- *Corresponding Author:
- A. Kumar
Department of Molecular Biology and Genetic Engineering, College of Basic sciences and Humanities, Pantnagar-263 145, India
E-mail: ak_gupta2k@rediffmail.com
| Date of Submission | 20 December 2016 |
| Date of Revision | 28 April 2017 |
| Date of Acceptance | 16 December 2017 |
| Indian J Pharm Sci 2018;80(1):161-172 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In the present study, the antidiabetic properties of Cinnamomum tamala were investigated. The ethanol extract of the leaf of C. tamala was used for investigating its effect against diabetes using a rat model. Different doses of the ethanol extract of C. tamala was administered orally to alloxan-induced diabetic rats for 15 d and the effect of treatment on blood glucose level, glycosylated haemoglobin and peroxidation products such as thiobarbituric acid reactive substances and serum lipids were monitored. Gas chromatography-mass spectroscopy and high performance liquid chromatography techniques were used for identification and quantification of the active principles of the ethanol extract responsible for exerting antioxidant and antidiabetic effects. Oral administration of the ethanol extract of C. tamala resulted in significant decrease in the blood glucose levels, glycosylated haemoglobin, thiobarbituric acid reactive substances and serum lipids in diabetic rats under investigation. It was also observed that the oral administration of ethanol extract of C. tamala significantly decreased the levels of reduced glutathione and superoxide dismutase in the hepatic and renal tissues of alloxan-induced diabetic rats. These results provided evidence for the antidiabetic, antioxidant and antihyperlipidemic effects of the ethanol extract of C. tamala when administered orally. On the basis of these findings it can be concluded that extracts of C. tamala should be investigated further for predicting their clinical potential as a phytomedicine for treating diabetes mellitus.
Keywords
Diabetes mellitus, C. tamala, alloxan, antidiabetic, antioxidant
Diabetes mellitus, characterized by chronic hyperglycaemia with disturbance of carbohydrate, fat and protein metabolism resulting from a defect in insulin secretion or insulin action or both has become one of the major challenges in contemporary world. Great rise in the prevalence of diabetes has made it a major public health challenge in India, which is now considered as the diabetic capital of the world with over 20 million diabetics, which is projected to cross the 87 million mark by the year 2030 [1]. The evolution of numerous chronic complications of diabetes mellitus correlates with the severity and duration of hyperglycaemia [2]. Prolonged occurrence of hyperglycaemia contributes to the dysfunction of several metabolic factors leading to increased glucose flux through the polyol pathway as well as increased production of reactive oxygen species by the mitochondrial respiratory chain, nonenzymatic glycations, protein kinase-C activation and increased flux through the hexosamine pathway [3]. Hyper-physiological burden of free radicals causes an imbalance between oxidants and antioxidants in the body. The coexistence of diabetes with the reduction in antioxidant capacity leads to the deleterious effects. Management of type-2 diabetes requires rational pharmacotherapy and a stepwise approach with lifestyle modifications, oral antidiabetic drugs including insulin therapy. Although there are a number of synthetic medicines available in the market for the diabetic patients, these oral hypoglycaemic agents are reported to produce adverse side effects on the body. These limitations of present pharmacotherapy have opened avenues for developing the alternative green medicines that are not only safe but also easily accessible and economic.
The Indian medicinal plant Cinnamomum tamala Nees and Eberm or Indian bay leaf is an evergreen, medium-sized tree belonging to the family Lauraceae. The leaves, known as tejpat, tejpatta, or tejpata in Hindi, tamalpatra in Marathi, and Indian Cassia in English, are usually olive green in colour, may have some brownish spots and have three veins running down the length of the leaf. The leaves of this tree have medicinal properties and are reported to be used in treatment of numerous ailments. The produce of the plant is also used as food, fodder, medicine and timber in Uttarakhand state as well as in other Himalayan regions of India [4]. Various kinds of leaf extracts of C. tamala have previously been reported to have antiinflammatory [5], antioxidant [6], antiulcer [7], anticarcinogenic [8], antidiarrhoeal [9] and antidiabetic [10] properties.
The present study investigated the chemical composition of polar and non-polar extracts of the leaves of C. tamala, with respect to its total flavonoid and total phenol contents. The antioxidant activity of the polar extract was examined through a 1,1-diphenyl- 2-picrylhydrazyl (DPPH) assay. The quantification of antioxidant compounds of C. tamala was investigated through high pressure liquid chromatographic technique, while the identification of various chemical constituents was conducted mainly with Gas chromatography-mass spectrometry (GC-MS). Finally, the potential of the ethanol extract of C. tamala (CTEE) leaves in the modulation of diabetic complications was explored in vivo in rat models.
Materials and Methods
Leaves of C. tamala were collected from Medicinal Plants Research and Development Centre (MRDC), G. B. Pant University of Agriculture & Technology, Pantnagar. Chemical reagents such as, DPPH, catechin, gallic acid, standard eugenol were procured from Sigma. All other chemicals and reagents used were of analytical and HPLC grade.
Extract preparation
For preparation of aqueous (CTCWE: C. tamala cold water extract, CTHWE: C. tamala hot water extract) and alcoholic extracts of C. tamala, properly washed and shade dried leaves were powdered in an electrical grinder and stored at room temperature until further used. About 10 g of dry leaf powder was mixed in 100 ml of distilled water at room temperature and at 60° for the preparation of aqueous cold/hot extracts, respectively, in conical flasks covered with parafilm. Ethanol extract was prepared by mixing 10 g of dry leaf powder in 100 ml of ethanol. The flasks were kept in shaker incubator overnight at 37° at 120 rpm. Supernatant was filtered with muslin cloth and filtered twice with Whatman No. 1 filter paper. The supernatant collected was dried to form gel in a fan equipped incubator at 50°. Water extract were lyophilized and all the extracts were stored at 4° until used.
Extraction yield of extracts
The yield (%, w/w) from all the dried extracts was calculated as: yield (%) = (W1×100)/W2, where W1 is the weight of the extract after lyophilization of the solvent and W2 is the weight of the plant powder initially taken for extract preparation.
Phytochemical analysis
Preliminary qualitative phytochemical tests were carried out on the aqueous and ethanol extracts to determine the biologically active compounds that contribute to the flavour, colour and other characteristics of C. tamala leaves using standard qualitative methods described by Trease and Evans [11].
Determination of the total phenolic content
Total phenolics in the extracts were determined using Folin-Ciocalteau reagents [12] using gallic acid as a standard. The amount of total phenolic compounds in the Cinnamomum extracts was determined in micrograms of gallic acid equivalent, using the equation obtained from the standard gallic acid graph: absorbance = 0.015×(total phenols (microgram of gallic acid equivalent)−0.055; R2= 0.994
Determination of the total flavonoid content
Flavonoid content was determined according to the colorimetric assay [13] using catechin as a standard. The amount of total flavonoid compounds in the Cinnamomum extracts was expressed in microgram of catechin equivalent, using the Eqn. obtained from the standard catechin graph, absorbance = 0.009×(total flavonoid (microgram of catechin equivalent)−0.01; R2= 0.999
Determination of the free radical scavenging activity
The free radical scavenging activity of aqueous and ethanol extracts was measured in terms of hydrogen donating or radical scavenging ability using the stable radical DPPH [14]. Five millilitres of freshly prepared DPPH (0.004 % w/v) solution in 95 % methanol was added to 1 ml of various concentrations (20-100 μg/ml) of extracts dissolved in their respective solvents. The absorbance was measured at 517 nm after 30 min of incubation. Percentage of scavenging activity was calculated using following formula: DPPH radical scavenging (%) = (Abs of control–Abs of test sample)/ Abs of control×100. The IC50 values of various extracts were calculated from the regression equations prepared from the concentrations of extracts and percentage inhibition of free radical formation. The results were compared with ascorbic acid taken as standard.
