- *Corresponding Author:
- S. Natkanktkul
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chiba, 260-8675, Japan
E-mail: surapolhsri@gmail.com
| Date of Submission | 17 May 2017 |
| Date of Revision | 13 December 2017 |
| Date of Acceptance | 10 July 2018 |
| Indian J Pharm Sci 2018;80(5): 795-801 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Systematic extraction and purification of β-glucan was attempted from molasses yeast (Saccharomyces cerevisiae) waste available from ethanol industry. The aim was to create value for the waste material by making it suitable for topical use in beauty industry. The process involved cell autolysis fractioning, solvent extraction and drying. The optimal extraction method of β-glucan gave an yield of 12.0 % (w/w) from molasses yeast waste. The β-glucan obtained was characterized using nuclear magnetic resonance spectroscopy and high-performance liquid chromatography. The effect of β-glucan on TNF-α and IL-6 levels was studied in vitro in lipopolysaccharide-activated RAW 264.7 cell line. The results indicated that β-glucan suppressed TNF-α and IL-6 production at 6 h and 24 h compared to the LPS control. Clinical evaluation of moisturizing potential of β-glucan was conducted on 22 volunteers over a period of 14 days. The results demonstrated an increase in hydration rate of 9%. The 48 h occlusive single patch test indicated that the β-glucan sample was non-irritating with mean cumulative irritation index value of 0. This study demonstrated that it is possible to create value from molasses yeast waste material, which could be industrially exploited to benefit the personal care industry in a sustainable economy.
Keywords
β-glucan, Saccharomyces cerevisiae, yeast waste material, extraction, immunomodulatory activity, moisturizing activity
Alternative eco-friendly sources of raw materials have been in focus especially in food and beauty industry to sustain the world environment. One of the interesting processes that generate large amount of valuable byprocess waste is fermentation of ethanol utilizing yeast. Saccharomyces cerevisiae is the most common species of yeast used in baker, brewer, and energy industry. Several studies have shown that the by-process yeast from the waste material is safe and beneficial. It is rich in protein, vitamin B, chitin and most importantly β-glucan. β-Glucan is a polysaccharide comprised of repeating glucose units and it is reported to be an immunomodulator and low-density lipoprotein cholesterol reducer[1-3]. Recently, most β-glucan skincare formulae claim that β-glucan provided moisture retention capability with an additional benefit of stabilizing the emulsion or gel-based products[4-6]. It is also shown to have wound healing property through mortality reduction resulting in lower infection and stronger tensile strength of scar tissue[7]. As a result, β-glucan could be used as immunomodulator that enhances macrophage activity leading to increased collagen biosynthesis in the wound healing process both in animals and human.
Lipopolysaccharide (LPS) is the major component of the outer membrane of Gram-negative bacteria such as Escherichia coli. Once LPS is recognized by toll-like receptor 4, it activates the innate immune system and promotes the secretion of proinflammatory cytokines including tumor necrosis factor (TNF-α). In addition, TNF-α is often induced together with interleukin-6 (IL-6). IL-6 regulates acute local and systemic inflammatory responses such as those elicited by either local lung or systemic exposure to endotoxin. IL-6 acts as both a pro-inflammatory cytokine and an antiinflammatory myokine. In addition, IL-6 plays an important pathological role in inflammatory and autoimmune diseases. Because of its pro-inflammatory character, it also has a significant function in the defence against pathogens and cancers[8].
In this study, the structure and composition of β-glucan obtained from the molasses yeast industry was determined using high-performance liquid chromatography (HPLC) and nuclear magnetic resonance spectroscopy (NMR). Furthermore, the effects of β-glucan on the production of IL-6 and TNF-α by the Abelson leukaemia virus transformed monocyte/ macrophage cell line (RAW 264.7) challenged with LPS were studied. Moreover, clinical evaluation of its moisturizing activity was also conducted on 22 volunteers over the period of 14 d. The purpose of this study is to evaluate the potential of industrial waste material to generate quality ingredients for health and beauty industries at a lower cost.
