- *Corresponding Author:
- Monica R. P. Rao
Department of Pharmaceutics, AISSMS College of Pharmacy, Near RTO, Kennedy Road, Pune 411001, India
E-mail: monicarp_6@hotmail.com
| Date of Submission | 11 August 2014 |
| Date of Revision | 09 February 2015 |
| Date of Acceptance | 22 September 2015 |
| Indian J Pharm Sci 2015;77(5):605-612 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The aim of the present study was to modify psyllium seed polysaccharide and evaluate the modified polysaccharide as release retardant in tablets employing ciprofloxacin hydrochloride as model drug. Studies on polysaccharide from psyllium husk has been reported but no work has been reported on characterization and modification of the polysaccharide present in the psyllium (Plantago ovata) seed and the use of the modified polysaccharide as a release retardant in tablets. In this study, the seed gum was modified using sodium trimetaphosphate as crosslinking agent. Sustained release matrix tablets of ciprofloxacin hydrochloride were prepared by wet granulation using various drug-polymer ratios. The polymers investigated were psyllium polysaccharide, phosphorylated psyllium polysaccharide and widely used release retardant hydroxypropyl methylcellulose K100M. The tablets were evaluated for hardness, friability, drug content, swelling profile and in vitro dissolution studies. The matrix tablets containing 1:3 proportion of drug-phosphorylated psyllium polysaccharide was found to have higher hardness as compared to tablets containing 1:1 and 1:2 proportions. The results of swelling behavior in water showed that the tablets containing 1:3 drug:phosphorylated psyllium polysaccharide ratio had swelling comparable to that of tablets containing 1:3 drug:hydroxypropyl methylcellulose ratio. The in vitro dissolution studies shows that the dissolution rate was retarded from 98.41 to 37.6% in 6 h with increase in concentration of phosphorylated psyllium polysaccharide from 100 to 300 mg. Formulations containing psyllium polysaccharide showed complete drug release in 8 h whereas those formulated with phosphorylated psyllium polysaccharide exhibited extended drug release over the 12 h period. Drug release kinetic studies revealed that drug release followed Korsmeyer-Peppas model.
Keywords
Psyllium, polysaccharide, ciprofloxacin hydrochloride, tri-sodium tri-metaphosphate, derivatization, sustained release
Polysaccharides are complex carbohydrate polymers consisting of two or more monosaccharides linked together covalently by glycosidic linkages in a condensation reaction. They are usually insoluble in water because of their large molecular size. Natural polysaccharides are widely used as excipients in conventional and novel dosage forms. These natural polysaccharides can be derivatised to obtain modified polymers which can compete with the synthetic excipients. Interest in the physical effects and properties of the excipients used in pharmaceutical formulations has increased in recent years due to the awareness of the fundamental effect that excipients can exert on the bioavailability, bioequivalence and stability of formulations [1].
The dried, ripe seeds of Plantago afra (Plantago psyllium), Plantago indica (Plantago arenaria) and Plantago ovata (Plantaginaceae) are used in medicine since ages for treatment of constipation, diarrhea, hemorrhoids and high blood pressure [2]. The polysaccharide from psyllium seed husk has been fractionated and evaluated for gelling ability. Desai et al. [3] formulated sustained release granules of amoxicillin trihydrate using various combinations of psyllium husk and hydroxypropyl methylcellulose K4M (HPMC K4M). A faster release of amoxicillin from the granules containing only psyllium husk was observed, while a combination of psyllium husk and HPMC K4M provided a sustained release of drug. Lawani and Parikh [4] prepared and evaluated isabgol based additive to be used as matrix for directly compressible tablets of acetaminophen using agglomeration technique.
However, the polysaccharide present in the seeds has not been evaluated for its properties as a multifunctional excipient. The present study focuses on phosphorylation of the psyllium seed polysaccharide (PPS) using sodium trimetaphosphate as the cross-linking agent. The phosphorylated psyllium seed polysaccharide (PhPPS) was then evaluated as a release retardant for the formulation of sustained release tablets of ciprofloxacin hydroxide (CIP) and compared with unmodified PPS and HPMC K100M.
Materials and Methods
Ciprofloxacin hydrochloride (CIP) was obtained as a gift sample from Aarti Drugs Ltd., Mumbai. Psyllium seeds were obtained from Manakarnika Aushadalay, Pune and authenticated at Agarkar Research Institute, Pune. Sodium trimetaphosphate was obtained as a gift sample from Sigma Aldrich. All the other chemicals, solvents and reagents used were of analytical grade and were procured locally.