Quantification of antioxidant component by high performance liquid chromatography (HPLC)
HPLC coupled to diode array detector was used to analyse antioxidant (eugenol) compound of Cinnamomum samples using a separation module (Agilent 1120 HPLC system) equipped with reverse phase Zorbax C18 (4.6×250 mm, particle size 5 μm) analytical column (Agilent) with injection volume of 20 μl. The samples were eluted using HPLC grade water:methanol:acetonitrile in ratios of 45:35:20 v/v at a flow rate of 1.0 ml/min at the run time of 15 min. The peak of eugenol was monitored at 210 nm wavelength. Identification of eugenol peak in C. tamala was achieved in comparison with the retention time of the standard. The eugenol was quantified from their peak areas and the calibration curves of the corresponding standard was made using appropriate dilutions of stock solution (1000 μg/ml each) i.e., in the concentration ranges of 0.02-0.2 μg/ml prepared in mobile phase and later expressed as μg/ml.
Experimental animals
Twenty four Sprague Dawley rats, weighing between 220 to 250 g were procured from Laboratory Animal Resource Centre, IVRI, Izzatnagar. Rats were divided randomly and equally into four groups (I, II, III and IV) and were maintained as per IAEC recommendations. IAEC-approved protocol was followed in conducting the experiments.
Induction of diabetes
For induction of diabetes, overnight fasted rats in groups II, III and IV were administrated a single intraperitoneal injection of a freshly prepared aqueous solution of alloxan (ALX) at a dose of 90 mg/kg within a minute. Group I rats were injected with distilled water as vehicle control. Fasting blood glucose (FBG) was estimated at the time of induction of diabetes and post-prandial glucose was checked regularly. The animals were considered as diabetic, if their blood glucose values were above 250 mg/dl on the 2nd d after ALX injection. The treatment was started on the 3rd d after ALX injection, which was considered as the 1st d of treatment. The treatment was continued for 15 d. Body weights were measured before induction of diabetes and on the day of sacrifice i.e., on the 15th d for diabetic, treated and normal rats.
Experimental design
Twenty four Sprague Dawley rats were divided into four groups of six rats in each. Group I rats were treated as control. Diabetes was induced by single intraperitoneal dose of ALX monohydrate (90 mg/kg) in rats of groups II, III and IV. Group II rats were treated as diabetic control. Metformin (300 mg/kg/bid) in 10 % ethanol solution was given to group III rats and C. tamala (250 mg/kg/bid) in 10 % ethanol solution was given to group IV rats.
Collection of rat liver, kidney and blood
After the experimental regimen, the animals were fasted overnight and sacrificed by cervical dislocation under mild anaesthesia. Blood was collected on decapitation and serum was separated by centrifugation at 2500 rpm for 15 min. The whole blood and serum collected was used for biochemical estimations. The liver and kidney were excised immediately and thoroughly washed with ice-cold physiological saline and stored at –20° until further used.
Biochemical parameters
Blood samples were obtained from tail vein of all experimental animals and FBG concentration was determined using one-touch ultra-glucometer (Ascensia Entrust) at regular time intervals i.e., 0 (before ALX injection), 2nd and 15th d (after ALX injection) of experiment. The results were expressed in terms of milligrams per decilitre of blood. Glycated haemoglobin (HbA1c) was estimated in whole blood (Excel diagnostics kit). Hepatic glycogen was measured according to the anthrone-H2SO4 method using glucose as standard [15].
Determination of serum lipid profile
Serum total cholesterol, high density lipoprotein (HDL) cholesterol and triglyceride levels were estimated by using ERBA Diagnostics kit. Very low density lipoprotein (VLDL) and low density lipoprotein (LDL) cholesterol were calculated as (mg/dl) per Friedwald’s Eqn., VLDL cholesterol = triglyceride/5; LDL cholesterol = total cholesterol–(VLDL+HDL cholesterol).
Determination of antioxidative enzyme in tissue homogenate
Frozen liver and kidney samples were partially thawed and 200 mg of each sample was weighed and taken in 2 ml of ice-cold saline for lipid peroxidation (LPO) and superoxide dismutase (SOD) estimation. Again, 200 mg of sample was weighed separately and taken in 2.0 ml of 0.02 M EDTA for reduced glutathione (GSH) estimation. Fresh samples from organ homogenates were prepared using homogenizer under cold condition. The homogenate was centrifuged for 10 min at 3000 rpm. The supernatant was used for estimation of LPO [16], SOD [17] and GSH [18] contents.
Histopathology
Liver and kidney pieces were processed for the histopathological examinations. Ten percent formalin fixed tissue pieces were first serially dehydrated in alcohol and acetone and then embedded in paraffin blocks. Then, micro sections (4-5 microns thick) of tissues were cut and stained in haematoxylin and eosin stain [19] and examined for histopathological changes.
Statistical analysis
Data were expressed as means±standard deviation (SD) of three replicate determinations. Correlation analyses of antioxidant activity (Y) versus the total phenolic/total flavonoid content (X) were carried out using the correlation and regression programme using Microsoft office Excel program. Statistical analysis of in vivo data was done by using ANOVA at 5 % level of significance [20].
Characterization of ethanol extracts via GCMS analysis
CTEE was dissolved in ethanol and filtered to obtain a stock solution of 1.0 mg/ml. The analysis of components of extract was performed using gas chromatograph 7890 A with mass selective detector MS 5975C (Agilent Technologies, USA) fitted with a DB-1MS fused silica column (25 m×0.2 mm; 0.33 μm film thickness) which was operated in EI mode. Ion source temperature was maintained at 250°. The mass spectrum of compounds in samples was obtained by electron ionization at 70 eV. Helium (99.99 %) was used as the carrier gas with a flow rate of 1.5 ml/min in the split mode (25:1). GC oven temperature started at 40° (for 5 min) rising 150° at a 5°/min without holding. The temperature was then allowed to increase to 250° at a 20°/min and maintained at 250° for 2 min. The oven temperature was finally ramped to 290° at a rate of 20°/min and kept for 5 min at 290°. An aliquot of 1 μl of CTEE was injected into the column with the injector temperature at 250°. Identification of components was based on the molecular structure, molecular mass and calculated fragments.
