis using TLC densitometry and TLC image analysis of aloe-emodin in Cassia grandis and Cassia garrettiana leaves were not statistically significant (p>0.05). Both validated methods can be used for evaluation of aloe-emodin contents in C. grandis and C. garrettiana leaves.
Keywords
Cassia grandis, Cassia garrettiana, TLC densitometry, TLC image analysis, aloe-emodin contents
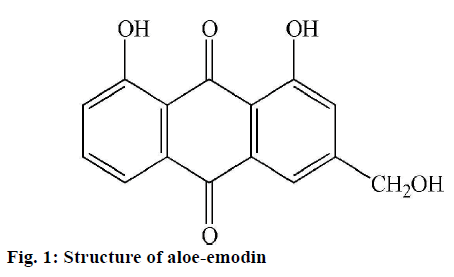
Cassia is a genus of tropical flowering plant belonging to the family Caesalpiniaceae. There are many Cassia species worldwide used in herbal medicine. Plants in genus Cassia contain mainly anthraquinone compounds, which are the largest group of natural quinones used as laxatives and antifungal drug for skin diseases [1]. Several anthraquinones and their derivatives from Cassia species have been documented such as rhein, emodin, sennosides and aloe-emodin. Aloeemodin (Figure 1) is an anthraquinone found in plants (Aloe sp., Rhamnus sp. and Cassia sp.), fungi, lichens and insects [2] has interesting biological activities such as antiviral, antimicrobial, anticancer and hepatoprotective activities [3-6]. In Thailand, C. grandis is known as “Kanlaphruek” or “Kalapaphruek” in Thai language. This plant is wildly distributed as ornamental plant throughout the country. C. garrettiana is also a traditional Thai medicine called “Sa mae san” which has been used as emmenagogue, blood tonic for women and used to treat herpes zoster, leukaemia, and constipation [7,8]. Previous study identified aloe-emodin as the main anthraquinone isolated from C. grandis and C. garrettiana leaves [9-10].
Thin-layer chromatography (TLC) is a fast screening method for compound separation and identification of herbal extracts. This method is frequently used as a qualitative and quantitative analysis as low cost of instrumentation, short time for analysis and easy to use [11,12]. Quantitative analysis can be performed using TLC densitometry and TLC image analysis. TLC densitometry is one of the suitable methods wildly used for quantitative analysis due to its accurate, precise and reliable procedure [13]. TLC image analysis using computer software technology has been in consideration as a simple, inexpensive and convenient quantitation method with good accuracy and precision for chemical compounds analysis in crude drug and medicinal plants [14]. Both methods successfully applied for quantification of chemical compounds in various herbal plants such as Artocarpus lakoocha, C. fistula, Senna siamia and Chromolaena odorata [15-18]. The quantification of aloe-emodin in C. grandis and C. garrettiana leaf extracts using TLC densitometry and TLC image analysis has not been reported. Thus, the objective of this study was to develop and validate TLC densitometry with winCATS software and TLC image analysis with ImageJ software for quantification of aloe-emodin contents in C. grandis and C. garrettiana leaves collected from different locations in Thailand.
Materials and Methods
The fresh mature leaves of C. grandis and C. garrettiana were collected from 15 locations in Thailand. Plant specimens were authenticated at the College of Public Health Sciences, Chulalongkorn University and Faculty of Pharmacy, Rangsit University, Thailand. Plant specimens were compared to the herbarium specimens at the Botanical Garden Organization, Ministry of Natural Resource. Voucher specimens were deposited at College of Public Health Sciences, Chulalongkorn University. Each authentic sample was dried in hot air oven at 45° and ground to a powder for phytochemical screening of anthraquinones.
Phytochemical screening of anthraquinones
Dilute hydrochloric acid (2 M) was added to the sample and the mixture was heated on a hot water bath for 15 min, then cooled and filtered. The filtrate was then extracted with dichloromethane. The dichloromethane layer was separated and shaken with ammonium hydroxide. Pink to red colour was developed in alkali layer [19].