Extraction of seed gum
Psyllium seeds were cleaned by washing them several times with deionized water. The seeds were then soaked in deionized water, kept on water bath at 80º, with occasional stirring for 2 h and allowed to cool to room temperature and kept overnight for soaking. Sodium hydroxide (NaOH) 0.5 M was added while stirring (200 rpm for 15 min) to separate the polysaccharide from seeds and resulting slurry passed through 12#. The PPS was reprecipitated by addition of 2 M hydrochloric acid to the filtrate. The precipitate was washed with deionized water to remove traces of acid and separated by centrifugation at 3000 rpm for 15 min. The residue was dried overnight in a tray drier at 50-60º.
Chemical cross linking of PPS
Sodium trimetaphosphate (STMP, 1 g) was dissolved in 50 ml of deionised water. This was added to 5 ml of 0.1 M NaOH. PPS (1 g) dispersed in 50 ml of water was then added slowly with stirring. The reaction mixture (100 ml) was stirred for 2 h, poured into five petri dishes (20 ml each) and dried at 60º for 24 h. The dried complex (modified gum) was powdered, passed through sieve 120# and used for evaluation. The same procedure was followed by using 0.2 M NaOH as catalyst. The PhPPS was prepared by using two different concentrations of NaOH solutions (0.1 and 0.2 M) as catalyst to study the effect of alkalinity on the swelling properties of the PhPPS [5,6].
Characterization of PPS and PhPPS
The PPS and PhPPS were characterized for physicochemical properties, spectral analysis (FTIR) and thermal analysis (DSC). The PhPPS was evaluated for the presence of phosphate by elemental analysis. The phosphorous content in the PhPPS prepared using 0.1 M NaOH and 0.2 M NaOH was determined by UV Spectrophotometer (Jasco V-530). The vanadate/molybdate yellow method was used for the determination of phosphate in PhPPS and analyzed at 380 nm using UV Spectrophotometer.
Physicochemical properties
The bulk density, tapped density, angle of repose, Hausner’s ratio and Carr’s index was evaluated. Water uptake studies were carried out at different time intervals (2, 4, 8, 12 and 24 h) and computed gravimetrically according to Equation, Water uptake (%)=(Ts–T)/T×10. Where Ts is the weight of the swollen polymer and T is the initial weight of the polymer. The pH of 1% solution of the PPS and PhPPS was determined using a digital pH meter (EI, DELUX 101).
Compatibility studies
Compatibility studies for CIP were carried out with potential formulation excipients to determine possibility of any drug-excipient interaction/incompatibility. Excipients studied include PPS, PhPPS, PVP K-30, HPMC K100M, microcrystalline cellulose, magnesium stearate, and talc. Drug was mixed with excipients in 1:1 ratio and their IR spectra were obtained. These samples were subjected to room temperature. IR spectra of these stored samples were then obtained after 30 days.
Preparation of calibration curve of CIP
The first stock solution was prepared by dissolving 100 mg of CIP in 100 ml 0.1 N hydrochloric acid in a 100 ml volumetric flask (1 mg/ml). From the stock solution 5, 10, 15, 20, 25, and 30 μg/ml dilutions were prepared. The absorbance of each sample was measured at 276 nm. Calibration curve of concentration Vs absorbance was plotted. The method was validated for accuracy, precision, repeatability and reproducibility.
Preparation of granules and formulation of CIP sustained release matrix tablets
Nine formulations were prepared by wet granulation method as per Table 1. The drug-polymer ratios selected were 1:1, 1:2 and 1:3 CIP and all the excipients except lubricants were passed through 150# and granulated with PVP K-30 in IPA (5% w/v PVP K-30 solution) as granulating agent. Wet granulation was done by adding small amount of PVP K-30 solution to the mixture to prepare damp mass which was then passed through 18# for granulation and dried in hot air oven at 50º for 15 min. The granules were then lubricated using magnesium stearate and talc and compressed using standard 12.5 mm flat punch using rotary tablet machine [7].