Results and Discussion
The extraction yields for the aqueous and CTEE are shown in Table 1. The ethanol extract showed the highest extraction yield while the lowest yield was found in cold water extract. Preliminary phytochemical screening of the aqueous (hot and cold) and ethanol extracts of C. tamala showed the presence of various phytoconstituents including alkaloid, steroids, terpenoids, flavonoids, saponins. As summarized in Table 2, alkaloids, steroids, terpenoids and flavonoids presence were detected in all the extracts in varying concentrations, however saponins were found only in aqueous extract while tannin was absent in all the extracts. Eugenol was quantified by HPLC method. Diversity of medicinal plants and spices containing various phytochemicals with biological activity serve as viable source of drugs for the world population. Several phenolics, flavonoids and alkaloids possess marked antidiabetic activities [21]. In a previous study, terpenoids and flavonoids have been reported to increase the concentration of antioxidants [22]. Rupasinghe et al. [23] have reported that saponins possessed hypocholesterolemic and antidiabetic properties. The presence of these phytochemicals supports the claim for the medicinal uses of C. tamala as potent antioxidant and hypoglycaemic agent.
| Samples | Extraction yield (%) | Phenolic content (mg GAE/g extract) |
Flavonoid content (mg CE/g extract) |
|---|---|---|---|
| CTEE | 1.6 | 435.1±3.72 | 296.16±0.00 |
| CTHWE | 3.8 | 212.94±5.6 | 92.5±0.00 |
| CTCWE | 8.6 | 112.94±3.72 | 55.5±0.00 |
Table 1: Extraction yield, Phenolic and Flavonoid content of both aqueous and alcoholic extracts of C. tamala
| Tests | Extracts | ||
|---|---|---|---|
| CTCWE | CTHWE | CTEE | |
| Alkaloids | + | + | + |
| Tannins | - | - | - |
| Steroids | +++ | +++ | ++ |
| Terpenoids | + | +++ | +++ |
| Saponins | +++ | ++ | - |
| Flavonoids | ++ | ++ | +++ |
Table 2: Phytochemical analysis of C. tamala extracts for the presence of bioactive constituents
Total phenolic and flavonoid contents of ethanol extracts were significantly higher than that of hot and cold aqueous extracts of C. tamala as represented in Table 1. Ethanol extract contains more phenolic and flavonoid compounds than aqueous extracts because ethanol is less polar than water and due its low polarity it can release the cell wall bound polyphenols from cells and also can neutralize the activity of polyphenol oxidase which degrades the polyphenols in plants [24]. Yin et al. [25] reported the similar results with greater phenolic and flavonoid contents in alcoholic extract than water extracts. These high concentrations of polyphenols in ethanol extract also lead to its higher antioxidant activity which could be due to its proton donating ability [26] and scavenging of both active oxygen species and electrophiles, inhibit nitrosation, chelate metal ions and have potential for autooxidation and the capability to modulate certain cellular enzyme activities [27]. Aljadi et al. [28] also reported higher potent antioxidant activity of methanol extract compared to water extract of C. tamala. Sofowora [29] reported a positive correlation between antioxidant activity and total phenols/flavonoid contents, which was in accordance with the present study inferring that the antioxidant activity of C. tamala is directly related to their phenolic constituents.
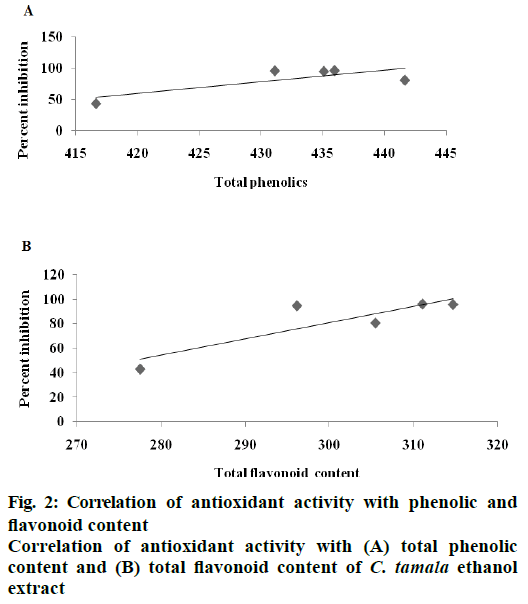
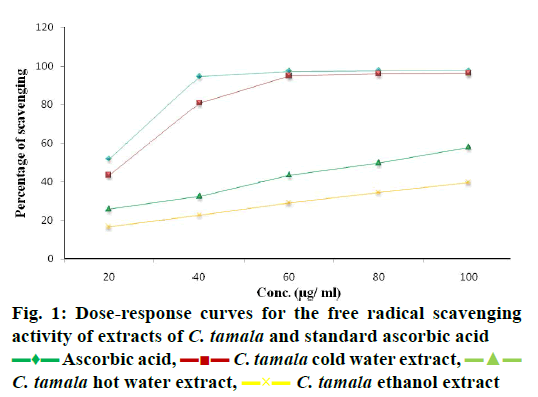
DPPH radical scavenging activity measured in terms of IC50 of the various extracts of C. tamala compared with ascorbic acid is depicted in Figure 1. The concentration dependent percent radical inhibition was higher in ethanol extract (96.18 %) than hot (57.82 %) and cold (39.57 %) water extracts which was comparable to ascorbic acid (97.5 %). The scavenging activities of different extracts of Cinnamomum and ascorbic acid were in the order as ascorbic acid>CTEE>CTHWE>CTCWE. An inverse relation was observed between IC50 values and the antioxidant activity as CTEE showed the lowest IC50 value in comparison to the aqueous extracts (Table 3). A significant positive correlation between total phenol/ flavonoid contents and DPPH with CTEE was also observed (Figure 2). Oxidative stress has been implicated in the pathology of many diseases and conditions including diabetes, hence, various extracts of C. tamala were subjected to quantitative analyses of polyphenols including phenolics and flavonoids as they majorly contribute to the resistance against the oxidative stress by scavenging free radicals, inhibiting LPO among many other mechanisms.