Preparation of standard solutions
A stock solution containing 0.5 mg/ml of standard aloe-emodin (Sigma-Aldrich, USA) was prepared in dichloromethane containing 10 % methanol and diluted to obtain a series dilution of 0.04, 0.08, 0.12, 0.16, and 0.20 mg/ml of standard solutions and then stored in refrigerator at 4°.
Preparation of dichloromethane extracts of C. grandis and C. garrettiana
Six grams of the leaf powder of C. grandis and C. garrettiana was extracted with dichloromethane using a Soxhlet apparatus. The extract was filtered and the solvent was evaporated by rotary evaporator. The yield of each plant sample was calculated and recorded. The extract was dissolved in dichloromethane containing 10 % methanol to make the final concentration 5 mg/ml of C. grandis and 20 mg/ml of C. garrettiana.
TLC densitometry method
Three microliters of 15 dichloromethane extracted samples of C. grandis and C. garrettiana and aloeemodin standard solutions were applied on the TLCsilica gel 60 GF254 20×10 cm plate (E. Merck, Germany) using a Camag Linomat 5 automatic sample spotter (Camag, Switzerland) under a flow of nitrogen gas. Each sample band was set at 10 mm and distance between bands was 8.9 mm. The TLC plates were developed in a Camag glass twin-through chamber (20×10 cm), which was pre-saturated in mobile phase of hexaneethyl acetate (1:1 v/v) for 1 h at room temperature. The plate was scanned under wavelength at 434 nm using TLC scanner 3 (Camag, Switzerland) with winCATS software. Aloe-emodin contents in C. grandis and C. garrettiana leaves extract were quantitated by peak area. The test was done in triplicate.
TLC image analysis using ImageJ software
TLC plate was photographed under ultraviolet light at 254 nm by a digital camera. Quantitative analysis of the aloe-emodin contents in C. grandis and C. garrettiana leaves extract and the colour intensity of spot on TLC plates were observed using ImageJ software (Department of Health and Human Services, National Institutes of Health, United State). The test was done in triplicate.
Method validation
TLC densitometry and TLC image analysis of aloeemodin contents in C. grandis and C. garrettiana leaves extracts were validated in terms of calibration range, accuracy, repeatability, intermediate precision, limit of detection (LOD), limit of quantitation (LOQ), specificity and robustness according to the International Council for Harmonisation guidelines (ICH Q2R1) [20].
The calibration range was constructed by analysis of five concentrations of standard aloe-emodin in the range 0.12, 0.24, 0.36, 0.48 and 0.60 μg/spot. A plot of average area under curve versus concentration was obtained. The calibration range was expressed as the correlation coefficient (r2). The accuracy of the analytical procedure was performed by recovery of spiking known three concentrations of standard aloeemodin in the sample. The accuracy was determined as recovery of aloe-emodin in percent. The repeatability and intermediate precision were determined in the same day and in the three different days and expressed as percent relative standard deviation (% RSD). LOD and LOQ were calculated from the calibration curve as 3.3 (SD/S) and 10 (SD/S), respectively where, SD was the standard deviation of regression line and S was the slope of regression line.
The specificity of the method was ascertained by analysing the standard solutions and sample extracts. The bands for aloe-emodin in samples were confirmed by comparing the Rf value and UV absorbance spectra of the bands with those of standard. The peak purity of sample was assessed by comparing the overlay spectra of standard aloe-emodin and sample extracts at three different positions, peak start, peak apex and peak end positions of the spot detected at 434 nm. The robustness of the method was done by slightly changing the suitable mobile phase ratio in the experiment and interpreted as % RSD of peak areas.
Data analysis of C. grandis and C. garrettiana
The aloe-emodin contents obtained using TLC densitometry and TLC image analysis were compared by paired t-test statistical analysis.