Materials and Methods
1H NMR spectra were recorded on a Jeol Resonance Delta T2 NMR spectrophotometer resonating at 600 MHz, and the chemical shifts were reported in parts per million (ppm). UV absorbance values were measured on a UV spectrophotometer (Tecan, Tokyo, Japan). LPS from E. coli 055:B5 (purified by phenol extraction) was purchased from Sigma-Aldrich, USA. Standard mouse TNF-α recombinant protein (1 μg/ml), standard mouse IL-6 recombinant protein (1 μg/ml), mouse TNF-α ELISA Ready-SET-Go and mouse IL-6 ELISA Ready-SET-Go were obtained from eBioscience, Inc., CA, USA. Dulbecco's modified eagle medium (DMEM) and 1 % penicillin-streptomycin solution were purchased from Nacalai Tesque, Inc., Kyoto, Japan. Fetal bovine serum 10 % was procured from Life Technology, Tokyo, Japan.
Raw material preparation
Yeast sludge was collected from ethanol production in Thailand. The mean±SE protein content of the starting molasses β-glucan (MBG) material was 32.10±1.25 %, pH of 4.33±0.31. Three batches of yeast cell were filtered and adjusted to 15 % dry solid content for the experiment. The solvent extraction of yeast β-glucan was optimized from Freimund’s method[9] to find the most optimal conditions. The yeast sludge was washed with distilled water twice and adjusted to 15 % dry matter at pH 5 prior to extraction. The autolysis process was performed under 4 conditions varying the time (16 or 24 h) and temperature (55° or 80°). After breaking the yeast cell wall with autolysis process and separating the yeast sludge through centrifugation, the concentration of the sediment was adjusted to 15 % dry solid. The sediment was then extracted with ethanol for 30 min and centrifuged at 10°. The pH of the collected sediment was adjusted to 7 to extract the enzyme with 5 g papain at 50° for 5 h followed by centrifugation at 7500 rpm for 10 min at 10°. The sediment was then washed with distilled water and acetone before drying at 70° to obtain the MBG.
Analysis of the extracted MBG
MBG was identified through NMR analysis on a Jeol Resonance Delta T2 NMR spectrophotometer resonating at 600 MHz. Five milligrams of MBG was dissolved in D2O (600 μl) and was kept at –76° for 15 min followed by lyophilization. The sample was then added to 600 μl D2O before NMR measurement. Quantification of MBG was performed by hydrolysis prior to HPLC measurement. One milliliter of 2.5 M trifluoro acetic acid in distilled water was added to 1 mg sample and purged with N2 gas. The solution was then heated at 100° for 4 h and evaporated under N2 gas at 40°. The obtained sample was then dissolved in 100 μl H2O for HPLC analysis. TSK Gel Sugar AXI (4.6 mm i.d.×150 mm) column was used under 70°. The eluent was 0.5 M boric acid-NaOH buffer (pH 8.5) and the flow rate was set at 0.4 ml/min. Reagents 1 and 2 were 0.5 % 2-cyanoacetamide (0.25 ml/min) and 0.25 M NaOH (0.25 ml/min.) with a reaction temperature of 110°. Fluorescence (Ex 331 nm; Em 383 nm) was used as the detector.
Cell culture preparation
The RAW 264.7 cell line was collected from the Chiba University. The cell line was incubated in DMEM cell culture medium with 10 % fetal bovine serum and 1 % penicillin-streptomycin solution at 36.5° in 5 % CO2 humidify incubator. RAW 264.7 cells (5×105) were treated in poly-D-lysine Cellware 6 well-plate with 2 ml cell suspension in each well for 24 h before the experiment. The plate was prepared in triplicate. After 24 h, the wells were replaced with new medium. LPS (1 μg/ml) and MBG sample (100 μg/ml) were added into the first and the second well accordingly. The third well was marked as a negative control. After 6 and 24 h, the cell solution was harvested and dead cells were removed from further experiment.