| Extracts/standards | IC50 (µg/ml) |
|---|---|
| Ascorbic acid | 16.13 |
| CTCWE | 134.32 |
| CTHWE | 80.02 |
| CTEE | 22.14 |
Table 3: Evaluation of IC50 values of standard and various extracts of C. tamala
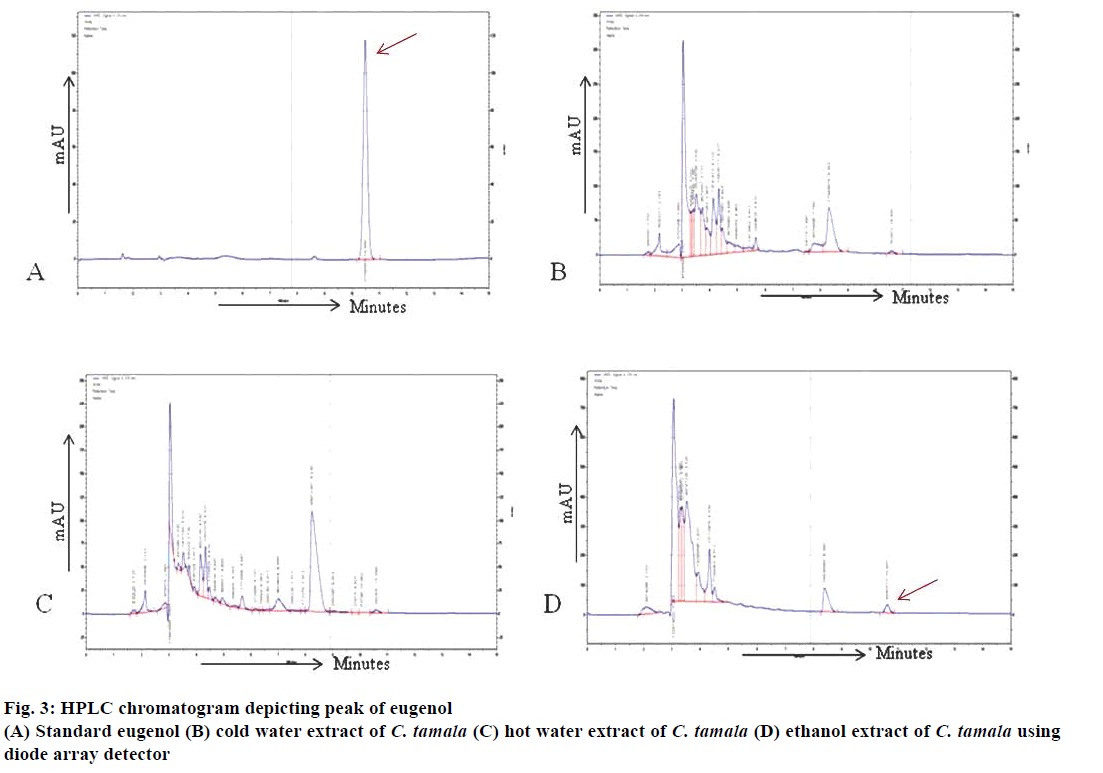
The retention time of eugenol was identified to be at 10.47 min in standard solution and at 10.57, 10.59 and 10.60 min in CTCWE, CTHWE and CTEE, respectively, under similar conditions as shown in (Figure 3A, B, C and D). The determination of eugenol content in extracts was performed by extrapolating standard concentration vs. peak area. The system was calibrated by quantifying the pure eugenol 99 % (Sigma) standard solutions (0.2-2.0 mg/l). The standard calibration curve was linear with a correlation coefficient of 0.993. The relative concentrations of the eugenol were estimated to be 0.03, 0.02 and 0.16 mg/l in cold water, hot water and ethanol extracts, respectively (Table 4). Kapoor et al. [30] also identified eugenol as the major compound while cinnamaldehyde was present in minor quantities in the oil and oleoresins extracted from the leaves of C. tamala. These compounds were reported to have potential antioxidant and antidiabetic activities.
| Component/extract | RT | RSD (%) | Quantity (mg/l) |
|---|---|---|---|
| Eugenol | 10.47±0.01 | 0.05 | - |
| CTCWE | 10.57±0.03 | 0.32 | 0.03 |
| CTHWE | 10.59±0.00 | 0.04 | 0.02 |
| CTEE | 10.60±0.00 | 0.04 | 0.16 |
Table 4: Amount of eugenol and percent relative standard deviation in different extracts of C. tamala
Phytoconstituent profiling of CTEE by GC-MS is one of the most accurate and available techniques to identify the constituents contributing to the medicinal quality of a plant. The studies on the active principles of ethanol extracts of C. tamala leaves by GC-MS analysis clearly showed the presence of forty constituents of phytochemical which included various hydrocarbons, aliphatic-saturated and unsaturated acids, alkyl halides, aromatic esters, aldehyde compounds, phenolic compounds, cinnamaldehyde compounds, palmitic acid ester and linoleic acid ester (Table 5). These components are known to possess many different biological activities. Some of them singly or in combination might be contributing for its antidiabetic effect as it is known that steroids, terpenoids and polysaccharides compounds possess antidiabetic activity [29].
| S. No. | R.T. | Name of the compound | Molecular formula | Molecular weight | Area % |
|---|---|---|---|---|---|
| 1 | 3.489 | 9,12,15-Octadecatrienoic acid, 2-[(trimethylsilyl)oxy] -1-[[(trimethylsilyl)oxy]methyl]ethyl ester,(Z,Z,Z)- | C27H52O4Si2 | 496 | 4.902 |
| 2 | 3.818 | Silane, triethylfluoro- | C6H15FSi | 134 | 2.202 |
| 3 | 3.885 | 9,10-Secocholesta-5,7,10(19)-triene-3,24,25-triol, (3ß,5Z,7E)- | C27H44O3 | 416 | 0.938 |
| 4 | 3.975 | Cetyl acetate | C18H36O2 | 284 | 0.667 |
| 5 | 4.050 | Deoxyspergualin | C17H37N7O3 | 387 | 0.632 |
| 6 | 4.211 | 9,12-Octadecadienoyl chloride,(Z,Z)- | C18H31ClO | 298 | 0.535 |
| 7 | 4.773 | Tetraethyl silicate | C8H20O4Si | 208 | 2.053 |
| 8 | 4.932 | 1,3,5-Pentanetriol,3-methyl- | C6H14O3 | 134 | 1.670 |
| 9 | 5.026 | 2-Butanol,3,3'-oxybis- | C8H18O3 | 162 | 1.586 |
| 10 | 5.053 | Dodecanoic acid,3-hydroxy- | C12H24O3 | 216 | 1.951 |
| 11 | 5.236 | Acetic acid,6-morpholin-4-yl-9-oxobicyclo[3.3.1]non-3-yl ester | C15H23NO4 | 281 | 1.035 |
| 12 | 5.270 | 1,2-Cyclopentanedicarboxylic acid,4-(1,1-dimethylethyl)-,dimethyl ester,(1a,2ß,4ß)- | C13H22O4 | 242 | 0.653 |
| 13 | 5.492 | 2-Myristynoyl pantetheine | C25H44N2O5S | 484 | 0.831 |
| 14 | 5.606 | 1,3-Isobenzofurandione,5-nitro- | C8H3NO5 | 193 | 1.165 |
| 15 | 5.656 | 6-(1-Methylethyl)-4,4,6-trimethyltetrahydro-1,3-oxazin-2-thione | C10H19NOS | 201 | 0.948 |
| 16 | 5.699 | 1-Ethyl-4-(4-phenyl-2-thiazolyl)piperidine | C16H20N2S | 272 | 0.792 |
| 17 | 5.968 | Dithiocarbamate,S-methyl-,N-(2-methyl-3-oxobutyl) - | C7H13NOS2 | 191 | 0.879 |
| 18 | 6.033 | a-D-Glucopyranoside,O-a-D-glucopyranosyl-(1.fwdarw.3)- ß-D-fructofuranosyl | C18H32O16 | 504 | 1.455 |
| 19 | 6.091 | Benzoic acid, 4-methyl-,[4-(methoxycarbonyl) phenyl]methyl ester | C17H16O4 | 284 | 1.400 |
| 20 | 6.680 | Cinnamaldehyde,ß-methyl- | C10H10O | 146 | 1.165 |
| 21 | 6.725 | 1-Dodecanol,3,7,11-trimethyl- | C15H32O | 228 | 0.276 |
| 22 | 6.795 | Geranylisovalerate | C15H26O2 | 238 | 0.941 |
| 23 | 6.866 | Thymol | C10H14O | 150 | 1.116 |
| 24 | 7.049 | Eugenol | C10H12O2 | 164 | 5.682 |
| 25 | 7.252 | Isoeugenol (E) | C10H12O2 | 164 | 2.142 |
| 26 | 7.600 | 7-Methyl-Z-tetradecen-1-ol acetate | C17H32O2 | 268 | 0.969 |
| 27 | 7.842 | Tetradecane,2,6,10-trimethyl- | C17H36 | 240 | 0.412 |
| 28 | 8.887 | 4,6-di-tert-butyl-m-cresol | C15H24O | 220 | 2.06 |
| 29 | 8.977 | Cholesta-8,24-dien-3-ol,4-methyl-,(3ß,4a)- | C28H46O | 398 | 0.126 |
| 30 | 9.739 | 3,6-Diazahomoadamantan-9-one hydrazone | C9H16N4 | 180 | 0.281 |
| 31 | 9.769 | 3',8,8'-Trimethoxy-3-piperidyl-2,2'-binaphthalene-1,1',4,4'-tetrone | C28H25NO7 | 487 | 0.453 |
| 32 | 10.575 | Cinnamic acid | C31H40O15 | 652 | 0.372 |
| 33 | 15.159 | Cyclopropanenonanoic acid,2-[(2-butylcyclopropyl) methyl]-,methyl ester | C21H38O2 | 322 | 0.337 |