Results and Discussion
The chromatographic condition for quantitating aloeemodin contents was examined using silica gel 60 GF254. The selected mobile phase, hexane-ethyl acetate (1:1 v/v) demonstrated the best separation of aloeemodin in C. grandis and C. garrettiana leaves with Rf value 0.50±0.007 and 0.50±0.009, respectively. The aloe-emodin band of the plant samples was confirmed by comparing an Rf value with standard aloe-emodin.
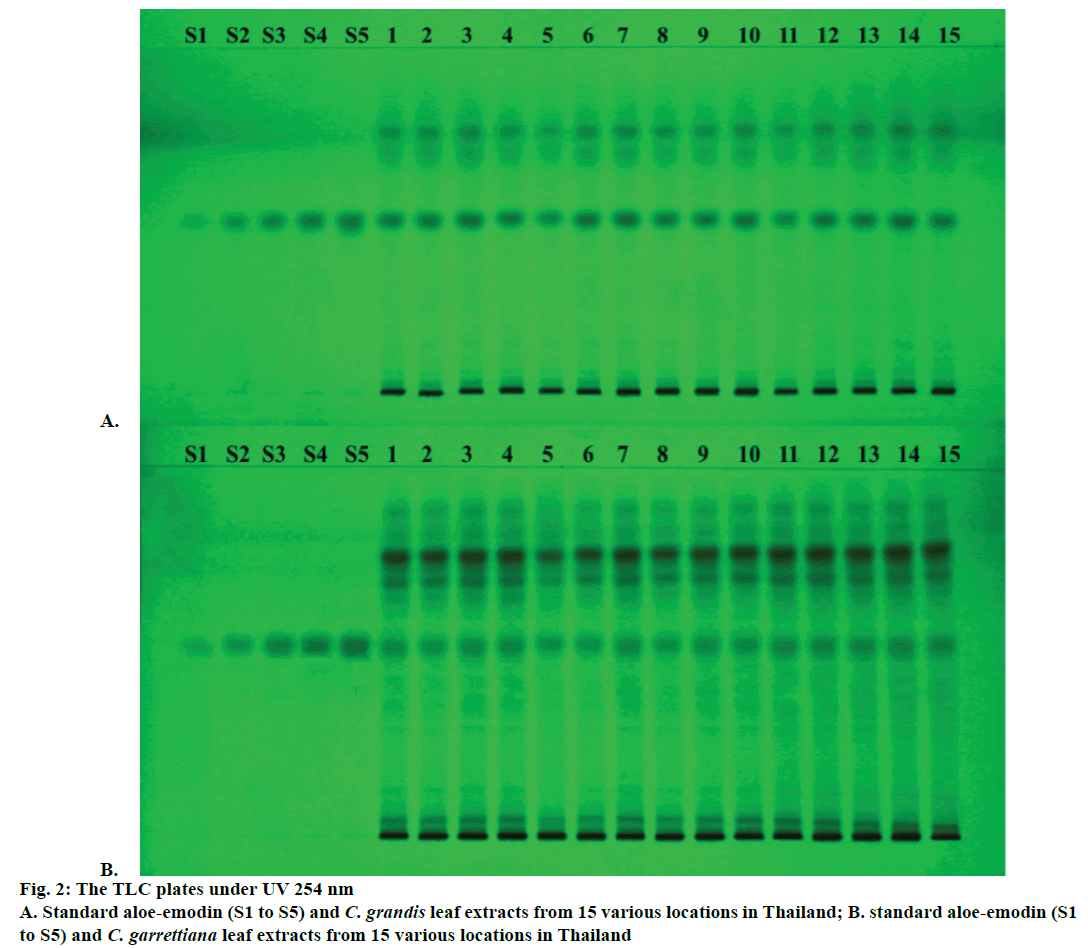
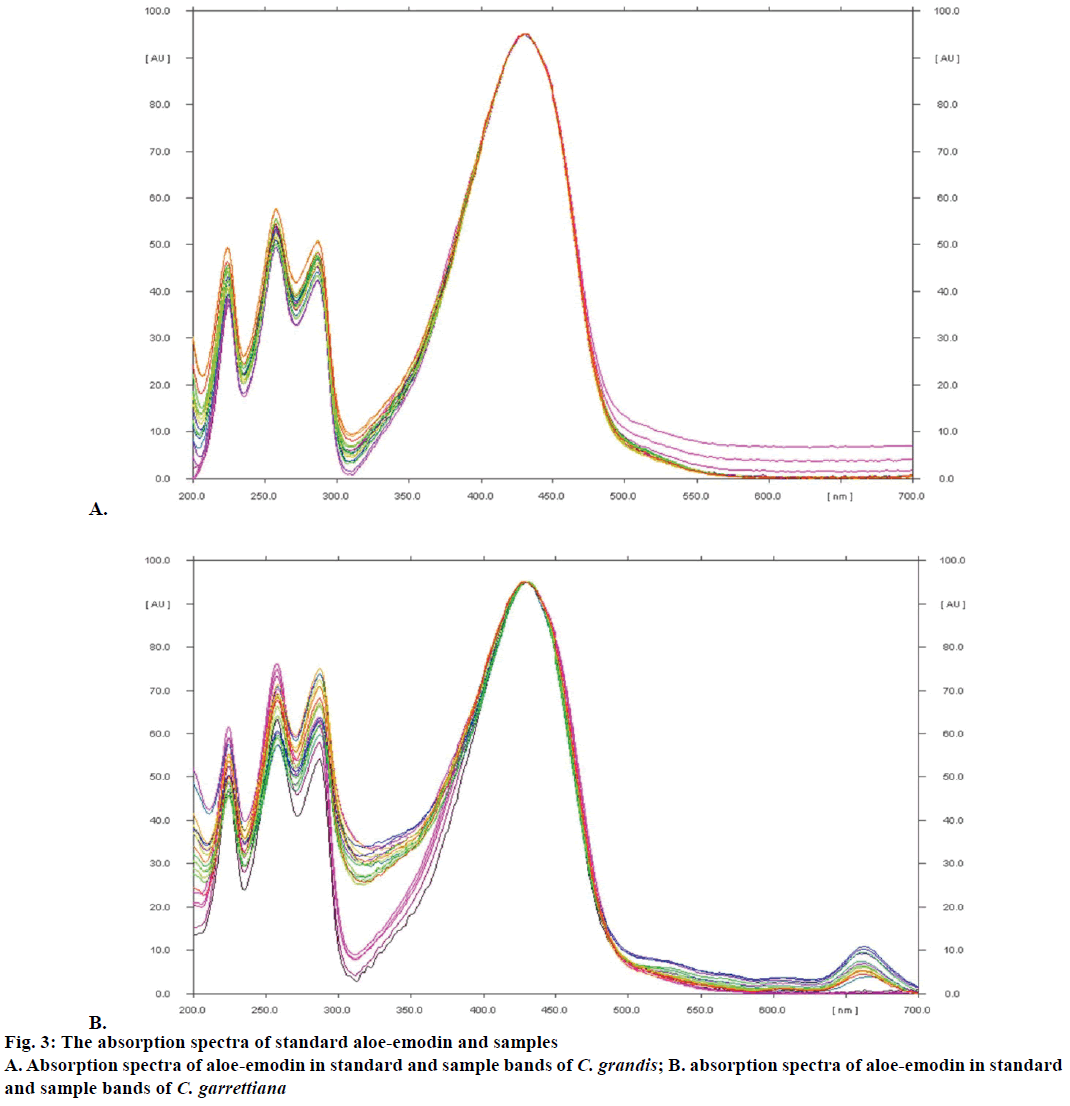
The yield of dichloromethane extract by Soxhlet extraction and aloe-emodin contents determination using TLC densitometry and TLC image analysis of C. grandis and C. garrettiana leaves were presented in Tables 1 and 2, respectively. TLC chromatogram of C. grandis and C. garrettiana under UV 254 was shown in Figure 2A and B, respectively. TLC densitogram of C. grandis and C. garrettiana scanned in the range of 200-700 nm was shown in Figure 3A and B, respectively. Aloe-emodin contents using TLC densitometry and TLC image analysis in C. grandis dried crude drug were 0.4122±0.0668 and 0.4130±0.0751 g %. The highest and lowest aloe-emodin contents were observed in the samples from Prachin Buri and Nakhon Ratchasima province, respectively. The aloeemodin contents in C. garrettiana dried crude drug using TLC densitometry and TLC image analysis were 0.0346±0.0067 and 0.0351±0.0056 g %. The highest aloe-emodin content was observed in the samples from Phetchabun province, whereas the lowest content was obtained from the samples from Khon Kaen province. A statistical comparison of the quantitative analysis using TLC densitometry and TLC image analysis of aloe-emodin contents in C. grandis and C. garrettiana leaves were not statistically significant (p>0.05) using paired t-test.