Quantitative determination of TNF-α and IL-6 levels with ELISA
Two reactions were performed in triplicate by ELISA using Mouse ELISA Ready-SET-Go kits to quantify the cytokines production of TNF-α and IL-6. The two coat Corning Costar 9018 ELISA plates were coated with 100 μl/well of 4 μg/ml TNF-α (lot# E12409-1634), 4 μg/ml IL-6 (lot# E05328-1633), captured antibody in coating buffer and incubated overnight at 4°. The plates were then aspirated and washed by 1X phosphate buffered saline, 0.05 % Tween 20 and ELISA diluent to remove excess antibody. Standard mouse TNF-α recombinant protein 1 μg/ml (lot# E08248-1643) and standard IL-6 (lot# 4280337) were prepared in 2-fold serial dilution to make 8-point standard curve. Samples were prepared to 1/20 dilution, directly injected before adding to 100 μl/well. After being aspirated and washed, 100 μl/well of 4 μl/ml detection antibody antimouse TNF-α (250X, lot# E12411-1634), 4 μl/ml detection antibody antimouse IL-6 (250X, lot# E12615-102) were added into each plate and were incubated at room temperature for 1 h. After being aspirated and washed, 100 μl/well of 4 μl/ml enzyme Avidin-HRP (250X; lot# E00005-1636) was added to each well and incubated at room temperature for 30 min. The plates were washed fourteen times and soaked for 2 min with wash buffer to increase the wash effectiveness before adding the 100 μl/well substrate 1X TMB solution (lot# E00007- 1642). After 15 min incubation, 1 M H3PO4 solution was added to each well and recorded at 450 nm on a Sunrise Thermo A-5082 (Tecan, Tokyo, Japan). Results were calculated in pg/ml.
In vivo moisturizing activity
The measurement of moisturizing activity was according to a modified Chang’s method[10]. The cutaneous hydration rates of the sample against the base control were evaluated. The study was conducted at Dermscan Asia, Bangkok, Thailand. Sample A used was 3 % MBG in a gel base and the control was the gel base, both were applied on the forearms twice a day for 14 d. The sample size was 20 Asian male and female subjects at age of 18 y or more.
The general inclusion criteria of the subjects were; healthy subjects who gave written consent of his or her free will, and were informed specific willingness to participate. They were aware and complied with the importance and the duration of the controls, enabling complete respect of the protocols established by the Clinical Center.
On day 1, the subjects were informed on the trial objectives and the protocol that will be followed. After their written consent, clinical examination of the scapular area was conducted under the supervision of a dermatologist. Then the skin humidity was measured on the defined zone using a corneometer (Courage and Khazaka, Electronic GmbH, Cologne, Germany). The measurement of skin hydration was carried out using the capacitance method, which depended entirely on the water content in the skin. The changes were then converted into arbitrary units, proportionally to the skin humidity. Due to a short measurement time, errors from skin deformations or evaporation buildup were ignored. Five repetitive measurements at different points of the forearms were performed to minimize errors. The subjects were assigned to apply the product twice a day for 14 d on their forearms. Measurements were performed 14 d afterward. The subjects must stop using the product on the last evening and return the daily log and remaining samples. Cutaneous hydration rate was measured after 15 min.
Forty eight-hour occlusive single patch test
The objective of the study was to determine the acute skin tolerance of MBG 3 % gel base sample against placebo and control. The MBG sample was incorporated into a water-base gel to get a 3 % MBG gel. The study was done according to the operational mode referenced “single patch tests”. The 22 included subjects were male and female of the age 18 y or more with normal skin. The subject must not have any previous intolerance to cosmetic products and must have given their information with written consent.
A certified dermatologist scored the clinical examination of the skin reaction at the application sites. Macroscopic skin examinations were carried out under the same conditions, specifically under the same light, 30 min after removal. If there was no local skin reaction at the 30-min reading after patch removal, the study was completed. Nevertheless, each subject was asked to confirm that there was no reaction. The clinical examination of all skin reactions was performed at the application sites and scored by a certified dermatologist.
On day 1, the subjects were informed on the trial objectives and sequence of events. After their written consent, clinical examination of the scapular area was conducted under supervision of the dermatologist. Afterward, patches containing 25 μl of the product, placebo or a control were applied. At 24 h after the application, clinical examination of the scapular area under dermatological control was conducted. The assessments included erythema and edematous reactions scored in the range of 0, 0.5, 1, 2, 3. The mean cumulative irritation index (MCII) was calculated according to the following formula: MCII = Σ of the grade (erythema+oedema)/number of readings. The obtained index was interpreted according to the scale (Table 1).
| MCII | Class |
|---|---|
| MCII<0.25 | Non irritating (NI) |
| 0.25≤MCII<0.50 | Very slightly irritating (VSI) |
| 0.50≤MCII<1 | Slightly irritating (SI) |
| 0.50≤MCII<2 | Moderately irritating (MI) |
| MCII≥2 | Irritating (I) |
Table 1: MCII for 48-H Occlusive Single Patch
Statistical analysis
Statistical analyses were carried out using GraphPad Prism version 4.0 for Windows 7 (GraphPad, USA). ANOVA with Tukey’s multiple comparison tests were applied for multiple comparisons. Values are indicated as means±SE, and significant differences are shown as probability values.