| 34 | 16.307 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 | 10.555 |
| 35 | 16.621 | Decanoic acid, methyl ester | C11H22O2 | 186 | 6.253 |
| 36 | 18.230 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284 | 1.751 |
| 37 | 20.563 | 9-Octadecenoic acid (Z)-, methyl ester | C19H36O2 | 296 | 5.621 |
| 38 | 20.614 | Methyl 13-octadecenoate | C19H36O2 | 296 | 1.304 |
| 39 | 20.679 | Linoleic acid ethyl ester | C20H36O2 | 308 | 1.891 |
| 40 | 20.714 | 10-Octadecenoic acid, methyl ester | C19H36O2 | 296 | 1.700 |
Table 5: Various phytochemical components identified in ethanol leaf extracts of C. tamala by gc-ms analysis
ALX-induced diabetic rats were chosen as the animal models because this type of diabetes resembles with many of the features of human diabetes mellitus [31]. As intraperitoneal injection of ALX causes a massive reduction of β cells of islets of langerhans and in turn, reduces the blood glucose levels in healthy rats; this route of administration was selected in this investigation. It was observed that ALX (90 mg/kg) administration decreased body weight in group II, which was partially restored or improved upon administration of metformin (300 mg/kg/bid) and CTEE (250 mg/kg/bid) in groups III and IV after 15 d of treatment (Table 6). An increase in the body weights in CTEE-treated diabetic rats was observed, which might be due to the improvement in insulin secretion and glycemic control. Body weight gain was also previously reported in rats treated with other plants such as Ficus bengalensis and Trigonella foenum greacum [32]. Treatment with CTEE for 15 d in ALXinduced diabetic rats significantly reduced the blood sugar level up to near normal in this study. Several previous studies indicated the glucose lowering effect of different extracts of Tinospora cordifolia [33] and Gymnema sylvestre [34], which were in accordance to hypoglycaemic effect of CTEE in this study. The possible hypoglycaemic mechanism might be through potentiation of pancreatic secretion of insulin from β-cell of islets or through extra pancreatic mechanism i.e. due to enhanced transport of blood glucose to the peripheral tissues, inhibition of gluconeogenesis or increased glycolysis by the liver cells, inhibition of renal glucose reabsorption [32], inhibition of insulinase activity in both liver and kidney [35].
| Group | Treatments | Initial (g) | Final (g) |
|---|---|---|---|
| I | Normal control | 228±8.6 | 244±6.8 |
| II | Diabetic control | 236±7.5 | 211±7.8 |
| III | Diabetic+metformin | 228±8.6 | 232±7.4* |
| IV | Diabetic+extract | 226±7.8 | 236±4.9* |
Table 6: Effect of ethanol extract of C tamala on body weight in alloxan-induced diabetic and treated rats
FBG, HbA1c and liver glycogen: the rats exposed to ALX developed diabetes as evident from the significant elevation of FBG level as compared to control. Blood sugar in metformin and CTEE-treated groups declined to normoglycemic level with values of 110±1.4 and 130±1.1 as compared to 355±3.6 in diabetic control after treatment at 15th d (Table 7).
| Group | Treatment | Glucose concentration (mg/dl) |
HbA1c (%) |
Glycogen content (µg/ml) | ||
|---|---|---|---|---|---|---|
| 0th | 2nd | 15th | ||||
| I | Normal control | 76.2±2.3 | 77.8±1.4 | 77.8±2.6 | 6.82±0.3 | 36.14±3.0 |
| II | Diabetic control | 76.8±2.4 | 344±5.6 | 355±3.6 | 11.32±0.4 | 3±0.8 |
| III | Diabetic+metformin | 83.8±3.5 | 351.6±4.8 | 110±1.4* | 7.30±0.4** | 15±2.5* |
| IV | Diabetic+extract | 84.8±3.5 | 341.6±5.4 | 130±1.1* | 8.28±0.2** | 8.71±1.7* |
Table 7: Effect of ethanol extract of C. tamala on fasting blood glucose, hba1c and liver glycogen in alloxan-induced diabeticrats
A significant (p<0.05) increase in glycosylated haemoglobin was observed in diabetic control when compared to control rats (Table 7). Administration of metformin and CTEE significantly (p<0.05) decreased the levels of glycosylated haemoglobin in group III and IV near to normal level after 15 d.
The glycogen content of rat liver decreased in diabetic rats of group II (3±0.83) when compared to normal control of group I (36.14±2.95) rats but these levels increased significantly (p<0.01) to near normal after administration of metformin and extract in group III and IV, respectively after 15 d (Table 7). Glycosylated haemoglobin is produced progressively and irreversibly through glycosylation of haemoglobin over a period of time and subsequent browning reaction is enhanced by increased glucose levels, and this glycation itself further induces the formation of oxygen-derived free radicals. It is stable till the life of the RBC and is unaffected by diet, insulin or exercise on the day of test. Therefore, it is an excellent marker of overall glycemic control. HbA1c was found to increase in patients with diabetes mellitus and is directly proportional to the FBG level. A significant decline in HbA1c in CTEE-treated rats observed in this investigation, which could be due to the result of improved glycemic control by the extracts. Udayakumar et al. [36] reported decrease in HbA1c after the treatment with Withania somnifera root and leaf extracts on ALX-induced diabetic rats.