| Source (Province in Thailand) |
Extract yield (%) | Aloe-emodin contents (g % of dried crude drug) | |
|---|---|---|---|
| TLC densitometry | TLC image analysis | ||
| Ubon Ratchathani | 13.5253 | 0.3738±0.0120 | 0.3891±0.0097 |
| Surin | 13.7510 | 0.3858±0.0066 | 0.4196±0.0134 |
| Si Sa Ket | 13.9805 | 0.3931±0.0076 | 0.4059±0.0055 |
| Chaiyaphum | 14.8775 | 0.4124±0.0073 | 0.4420±0.0158 |
| Nakhon Ratchasima | 12.0755 | 0.2663±0.0052 | 0.2592±0.0146 |
| Nakhon Sawan | 13.6750 | 0.4321±0.0137 | 0.3905±0.0127 |
| Phichit | 13.6764 | 0.4670±0.0125 | 0.4428±0.0168 |
| Phitsanulok | 14.6409 | 0.4400±0.0154 | 0.4456±0.0114 |
| Sukhothai | 14.3488 | 0.4272±0.0071 | 0.4318±0.0275 |
| Uttaradit | 14.2157 | 0.4548±0.0119 | 0.4431±0.0138 |
| Pathum Thani | 12.2809 | 0.3286±0.0067 | 0.3071±0.0128 |
| Nakhon Pathom | 15.1001 | 0.4669±0.0238 | 0.4778±0.0159 |
| Prachin Buri | 15.7777 | 0.5228±0.0160 | 0.5192±0.0070 |
| Phra Nakhon Si Ayutthaya | 15.2663 | 0.4776±0.0142 | 0.5182±0.0266 |
| Bangkok | 12.2121 | 0.3349±0.0054 | 0.3023±0.0139 |
| Average | 13.9602±1.1229 | 0.4122±0.0668 | 0.4130±0.0751 |
Table 1: Extract yield and aloe-emodin contents in C. grandis leaves
| Source (Province in Thailand) |
Extract yield (%) | Aloe-emodin contents (g % of dried crude drug) | |
|---|---|---|---|
| TLC densitometry | TLC image analysis | ||
| Bangkok | 9.4392 | 0.0429±0.0013 | 0.0442±0.0014 |
| Prachin Buri | 9.1868 | 0.0406±0.0016 | 0.0409±0.0009 |
| Nakhon Pathom | 8.9384 | 0.0387±0.0021 | 0.0386±0.0018 |
| Kanchanaburi | 7.9699 | 0.0346±0.0015 | 0.0307±0.0009 |
| Chon Buri | 7.0660 | 0.0244±0.0007 | 0.0267±0.0013 |
| Nonthaburi | 8.9855 | 0.0346±0.0023 | 0.0400±0.0028 |
| Prachuap Khiri Khan | 9.5884 | 0.0415±0.0031 | 0.0375±0.0029 |
| Lop Buri | 8.5373 | 0.0356±0.0015 | 0.0355±0.0020 |
| Phetchabun | 9.8464 | 0.0460±0.0009 | 0.0424±0.0018 |
| Uttaradit | 7.9638 | 0.0326±0.0016 | 0.0365±0.0014 |
| Maha Sarakham | 8.1402 | 0.0328±0.0008 | 0.0336±0.0016 |
| Khon Kaen | 7.2331 | 0.0240±0.0024 | 0.0279±0.0024 |
| Nakhon Ratchasima | 7.9457 | 0.0272±0.0020 | 0.0285±0.0021 |
| Rayong | 8.0455 | 0.0278±0.0030 | 0.0290±0.0012 |
| Sukhothai | 9.6732 | 0.0356±0.0015 | 0.0343±0.0006 |
| Average | 8.5706±0.8846 | 0.0346±0.0067 | 0.0351±0.0056 |
Table 2: Extract yield and aloe-emodin contents in C. garrettiana leaves
The method was validated for its linearity, specificity, accuracy, precision, LOD, LOQ and robustness. The linear calibration curves of aloe-emodin contents were ranged from 0.12-0.60 μg/spot. The specificity was confirmed by comparing UV spectrum of the peak in standard aloe-emodin and all 15 samples. The result showed the maximum absorbance at the wavelength of 434 nm (Figure 2B). Good correlation of both samples was also obtained between standard and sample overlay spectra (r2>0.9950). The recovery values of both methods within accepted limit (98.16-103.38 and 97.58-107.86 % for C. grandis and 98.17-105.53 and 97.35-105.94 % for C. garrettiana). The repeatability and the intermediate precision of both methods of C. grandis and C. garrettiana were less than 3 % RSD.