Results and Discussion
Modified extraction from Freimund’s method[9] was compared and evaluated to find the most optimal extraction conditions of the molasses yeast waste material. Yeast cell wall is composed of an outer layer of polysaccharides including glycosylated mannoproteins covalently linked to peptides (approximately 40 % of the mass and the inner layer of β-glucan and chitin). The cell wall functions as a retainer of the periplasmic proteins and as a guard against foreign enzymes to the cell. The synthesis of β-1,3-glucan and chitin is performed by plasma membrane bound enzyme complexes[11]. Therefore, slight acidic autolysis condition will promote the cell break down. The obtained cell wall went through a series of extraction to purify the β-(1-3), (1-6)-glucan by breaking layers of chitin and separating fatty acid, vitamins and amino acids. The experiment was designed to vary the three most important factors in the autolysis process i.e. temperature, time, and pH.
Results revealed the extraction yield and the protein content of each extraction process as shown in Table 2. The autolysis process (step 1) was aimed to break the yeast cell wall in order to separate the inner protein liquid from the insoluble cell wall. During the process, yeast cell went through 24 h exposure to pH 5 at 50° and papain. The final yield of the β-glucan obtained was 12.0 % (w/w). The content and the structure of the β-glucan were further analysed.
| Molasses yeast | Weight (g) | SE | Weight (%) | Protein content |
|---|---|---|---|---|
| Step 0: Yeast sludge | 300.0 | 100.0 | 23.6 | |
| Step 1: Autolysis Sediment: Yeast cell wall |
144.3 | ±15.04 | 48.7 | 46.4 |
| Step 2: Glucan extraction Sediment: Yeast cell wall |
43.5 | ±3.53 | 14.5 | 12.5 |
| Step 3: Glucan purification Supernatant: Yeast cell wall |
36.0 | ±2.56 | 12.0 |
Table 2: Yield and Protein Content of Β-Glucan Obtained from each step of Solvent Extraction
In comparison to previous reports, the overall yield of the extraction process was lower due to the condition of yeast cell. The yeast waste material was damaged and partially deactivated prior to the extraction process, therefore the reaction is less effective compared to the freshly cultivated yeast in laboratory.
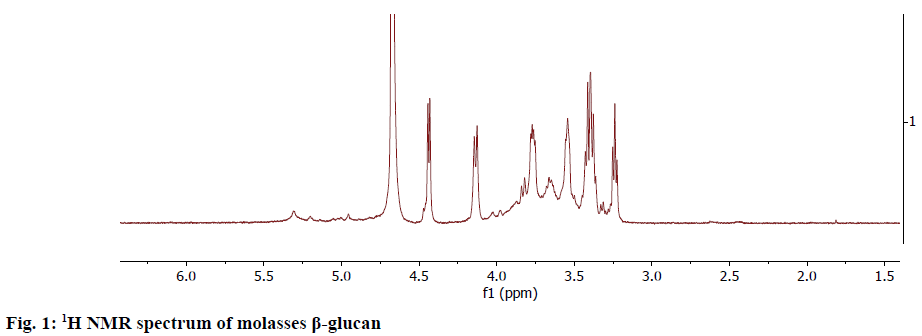
The NMR spectrum of the obtained β-glucan sample from the molasses yeast waste material was taken at 600 MHz. The 1H-NMR spectrum (Figure 1) showed the anomeric protons to resonate at the region 4.10-4.5 ppm. The α-anomeric configuration showed 3J1,2 coupling constants of less than 4 Hz whereas β configuration gave coupling constants greater than 7.0 Hz[12]. This data on comparison with the literature confirmed that the extracted glucan from molasses yeast waste material was β-glucan.