Increase in triglycerides, total cholesterol, LDL-c, VLDL-c and fall in HDL-c was observed in diabetic rats of group II in comparison to normal control (group I), which indicated that rise in blood sugar is accompanied by the increase in triglyceride, total cholesterol, LDL-c, VLDL-c and fall in HDL-c. Administration of metformin and CTEE significantly (p<0.05) decreased the level of these lipids along with increase of HDL-c in group III and IV after 15 d as shown in Table 8. Hyperlipidaemia is a common finding in patients with diabetes mellitus and is responsible for vascular complications [37]. Administration of CTEE reduced the levels of total cholesterol, triglyceride, LDL-c, VLDL-c and increased HDL-c in diabetic rat in our findings, which might be due to improvement in insulin release. Hypolipidemic activities of ethanol extract of Coccinia cordifolia and Catharanthus roseus were also reported earlier by Akhtar et al. [38] in ALXinduced diabetic rat. Though these plants belong to different families but possess antidiabetic as well as antilipidemic properties. Considering the diversity in plants led to differ in chemotyping of such plants. However, such plants possess several different types of antidiabetic compounds but their mode of action may be due to interaction with different components of insulin signalling pathways and thus exerting similar hypoglycaemic effects by these plants also.
| Group | Treatment | TC (mg/dl) |
TG (mg/dl) |
HDL (mg/dl) |
LDL (mg/dl) |
VLDL (mg/dl) |
|---|---|---|---|---|---|---|
| I | Normal control | 94.03±3.8 | 101.32±3.0 | 34.38±2.0 | 39.4±1.3 | 20.26±0.6 |
| II | Diabetic control | 124.03±3.6 | 247.84±6.2 | 14.16±1.2 | 60.3±1.2 | 49.57±1.2 |
| III | Diabetic+metformin | 88.87±5.1* | 119.52±7.0* | 31.8±2.4* | 33.17±1.3* | 23.91±1.4* |
| IV | Diabetic+extract | 87.23±2.8* | 147.78±3.3* | 27.37±0.9* | 30.3±1.3* | 29.56±0.7* |
Table 8: Effect of ethanol extract of C. tamala on TC, TG, HDL, VLDL and LDL in alloxan-induced diabetic rats
Levels of GSH and SOD was significantly (p<0.05) lower in group II diabetic rats than control which subsequently returned to normalcy in treatment groups III and IV both in liver and kidney (Table 9). Hyperglycaemia can increase oxidative stress and changes the redox potential of GSH [39] due to increased utilization of antioxidants. An increase in GSH level after administration of CTEE in ALX-induced diabetic rats might be either due to improvement in biosynthesis of GSH or reduced oxidative stress suppressing degradation of GSH or by combination of both effects. Sefi et al. [40] reported an increase in GSH content in pancreas after administration of Centaurium erythrea in streptozotocin-induced oxidative stress.
SOD has an antitoxic effect against the superoxide anion. Activation of SOD accelerates dismutation of superoxide radicals to H2O2 which is removed by catalase [41]. H2O2, a reaction product of the SOD reaction, inactivates SOD, as it acts like a pro-oxidant in the presence of H2O2. The increased SOD activity observed in this study could have been due to its induction by increased production of superoxide. Feshani et al. [42] reported an increased SOD content after administration of single dose of Vaccinium arctostaphylos, extract in ALX-induced diabetic rats. The extract of Rosmarinus officinalis activated various antioxidant enzymes including SOD in diabetic rats [43].
The ALX diabetic rats (group II) showed a significant (p<0.05) increase in thiobarbituric acid reactive substance (TBARS) when compared with control (group I) and subsequently decreased to normalcy in treatment groups III and IV both in liver and kidney (Table 9). LPO is the process where oxygen interacts with polyunsaturated fatty acids. When this process occurs in biological membrane, gross alteration of structural, organizational and enzyme function may result. Previous studies reported that administration of herbal medication including Terminalia arjuna [44]; Ficus bengalensis [45] reduced the TBARS. The outcome of the present study also showed that, oral administration of Cinnamomum tamala significantly decreased the level of LPO. This indicated that CTEE had potential to scavenge or inhibit the free radical formation and stabilize the membrane lipids effectively and prevent the liver and kidney damage from oxidative stress.
| Groups | Treatments | Parameters | |||||
|---|---|---|---|---|---|---|---|
| GSH (nM/g wet tissue) |
SOD (U) |
LPO (nM/g wet tissue) |
|||||
| Kidney | Liver | Kidney | Liver | Kidney | Liver | ||
| I | Normal control | 0.28±0.01 | 0.29±0.01 | 4.32±0.07 | 4.22±0.5 | 7.17±0.7 | 5.81±0.4 |
| II | Diabetic control | 0.08±0.008 | 0.08±0.01 | 1.36±0.08 | 1.35±0.1 | 20.2±1.7 | 19.37±1.2 |
| III | Diabetic+metformin | 0.35±0.05* | 0.44±0.05* | 4.23±0.0* | 4.07±0.1* | 7.40±0.8* | 6.30±0.3* |
| IV | Diabetic+extract | 0.26±0.02* | 0.24±0.01* | 4.02±0.1* | 3.7±0.1* | 9.05±0.4* | 8.28±0.4* |
Table 9: Effect of Ethanol Extract Of C.tamala on Antioxidative Enzymes In Alloxan-Induced Diabetic Rats
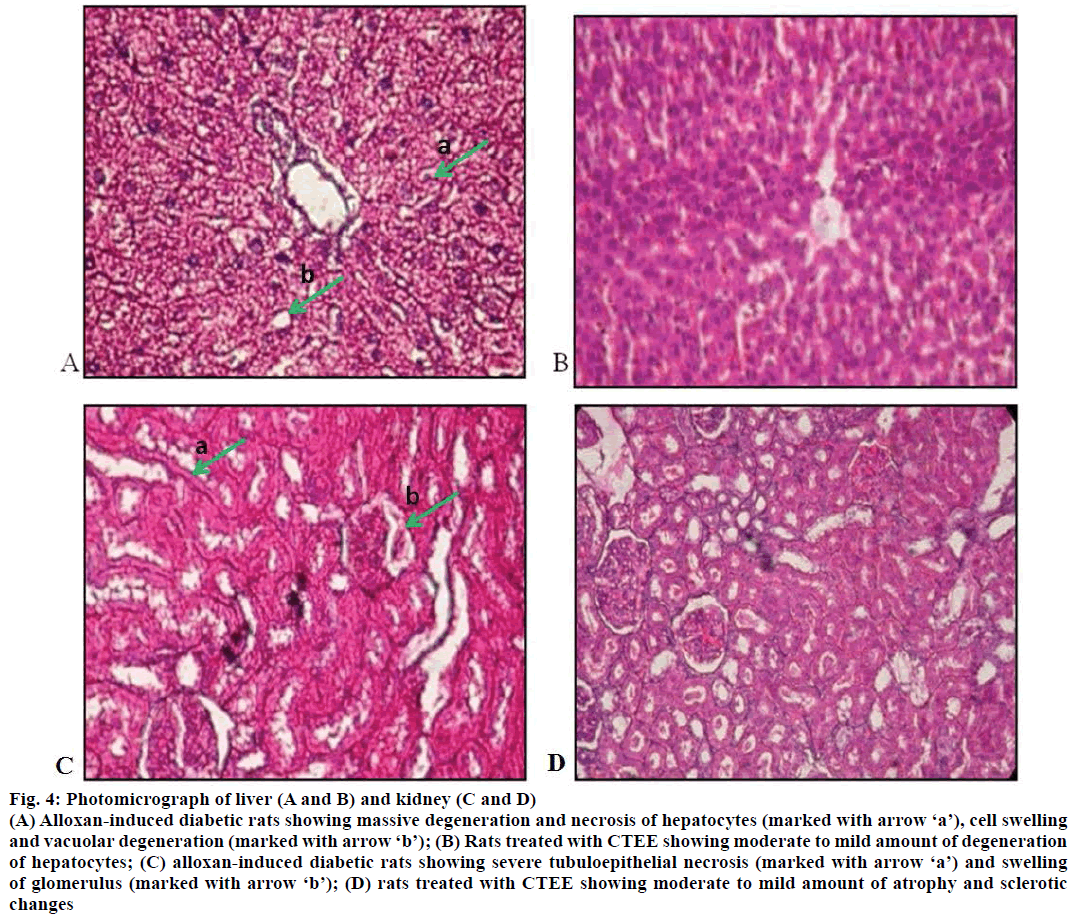
The effects of CTEE on histopathology of liver and kidney in ALX-induced diabetic rats are shown in Figure 4. Microscopic examination of liver of ALXinduced diabetic control rats revealed massive degeneration and necrosis of hepatocytes, and cell swelling with vacuolar degeneration (Figure 4A). Rats treated with CTEE showed moderate to mild amount of degeneration of hepatocytes (Figure 4B). Histopathological changes in kidney of ALX-induced diabetic control rats were characterized by severe tubulo-epithelial necrosis and swelling of glomerulus (Figure 4C), however CTEE-treated rats showed moderate to mild atrophy and sclerotic changes (Figure 4D).