The LOD and LOQ of TLC densitometry and TLC image analysis were found to be 0.0198 and 0.0601 μg/ spot and 0.0171 and 0.0517 μg/spot for C. grandis and 0.0214 and 0.0648 μg/spot and 0.0188 and 0.0568 μg/ spot for C. garrettiana. The robustness was performed by slightly changing composition of mobile phase (hexane-ethyl acetate 1:1, 0.9:1.1, 1.1:0.9 v/v) showed the values of 0.28 % RSD in TLC densitometry and 0.50 % RSD in TLC image analysis of C. grandis, whereas these values were 0.56 and 0.58 % RSD in C. garrettiana. The validity of TLC densitometry and TLC image analysis of C. grandis and C. garrettiana was presented in Table 3, respectively.
| Parameter | TLC densitometry | TLC image analysis |
|---|---|---|
| C. Grandis | ||
| Accuracy (% recovery) | 98.16-103.38 | 97.58-107.86 |
| Precision: Repeatability (% RSD) | 0.42-1.09 | 0.42-0.97 |
| Precision: Intermediate precision (% RSD) | 0.84-2.20 | 0.91-2.40 |
| Limit of detection (µg/spot) | 0.0198 | 0.0171 |
| Limit of quantitation (µg/spot) | 0.0601 | 0.0517 |
| Robustness (% RSD) | 0.28 | 0.50 |
| C. Garrettiana | ||
| Accuracy (% recovery) | 98.17-105.53 | 97.35-105.94 |
| Precision: Repeatability (% RSD) | 0.55-1.08 | 0.30-0.50 |
| Precision: Intermediate precision (% RSD) | 0.87-1.28 | 1.03-1.30 |
| Limit of detection (µg/spot) | 0.0214 | 0.0188 |
| Limit of quantitation (µg/spot) | 0.0648 | 0.0568 |
| Robustness (% RSD) | 0.56 | 0.58 |
Table 3: Method validity of tlc densitometry and tlc image analysis of aloe-emodin contents in C. grandis and C. garrettiana leaves
Aloe-emodin is a major component in the leaf extracts of C. grandis and C. garrettiana and this compound was employed as marker for TLC densitometry and TLC image analysis in the present study. The amounts of aloe-emodin showed a variation quantities in plant materials collected from various locations in Thailand as the chemical constituent contents in herbal plant can be vary with the plant origin, harvest season, environmental factor and herbal preparation method [21]. This data will be useful as guidance for sample collection of C. grandis and C. garrettiana that contained high aloe-emodin contents in Thailand. The aloe-emodin contents of C. grandis and C. garrettiana leaves from 15 various locations in Thailand obtained from TLC densitometry and TLC image analysis were compared using paired t-test statistical analysis. It was indicated that the aloe-emodin contents in C. grandis and C. garrettiana leaves from both methods were not significantly different with p>0.05. Both methods could be used as an alternative method for routine quantitative analysis of major compounds in the C. grandis and C. garrettiana leaves extracts. According to ICH guidelines, the analytical method was validated to confirm that the analytical procedure employed reliable and accurate data. The linear calibration curves of aloe-emodin contents in C. grandis and