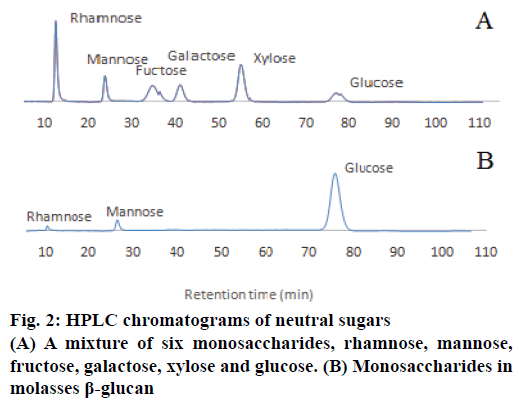
In order to quantify the concentration of β-glucan obtained, the samples were hydrolysed prior to the HPLC analysis to obtain monosaccharaides, rhamnose, mannose and glucose. These monosaccharaides were detected using the HPLC. D-Glucosamine, D-galactosamine and D-mannosamine at 100 ppm were used as standards for amino sugar composition analysis. The HPLC data revealed that the isolated β-glucan from the molasses yeast waste material contained mainly glucose (99.30 %) with minor amount of mannose and rhamnose (Figure 2).
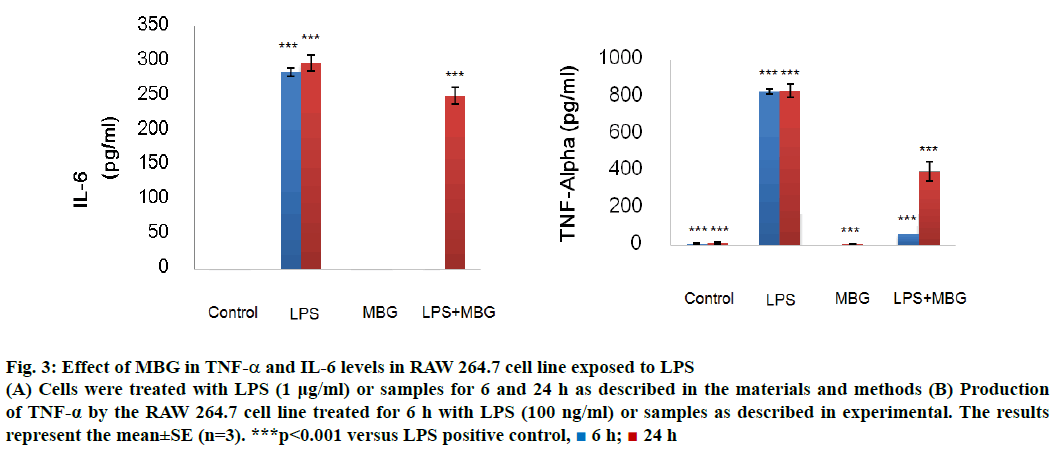
The immunomodulatory study on RAW 264.7 cell lines of obtained β-glucan was performed on TNF-α and IL-6 cytokine (Figure 3). β-Glucan sample demonstrated very effective immune modulating property performed by on LPS-activated macrophages RAW 264.7 cell of TNF-α and IL-6. TNF-α involved in a systemic inflammation where it stimulates the acute phase reaction by activated macrophages. At 6 h, MBG significantly suppressed the TNF-α (65.19±2.33), approximately twelve times less TNF-α compared to the positive control (831.48± 13.47 pg/ml). The suppression became less effective at 24 h, (395.90±31.88 pg/ml) approximately half of the TNF-α was produced comparing with the positive control (838.33±35.78 pg/ml).
Figure 3: Effect of MBG in TNF-α and IL-6 levels in RAW 264.7 cell line exposed to LPS
(A) Cells were treated with LPS (1 μg/ml) or samples for 6 and 24 h as described in the materials and methods (B) Production of TNF-α by the RAW 264.7 cell line treated for 6 h with LPS (100 ng/ml) or samples as described in experimental. The results represent the mean±SE (n=3). ***p<0.001 versus LPS positive control,  6 h;
6 h;  24 h
24 h
IL-6 is a pleiotropic cytokine and accounts in pathological role in inflammatory and autoimmune diseases. The cytokine associates with proinflammation activity against pathogens and cancer. At 6 h, MBG significantly suppressed the IL6 production (0.30±0.12), approximately 103 times less IL-6 production compared to the positive control (285.01±5.97 pg/ml). The suppression became less effective at 24 h, (134.07±25.78) approximately 50 and 20 % less IL-6 production compared to the positive control (297.95±11.60 pg/ml). The result suggested that IL-6 suppression at 24 h became less effective, possibly due to IL-6 was generated to suppress TNF-α. IL-6 was thought to have both pro- and antiinflammatory effects[13].