| Ingredients (mg) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| CIP | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| PPS | 100 | 200 | 300 | - | - | - | - | - | - |
| PhPPS | - | - | - | 100 200 300 | - | - | - | ||
| HPMC K100M | - | - | - | - | - | - | 100 | 200 | 300 |
| Microcrystalline cellulose | 268 | 268 | 268 | 268 | 268 | 268 | 268 | 268 | 268 |
| Magnesium stearate | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Talc | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| PVP K-30 (5% w/v) | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
All quantities in mg, total weight of the tablet is 500 mg. F1–F9: formulation batches. CIP: ciprofloxacin, PPS: psyllium seed polysaccharide, PhPPS: phosphorylated psyllium seed polysaccharidem, HPMC K100M: hydroxypropyl methylcellulose K100M
Table 1: Formulae Of Cip Sustained Release Matrix Tablets
Evaluation of granules
The bulk density and tapped density of granules were evaluated. Angle of repose of the granules was evaluated by fixed funnel method. Carr’s compressibility index and Hausner’s ratio was also determined. [8]
Heckel plot
Heckel plots were constructed to evaluate the compressibility of the granules. 500 mg of the granules were subjected to different compression pressures ranging from 300–1800 psi using hydraulic press (Mini Press-II MT, RIMEK). The diameter and thickness of the compacts were determined immediately after and 24 h after compression. Hardness was also determined after 24 h. The Heckle constants and yield pressures were determined using BVCPK Heckel Software. [9]
Kawakita plot
The compactibility of granules was determined by Kawakita analysis. The method involved pouring 10 g of granules into 50 ml glass measuring cylinder and the heap of the particles in the cylinder was leveled off and the bulk volume, V0 was accurately measured. The granules were subjected to gradual incremental tappings (N) using bulk density apparatus (BDA 001, Lab. Hosp. make) till the mass achieved constant volume, VN. The compactibility behavior of the granules was compared using numerical constants obtained from the Kawakita plots [10].
Physical properties of tablets
Tablets were evaluated for hardness using Monsanto Hardness Tester (Nevtex) and friability using Roche Friabilator. The tablets were also evaluated for weight variation. Thickness of the tablet was tested using vernier calipers (Besto). For estimation of drug content, ten tablets from each formulation were powdered. The powder equivalent to 100 mg of CIP was weighed and dissolved in phosphate buffer pH 7.4, suitable dilutions was prepared and the solution was analyzed in UV/ Vis double beam spectrophotometer at 278 nm using phosphate buffer pH 7.4 as blank. [11-14]
Swelling behavior of CIP sustained release matrix tablets
The swelling index of F3, F6 and F9 formulations were determined [15]. The pre-weighed tablets from each batch were placed in pH 6.8 phosphate buffer in petri plate. At time intervals of 2, 4, 6, 8, 10 and 12 h tablets were removed from buffer medium and excess water on their surface was carefully absorbed with filter paper. The swollen tablets were weighed and the % weight gain of each tablet was calculated by using follow equation: S.I.= [(Mt–M0)/M0]×100. Where, S.I. is swelling index, M is weight of tablet at time ‘t’ and M0 is weight of tablet at time 0.
In vitro dissolution study
Dissolution test for all the formulations was carried out using USP type I apparatus (model TDT-08l, Electrolab, Mumbai, India) at 100 rpm and 37º for the first 2 h in 900 ml of 0.1 N HCl and then continued in 900 ml of phosphate buffer (pH 7.4). The dissolution fluid was withdrawn at regular time intervals during the studies and replaced with an equal volume of drug-free dissolution fluid to maintain sink conditions. The samples were then analyzed on a Jasco UV/Vis spectrophotometer at 276 nm. Absorbance was measured and percent drug release was determined [16].
Drug release kinetics
The data obtained from in vitro release studies were fitted in to various kinetic equations zero order, first order, Higuchi matrix, Peppas and Hixson Crowell to know the mechanism of drug release. The equation with high regression coefficient (r2) and n values for formulation will be the best fit of release data. Goodness-of-fit was evaluated using DD Solver 1.0 software.