C. garrettiana leaves using both methods showed good linearity relationships in range of 0.12-0.60 μg/ spot with correlation coefficient (r2) more than 0.99. The identical absorption spectrum of standard aloeemodin in this study showed the maximum absorbance at 434 nm, which is in accordance to the previous study indicated that maximum UV absorption spectrum of aloe-emodin could be detected at 430 nm [22]. The recovery assay in both methods was accurate. Determination of aloe-emodin contents in C. grandis and C. garrettiana repeatedly within and between set of experiments by both methods revealed acceptable precisions. LOD and LOQ value from both methods of C. grandis and C. garrettiana confirmed that the lowest concentration of standard aloe-emodin (0.12 μg/spot) used in this study were suitable. The robustness studied by changing composition of mobile phase indicated that changing composition of mobile phase was not affected in both methods.
This is the first report of the validated TLC densitometric method and TLC image analysis using ImageJ software for quantitation of aloe-emodin contents in dichloromethane extract of C. grandis and C. garrettiana leaves collected from 15 different locations in Thailand. The data obtained from this study may be valuable for indicating alternative sources of aloe-emodin. Due to C. grandis and C. garrettiana distributed throughout Thailand, the supply of the leaves material will be easily available. Statistical analysis indicated that the aloe-emodin contents determined using TLC densitometric method and TLC image analysis showed no significantly different with p>0.05 hence TLC image analysis may be used as an alternative method for quantitative analysis of C. grandis and C. garrettiana leaves due to its rapid, simple, precise, accurate and cost effectiveness.
Acknowledgements
The authors are grateful to Naresuan University for a Ph. D. scholarships and research fund; the 90th Anniversary of the Chulalongkorn University fund (Ratchadaphiseksomphot Endowment Fund). The authors wish to thank Mr. Woratouch Thitikornpong and College of Public Health Sciences, Chulalongkorn University for providing research facilities. The authors also wish to thank Dr. Nijsiri Ruangrangsi, College of Public Health Sciences, Chulalongkorn University and Faculty of Pharmacy, Rangsit University, Thailand for authenticating the plant specimens.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Gritsanapan W, Somsak N. Variation of anthraquinone content in Cassia surattensis. Warasan Phesatchasat 2001;28:28-34.
- Kumar T, Ahmed R, Nagori K, Singh M, Dewangan D. Phytochemical estimation of anthraquinones from Cassia species. Int J Res Ayurveda Pharm 2011;2:1320-23.
- Yeh FT, Wu CH, Lee HZ. Signalling pathway for aloe-emodin-induced apoptosis in human H460 lung nonsmall carcinoma cell. Int J Cancer 2003;106:26-33.
- Eshun K, He Q. Aloe vera: a valuable ingredient for the food, pharmaceutical and cosmetic industries. Crit Rev Food Sci Nutr 2004;44:91-6.