Figure 4: Photomicrograph of liver (A and B) and kidney (C and D)
(A) Alloxan-induced diabetic rats showing massive degeneration and necrosis of hepatocytes (marked with arrow ‘a’), cell swelling and vacuolar degeneration (marked with arrow ‘b’); (B) Rats treated with CTEE showing moderate to mild amount of degeneration of hepatocytes; (C) alloxan-induced diabetic rats showing severe tubuloepithelial necrosis (marked with arrow ‘a’) and swelling of glomerulus (marked with arrow ‘b’); (D) rats treated with CTEE showing moderate to mild amount of atrophy and sclerotic changes
The study showed that, although the levels of glycogen in CTEE-treated diabetic rats could not achieve the level same as that in control, yet it significantly elevated the hepatic glycogen contents in comparison to diabetic control. This might be because of enhanced rate of glycogenesis or inhibited glucogenolysis due to stimulation of insulin release resulting in improved mobilization of blood glucose towards liver glycogen reserve or storage [46]. Sharma et al. [47] observed elevated glycogen content in Aegle marmelos-treated diabetic mice. Treatment of streptozotocin-induced diabetic rats with Aloe vera extract was also forced to increase glycogen amount in liver and muscle tissues by Kumar et al. [48] Administration of CTEE in ALXinduced diabetic rats helped in rapid recovery from damage to kidney and liver. Shairibha and Rajadurai [49] also observed the protective effects of glymin on pathological changes in streptozotocin-induced diabetic rats. Histological examination of pancreatic islets, kidney glomeruli, and liver was restored to its normal size after administration of Helicteres isora Linn. (Sterculiaceae) root extracts in ALX-induced diabetic rats [50].
The phytochemical screening including total phenolics, total flavonoids and the DPPH radical scavenging activity proved the antioxidant activity of Cinnamomum tamala extract. These findings revealed the potential of the extract as a source of natural antioxidants. The ethanol extract was found to have high concentrations of active principles, which upon furthur investigation appeared to have potential antidiabetic activity. It is concluded from this study that CTEE (250 mg/kg bid) for 15 d in ALX-induced diabetic rats revealed potent antioxidant, hypolipidemic and antidiabetic efficacy and thus, indicates towards its potential prospects for being developed as a phytomedicine for use in therapeutics of diabetes mellitus in humans. In this order, we recommend further evaluation of the antidiabetic efficacy of C. tamala extract on human subjects under proper ethical guidelines to launch a new orally administered herbal antidiabetic medicine in the market.
Acknowledgements
The authors are gratefully acknowledged to the Dean, College of Basic Sciences and Humanities, and the Director, Experiment Station, G. B. Pant University of Agriculture and Technology, Pantnagar for providing all necessary help and facilities for carrying out this research.
Conflict of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- http://www.diabetesatlas.org/.
- https://pdfs.semanticscholar.org/6161/8e6d4d2977a8f7b4fa4429d0ef0b0d4d782b.pdf.
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813-20.
- Nautiyal S, Kaechele H. Adverse impact of pasture abandonment in Himalayas of India: testing efficiency of a natural resource management plan (NRMP). Environ Impact Assess Rev 2007;27:109-25.
- Gambhire MN, Juvekar AR, Wankhede SS. Anti-inflammatory activity of aqueous extract of Cinnamomum tamala leaves by in vivo and in vitro methods. J Pharm Res 2009;2:1521-4.
- Gupta V, Sharma M. Protective Effect of Cinnamomum tejpata on lipid peroxide formation in isolated rat liver homogenate. Curr Res J Biol Sci 2010;2:246-9.
- Lima ZP, Severi JA, Pellizzon CH, Brito AR, Solis PN. Gastroprotective activity of Cinnamomum tamala leaves on experimental gastric ulcers in rats. J Ethnopharmacol 2010;128:537-40.
- Rao AR, Hashim S. Chemopreventive action of oriental food-seasoning spices mixture Garam masala on DMBA-induced tranplacental and translactational carcinogenesis in mice. Nutr Cancer 1995;23:91-101.
- Rao CV, Vijayakumar M, Sairam K, Kumar V. Anti-diarrhoeal activity of the standardized extract of Cinnamomum tamala in experimental rats. J Nat Med 2008;62:396-402.
- Kumanan R, Manimaran, S, Khan, S, Dhanabal, SP, Nanjan MJ. Screening of bark of Cinnamomum tamala (Lauraceae) by using a-amylase inhibition assay for anti-diabetic activity. Int J Pharmacol Biomed Res 2010;1:69-72.
- Trease GE, Evans WCA. Physician?s Guide to Herbal Medicine. In: Evans WC, Trease GE, editors. Trease and Evans? Pharmacognosy 13th ed. London: Bailliere Tindall; 1989. p. 176-80.
- Singleton VL, Rossi JA Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1965;16:144-58.
- Kim D, Jeond S, Lee CH. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem 2003;81:321-6.
- Hasan MS, Ahmed MI, Mondal S, Uddin SJ, Masud MM, Samir Kumar S, et al. Antioxidant, antinociceptive activity and general toxicity study of Den-drophthoe falcata and isolation of quercitrin as the major component. Orient Pharm Exp Med 2006;6:355-60.
- Sadasivam S, Manickam A. Carbohydrates. In: Sadasivam S, Manickam A. Methods in Biochemistry. New Delhi: New Age International Pvt Ltd.; 1996. p. 11-2.
- Rehman S. Lead induced regional lipid peroxidation in brain. Toxicol Lett 1984;21:333.
- Madesh M, Balasubramanian KA. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys 1998;35:184-8.
- Sedlak J, Linday RH. Estimation of total, protein bound, and nonprotein sulfhydryl groups in tissue with Ellman?s reagent. Anal Biochem 1968;25:192-205.