Results and Discussion
Phosphorylation using STMP as crosslinking agent has been used as a method of chemical derivatization for starches. STMP has also been reported as an effective cross-linker of hyaluronan and karaya gum for the purpose of synthesizing gels and sustained release tablets [17-19]. The crosslinking reaction occurred through the hydroxyl groups of the polysaccharide and led to ester linkages. Hence, STMP was selected as the cross linker. The advantage of phosphorylation crosslinking method is that di or tri esters can be formed by varying the pH conditions and different grades of crosslinked polymer can be obtained. Changing the amount of phosphorylating agent can regulate the degree of substitution of the products and polymers with desired degree of substitution can be obtained. Sodium hydroxide provides the necessary alkalinity for the crosslinking reaction with STMP. The crosslinking involves reaction between hydroxyl groups of PPS and metaphosphate groups in STMP leading to phosphate ester (O-P-O) linkages in between two PPS moieties.
The effect of sodium hydroxide concentration on the swelling index of PhPPS was observed and given in Table 2. The percent swelling of PhPPS was found to increase with increase in concentration of sodium hydroxide from 0.1 to 0.2 M NaOH. This may be due to increase in crosslinking between PPS and STMP at 0.2 M NaOH (confirmed in elemental analysis for phosphate). Thus, we can propose that two grades of PhPPS can be prepared based on the swelling as well as release retarding needs. At a higher pH, there is an increase in the number of ionized phosphate groups of STMP. This may be creating favorable conditions for phosphorylation of the polymer. Hence, we may presume that there is an increase in the degree of crosslinking and a mixture of psyllium grafted with mono and diester phosphates are obtained. This may account for the higher swelling index of PhPPS in all media as compared to PPS.
| Medium | Percent swelling index of PhPPS | |
|---|---|---|
| 0.1 M NaOH as catalyst | 0.2 M NaOH as catalyst | |
| Deionised water | 1720 ± 16.12 | 1876 ± 14.18 |
| Phosphate buffer pH 6.8 | 412 ± 9.23 | 441 ± 8.19 |
| 0.1 N HCl | 846 ± 21.13 | 948 ± 19.24 |
| 0.5 N NaOH | 292 ± 11.25 | 341 ± 12.23 |
Values are expressed as mean±SD of n=3 observations. PhPPS: phosphorylated psyllium seed polysaccharidem, SD: standard deviation
Table 2: Percent Swelling Index
The physicochemical properties of PPS and PhPPS were evaluated. The micromeritic properties reveal good flow properties of PhPPS as compared to PPS. No remarkable difference was observed in tap and bulk density of PPS and PhPPS. The pH of PPS in deionised water was found to be 4.46 and that of PhPPS was found to be 7.90. The low pH of PPS could be due to its acidic constituents. It may be proposed that due to crosslinking reaction of PPS with STMP, sodium and phosphate groups are introduced in the PPS, due to which the pH of PhPPS increased.
The phosphorous content in PhPPS was determined by the vanadate/molybdate yellow method which is as follows: In acids, orthophosphate ions react with ammonium molybdate and ammonium vanadate to form yellow ammonium phosphoric vanadomolybdate, which was analyzed at 380 nm using UV spectrophotometer. The phosphorous content of PhPPS prepared using 0.1 M NaOH as catalyst was found to be 4.98%. The phosphorous content of PhPPS prepared using 0.2 M NaOH as catalyst was found to be 6.52%. The results confirm that PPS was phosphorylated by using STMP as the cross linking agent.
Infra red spectrum of the PPS and crosslinked PhPPS was recorded on Jasco FTIR-401, Japan at transmittance mode. FTIR studies revealed the various characteristic peaks of –OH, -C=O, O-O-, and –CH2. As the original material does not contain any phosphate group in its structure, the typical stretching vibrations of P=O and P-O (at about 1200-1100 cm-1) were observed in the spectra of crosslinked sample. It was also noticeable that bands assigned to hydroxyl stretching vibration (3400 cm-1) were decreased in intensity after the crosslinking reaction.