Previous study reviewed inflammation as a complex defence mechanism regulated by cytokine produced from activated macrophages influenced leucocytes to migrate from vasculature into damaged tissue to destroy agent, which cause tissue injury. Upon the stimulation of LPS, macrophages activated intracellular signalling pathways: the myeloid differentiation factor 88-independent path way and myeloid differentiation factor 88-dependent pathway[14]. As a result, NF-κB in the cell nucleus induced the transcription of IL-6[15]. IL-6 is a pleiotropic cytokine. It accounts in pathological role in amplifying inflammatory and autoimmune disease[16]. In addition, the blockage of IL-6 production was accounted as a treatment to inflammation disease.
The activity of IL-10 was the inhibition of proinflammatory cytokines including TNF-α and IL-6 production. The expression of MLCK-p-MLC signalling induced by TNF-α was regulated by NF-κB signalling. The TNF-α/IL-10 ratio and IL-6/ IL-10 should be further analysed. Moreover, possible mechanism might be from the acceptance of immune receptor on the β-glucan structure. Further the study of in vivo antiinflammatory effect should be considered.
The measurement of moisturizing activity on twenty volunteers was conducted by third party clinical testing institution at Dermscan Asia, Bangkok, Thailand. The testing procedure and safety review were approved by the ethic committee prior the experiment. The moisturizing property was measured by the retention of water in the stratum corneum of skin. The measurement was performed by the presence of NMF within thecorneocytes and the stratum corneum intercellular lipids barrier to transepidermal water loss from the epidermis. The cutaneous hydration rate was analysed against the control. The results are shown in Table 3.
| Parameter | Product | Kinetic | Δ | Average hydration (%) | p | Significance | Subjects with the expected effect (%) | |
|---|---|---|---|---|---|---|---|---|
| (mean±SEM) | ||||||||
| Cutaneous hydration rate |
Gel base | Δ | Da 14 | 1.30±0.61 | 5 % | 0.048 | Yes | 50 % |
| Gel base+MBG | Δ | Da 14 | 3.18±1.23 | 9 % | 0.019 | Yes | 65 % | |
Table 3: Hydration Rate of Skin Applied with Gel Base or MBG Gel Base for 14 Days
The result shows increase in skin hydration measured by Corneometer. The increase in the % skin hydration was partly affected by the water gel base, the % hydration of the placebo (gel base only) increased by 5 %. The subjects who used MBG samples exhibited 9 % increase in the skin hydration compare to control. This can be concluded that the increasing of skin moisture was from β-glucan.
The irritation test was performed by Dearmscan Asia. The 22 subjects included consisted of females and males with normal skin and of the age 18 y or more. Each subject must not have any previous experience of intolerance to cosmetic products and must have given this information along with the written consent. The readings at 30 min and 48 h after removal of the patch produced the MCII values as shown in Table 4.
| MCII | 30 min | 48 h |
|---|---|---|
| MBG | 0.000 | 0.000 |
| Results | Non-irritating | Non-irritating |
Table 4: The MCII Values of Molasses Β-Glucan at 30 Min and 48 h After Treatment
The result showed that the application of MBG under occlusive patch for 48 h did not lead to any skin irritation in all the 22 adult subjects at the 30-min reading as well as at the 48 h-reading. Therefore, MBG was considered as safe for topical use at 3 % concentration.