- Fenig E, Nordenberg J, Beery E, Sulkes J, Wasserman L. Combined effect of aloe-emodin and chemotherapeutic agents on the proliferation of an adherent variant cell line of Merkel cell carcinoma. Oncol Rep 2004;11:213-7.
- Lin KY, Uen YH. Aloe-emodin, an anthraquinone, in vitro inhibits proliferation and induces apoptosis in human colon carcinoma cells. Oncol Lett 2010;1:541-7.
- Tewtrakul S, Subhadhirasakul S, Rattanasuwan P. HIV-1 protease inhibitory effects of some selected plants in Caesalpiniaceae and Papilionaceae families. Songklanakarin J Sci Technol 2003;25:509-14.
- Boonyapraphatsara N, Chokchaicharoenporn A. Thai Native Herbs. Vol IV. Bangkok: Prachachon Press; 1998. p. 1-683.
- Gritsanapan W, Jirawongse V, Tantiseawie B. Cassia grandis L. a new source of aloe-emodin. J Pharm Sci 1983;10:6-8.
- Mondranondra I, Tantivatana P, Jirawongse V, Reutrakool V. A phytochemical study of the leaves of Cassia garrettiana Craib. J Health Res 1978;5:119-24.
- Shewiyo DH, Kaale E, Risha PG, Dejaegher B, Smeyers VJ, Heyden YV. HPTLC methods to assay active ingredients in pharmaceutical formulations: A review of the method development and validation steps. J Pharm Biomed Anal 2012;66:11-23.
- Soponar F, Mot AC, Sârbu C. Quantitative determination of some food dyes using digital processing of images obtained by thin-layer chromatography. J Chromatogr A 2008;1188:295-300.
- Padumanoda T, Suntornsuk L, Gritsanapan W. Quantitative analysis of barakol content in Senna siamea leaves and flowers by TLC-densitometry. Med Princ Pract 2007;16:47-52.
- Ketmongkhonsit P, Chaichantipyuth C, Palanuvej C, Thitikornpong W, Sukrong S. A validated TLC-image analysis method for detecting and quantifying bioactive phyllanthin in Phyllanthus amarus and commercial herbal drugs. Songklanakarin J Sci Technol 2015;37:319-26.
- Maneechai S, Likhitwitayawuid K, Sritularak B, Palanuvej C, Ruangrungsi N, Sirisa-ard P. Quantitative analysis of oxyresveratrol content in Artocarpus lakoocha and Puag-Haad. Med Princ Pract 2009;18:223-7.
- Chewchinda S, Sithisarn P, Gritsanapan W. Thin layer chromatography (TLC)-densitometric method for chemical stability evaluation of Cassia fistula pod pulp extract: an alternative laxative drug. J Med Plant Res 2012;6:4940-5.
- Sakunpak A, Suksaeree J, Monton C, Pathompak P. Development and quantitative determination of barakol in Senna siamea leaf extract by TLC-image analysis method. Int J Pharm Pharm Sci 2014;6:267-70.
- Pitakpawasutthi Y, Thitikornpong W, Palanuvej C, Ruangrungsi N. Chlorogenic acid content, essential oil compositions, and in vitro antioxidant activities of Chromolaena odorata leaves. J Adv Pharm Technol Res 2016;7:37-42.
- Sakulpanich A, Gritsanapan W. Extraction method for high content of anthraquinones from Cassia fistula pods. J Health Res 2008;22:167-72.
- ICH Harmonized Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology, Q2 (R1). Geneva: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; 2005.
- Kunle OF, Egharevba HO, Ahmadu PO. Standardization of herbal medicines-A review. Int J Biodivers Conserv 2012;4:101-2.
- Nadia M, Giancarlo lM, Marzia I, FrancoF V, Nicoletta C-P, Anacleto M. Cell cultures of Ajuga reptans L. to bio convert emodin and aloe–emodin: an HPLC/ESI/MS investigation. Enz Microb Technol 2005;36:399-408.