- Lillie RD. Histopathologic technic and practical histochemistry. 3rd ed. New York: McGrawHill Co.; 1965, p.715.
- Snedecor GW, Cochran WG. Statistical Methods. 8th ed. Ames: Iowa State University Press; 1989.
- Hsu F, Chen Y, Cheng J Caffeic. Acid as active principle form the fruit of Xanthium strumarium to lower plasma glucose in diabetic rats. Planta Medica 2000;66:228-30.
- Luo J, Cheung J, Yevich E. Novel terpenoidtype quinones isolated from Pycnanthu angolensis of potential utility in the treatment of type-2 diabetes. J Pharm Exp Ther 1999;288:529-34.
- Rupasinghe HP, Jackson CJ, Poysa V, Di Berardo C, Bewley JD, Jenkinson J. Soyasapogenol A and B distribution in Soybean (Glycine max L.Merr) in relation to seed physiology, genetic variability and growing location. J Agric Food Chem 2003;51:5888-94.
- Nagavani V, Madhavi Y, Bhaskar Rao D, Koteswara Rao P, Raghava Rao T. Free radical scavenging activity and qualitative analysis of polyphenols by RP-HPLC in the flowers of Couroupita guianensis Abul. Electron. J Enviorn Agric Food Chem 2010;9:1471-84.
- Yin J, Heo S, Wang MH. Antioxidant and antidiabetic activities of extracts from Cirsium japonicum roots. Nutr Res Pract 2008;2:247-51.
- Adesegun SA, Fajana A, Orabueze CI, Cooker HAB. Evaluation of antioxidant properties of Phaulopsis fascisepala C.BC.I. (Acanthaceae). Evid Based Complement Altern Med 2007;4:1-5.
- Zhang D, Hamauzu Y. Phenolics compounds and their antioxidant properties in different tissues of carrots (Daucus carota L.). J Food Agric Environ 2004;2:95-100.
- Aljadi AM, Kamaruddin MY. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem 2004;85:513-18.
- Sofowora LA. Medicinal plants and traditional medicine in Africa. Ibaban: Spectrum Books Ltd.; 1993. p. 55-71.
- Kapoor IPS, Singh B, Singh G, Isidorov V, Szczepaniak L. Chemistry, antimicrobial and antioxidant potentials of Cinnamomum tamala Nees and Eberm. (Tejpat) essential oil and oiloresins. Nat Prod Rad 2009;8:106-16.
- Etuk EU, Muhammed BJ. Evidence based analysis of chemical method of induction of diabetes mellitus in experimental rats. Int J Res Pharm Sci 2010;1:139-42.
- Solomon G, Raosaheb KK, Najma ZB. Effects of vanadate, insulin and fenugreek (Trigonella foenum graecum) on creatinine kinase levels in tissues of diabetic rats. Indian J Exp Biol 1999;37:200-2.
- Prince PSM, Menon VP. Hypoglycaemic and other related actions of Tinospora cardifolia roots in alloxan-induced diabetic rats. J Ethnopharmacol 2000;70:9-15.
- Shanmugasundaram ERB, Rajeshwari G, Baskaran X, Kumar BRR, Shanmugasundaram KR, Ahmath BK. (Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin dependent diabetes mellitus. J Ethnopharmacol 1990;30:281-94.
- Achrekar B, Kakij GS, Pote MS, Kelkar SM. Hypoglycaemic activity of Eugenia jambolana and Ficus bengalensis. In Vivo 1991;5:143-7.
- Udayakumar R, Sampath K, Thankaraj SM, Rajesh M, Anbazhagan VR, Kim SC, et al. Hypoglycaemic and Hypolipidaemic Effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int J Mol Sci 2009;10:2367-82.
- Valdivielso P, Puerta S, Rioja J. Postprandial apolipopreotein B48 is associated with asymptomatic peripheral arterial diseases, a study in patients with type 2 diabetes and controls. Clin Chim Acta 2010;411:433-7.
- Akhtar MA, Rashid M. Comparison of long-term antihyperglycemic and hypolipidemic effects between Coccinia cordifolia (Linn.) and Catharanthus roseus (Linn.) In alloxan-induced diabetic rats. J Med Med Sci 2007;2:29-34.
- Irshad M, Chaudhuri PS. Oxidant-antioxidant system; role and significance in human body. Indian J Exp Biol 2002;40:1233-9.
- Sefi M, Fetoui H, Lachkar N, Tahraoui A, Lyoussi B, Boudawara T, et al. Centaurium erythrea (Gentianaceae) leaf extract alleviates streptozotocin-induced oxidative stress and ß-cell damage in rat pancreas. J Ethnopharmacol 2011;135:243-50.
- Aebi H. Catalase In vitro. In: Colowick SPN, Kaplane O, editors. Methods in Enzymology. New York: Academic Press; 1984. p.121-26.
- Feshani AM, Kouhsari SM, Mohammadi S. Vaccinium arctostaphylos a common herbal medicine in Iran: Molecular and biochemical study of its antidiabetic effects on alloxan-diabetic Wistar rats. J Ethnopharmacol 2011;133:67-74.
- Bakirel T, Bakirel U, Keles¸ OU, Gunes¸ SU, Yardibi, H. In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan diabetic rabbits. J Ethnopharmacol 2008;116:64-73.
- Raghavan B, Kumari SK. Effect of Terminalia arjuna stem bark on antioxidant status in liver and kidney of alloxan diabetic rats. Indian J Physiol Pharm 2006;50:133-42.
- Nagalakshmi GCD, Rao SS, Fareeda G. Hypoglycemic effect of aqueous fruit extract of Ficus bengalensis in normal and streptozotocin induced diabetes rats. Bioscan 2010;5:197-200.
- Chakrabarti S, Biswas TK, Rokeya B, Ali L, Mosihuzzaman M, Nahar N, et al. Advanced studies on the hypoglycemic effect of Caesalpinia bonducella in type 1 and type 2 diabetes in Long Evans rats. J Ethnopharmacol 2003;84:41-6.
- Sharma B, Sarapathi SK, Roy P. Hypoglycemic and hypolipidemic effect of Aegle marmelos (L.) leaf extract on streptozotocin induced diabetic rats. Int J Pharm 2007;3:444-52.
- Kumar R, Sharma B, Tomar NR, Roy P, Gupta AK, Kumar A. In vivo evalution of hypoglycemic activity of Aloe vera spp. and identification of its mode of action on GLUT-4 gene expression in vitro. Appl Biochem Biotech 2011;164:1246-56.
- Shairibha SM, Rajadurai M. Effect of glymin, a polyherbal formulation on lipid profile and histopathological examination in streptozotocin-induced diabetic rats. Int J Pharm Sci Drug Res 2012;4:49-55.
- Venkatesh S, Reddy BM, Reddy GD, Mullangi R, Lakshman M. Antihyperglycemic and hypolipidemic effects of Helicteres isora roots in alloxan-induced diabetic rats: a possible mechanism of action. J Nat Med 2010;64:295-304.
 Ascorbic acid,
Ascorbic acid,  C. tamala cold water extract,
C. tamala cold water extract, C. tamala hot water extract,
C. tamala hot water extract,  C. tamala ethanol extract
C. tamala ethanol extract