The industrial yeast waste material appears to be beneficial in cosmetic applications after purification. The modified purification process for industrial scales under the condition of 24 h, pH 5 at 55° with papain showed the most effective cell breakdown. Series of extractions including ethanol extraction, NaOH and enzymatic extraction, acetone filtration, water extraction yielded 12 % (w/w) β-glucan from molasses yeast waste material. The extracted β-glucan was characterized by 1D NMR and HPLC. The β-glucan sample was found effective on LPS-activated RAW 264.7 cell lines by reducing TNF-α and IL-6 levels. These results demonstrated immunomodulating property of the β-glucan isolated from molasses yeast waste material. ELISA data indicated that TNF-α and IL-6 levels were reduced due to the treatment with β-glucan samples. In vivo clinical evaluation of moisturizing activity was conducted on 22 volunteers for fourteen days. The result showed beneficial effects against the control and the placebo. Skin hydration after treating with 3 % MBG sample, has increased by 13 %. Lastly, the single patch irritation test demonstrated the obtained β-glucan as safe for skin tropical use.
Therefore, this study suggested that molasses yeast waste material from ethanol production is effective alternatives to natural ingredients, which could be used as immunomodulatory agents in eco-friendly cosmetics. The feasibility study of the variable cost of production also showed profitable outcome compared to the conventional wastewater treatment.
Acknowledgements
The authors would like to thank the Faculty of Pharmacy, Chiang Mai University, Thailand for financial support from CMU 50th Anniversary Ph.D. Grant from Chiang Mai University, Graduate School of Pharmaceutical Sciences, Chiba University, Japan and Specialty Biotech Co., Ltd. for experimental equipment of the experiment. The authors also thank Dr. Atsushi Ichikawa, Chiba University for providing the RAW 264.7 cell line.
Conflicts of interest
There are no conflicts of interest.
References
- Wheatcroft R, Kulandai J, Gilbert RW, Sime KJ, Smith CG, Langeris WH. Production of β-glucan-mannan preparations by autolysis of cells under certain pH, temperature and time conditions. US Patent Number; 6444448. 2002.
- Kim SK, Yun HS. Production of soluble β-glucan from the cell wall of Saccharomyces cerevisiae. Enzyme Microb Technol 2006;39:496-500.
- Mantovani MS, Bellini MF, Angeli JP, Oliveira RJ, Silva AF, Ribeiro LR. β-Glucans in promoting health: prevention against mutation and cancer. Mutat Res 2008;658:154-61.
- Gardiner T, Carter G. β-glucan biological activities. A review condensed version. Glyco Sci Nutr 2000;1:1-6.
- Donzis BA. Method for revitalizing skin by applying topically water insoluble glucan. US Patent Number: US5223491A. 1993.
- Bohn JA, BeMiller JN. (1-3)-β-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydr Polym 1995;28:3-14.
- Browder W, Williams D, Pretus H, Olivero G, Enrichens F, Mao P, et al. Benefical effect of enhanced macrophage function in trauma patients. Ann Surg 1990;21:605-13.
- Suabjakyong P, Nishimura K, Toida T, Van Griensven LJ. Structural characterization and immunomodulatory effects of polysaccharides from Phellinus linteus and Phellinus igniarius on the IL-6/IL-10 cytokine balance of the mouse macrophage cell lines (RAW 264.7). Food Funct 2015;6:2834-44.
- Freimund S, Sauter M, Ka ̈ppeli O, Dutler H. A new non-degrading isolation process for 1,3-β-Dglucan of high purity from baker’s yeast Saccharomyces cerevisiae. Carbohydr Polym 2003;54:159-71.
- Chang MJ, Huang HC, Chang HC, Chang TM. Cosmetic formulations containing Lithospermum erythrorhizon root extract show moisturizing effects on human skin. Arch Dermatol Res 2008;300:317-23.
- Fesel PH, Zuccaro A. β-glucan: crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet Biol 2016;31:53-60.
- Laws AP, Chadha MJ, Chacon-Romero M, Marshall VM, Maqsood M. Determination of the structure and molecular weights of the exopolysaccharide produced by Lactobacillus acidophilus 5e2 when grown on different carbon feeds. Carbohydr Res 2008;343:301-7.
- Hodes GE, Ménard C, Russo SJ. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol Stress 2016;4:15-22.
- Lampiasi N, Montana G. The molecular events behind ferulic acid mediated modulation of IL-6 expression in LPS-activated Raw 264.7 cells. Immunobiology 2016;221:486-93.
- Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, et al. NF-κB and AP-1 connection: mechanism of NF-κB-dependent regulation of AP-1 activity. Mol Cell Biol 2004;24:7806-19.
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Ann Rev Immunol 2001;19:683-765.