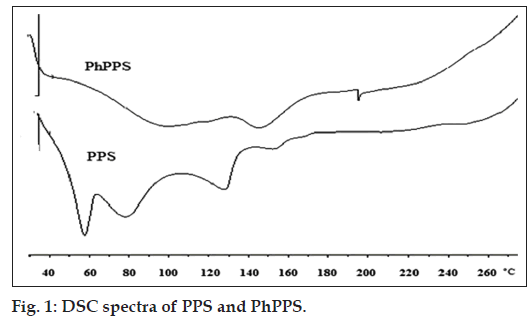
The DSC thermograms (fig. 1) of unmodified PPS showed endothermic peaks at 57.59º, 77.95º, 127.49º and 151.74º, while modified PPS i.e. PhPPS showed endothermic peaks at 99.49º, 144.97º and 194.54º. The DSC thermogram of PhPPS when compared to PPS displayed three endothermic peaks. Disappearance of the peak, larger enthalpy value and a shift in the last endothermic peak is observed in the thermogram of PhPPS. The difference in decomposition temperature for PhPPS was more as compared to PPS. Hence the rate of decomposition with respect to temperature was slower for PhPPS [20]. This suggests that a change in the structure of PPS occurred as a result of crosslinking. The enthalpy values for PPS were found to be -5.58, -4.71, -3.51 and -1.64 mW and that of PhPPS were found to be -3.61, -3.67 and -2.23 mW. The enthalpy of PhPPS was increased which indicated that PhPPS is more thermostable as compared to PPS. Thus, both FT-IR and DSC indicated that PPS structure was modified by crosslinking.
A pharmaceutical formulation is considered appropriate when no drug-excipient or excipient-excipient interactions occur [21]. Compatibility study for CIP was carried out with potential formulation excipients to determine possibility of any drug-excipient interaction/incompatibility. FTIR analysis showed no evidence of any interaction between drug and studied excipients.
The size and surface area of a particle can be related to the physical, chemical and pharmacologic properties of drugs [22]. The bulk density for all the granules was found to be between 0.3112 and 0.467 g/ml and the tapped density was found to be between 0.3756 and 0.576 g/ml (Table 3). The granules were studied for their angle of repose. From the observations it was concluded that the granules made using the PPS and PhPPS had flowability comparable to that of the granules prepared using HPMC K100M. The study of Carr’s index and Hausner’s ratio further supported the results for angle of repose. The Hausner’s ratio for all the granules was found to be between 1.11 and 1.3002 which indicated good compressibility.
| Property | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Bulk density (g/cc) | 0.443 ± 0.039 | 0.371 ± 0.038 | 0.458 ± 0.027 | 0.311 ± 0.045 | 0.331 ± 0.034 | 0.447 ± 0.028 | 0.32 ± 0.058 | 0.347 ± 0.049 | 0.467 ± 0.027 |
| Tapped density (g/cc) | 0.576 ± 0.22 | 0.433 ± 0.21 | 0.508 ± 0.18 | 0.387 ± 0.16 | 0.375 ± 0.12 | 0.482 ± 0.14 | 0.40 ± 0.24 | 0.407 ± 0.25 | 0.488 ± 0.18 |
| Angle of repose (°) | 33.14 ± 2.15 | 32.36 ± 2.06 | 31.27 ± 2.18 | 28.34 ± 2.13 | 27.18 ± 2.04 | 25.46 ± 2.23 | 27.2 ± 2.16 | 26.84 ± 2.22 | 25.32 ± 2.17 |
| Carr’s index (%) | 23.09 ± 1.05 | 14.22 ± 1.12 | 10.14 ± 1.06 | 19.75 ± 1.13 | 11.66 ± 1.11 | 7.41 ± 1.09 | 18.7 ± 1.03 | 14.11 ± 1.07 | 10.76 ± 1.08 |
| Hausner’s ratio (%) | 1.300 ± 0.11 | 1.165 ± 0.19 | 1.11 ± 0.12 | 1.246 ± 0.13 | 1.132 ± 0.26 | 1.08 ± 0.17 | 1.23 ± 0.28 | 1.17 ± 0.23 | 1.12 ± 0.17 |
Values are expressed as mean±SD of n=3 observations, SD: standard deviations
Table 3: Micromeritic Properties Of Granules
The Heckel plot is widely used for relating the relative density of the material during compression to the applied pressure. Deformability of the powder can be characterized using Heckel plot. Process of tableting involves application of massive compressional forces which induce considerable deformation in the solid particles. With many pharmaceutical solids these forces are large enough to exceed the elastic limit of the solid. Plastic deformation and/or brittle fracture results in generation of new, clean surfaces, which when pressed against one another undergo cold welding (fusion bonding if the material melts). In 1961, Heckel postulated a linear relationship between the relative porosity of a powder and applied pressure. Slope of linear regression the Heckel constant, a material dependent parameter inversely proportional to the mean yield pressure (Py). Materials that are comparatively soft readily undergo plastic deformation. Conversely, materials with higher mean yield pressure values undergo compression by fragmentation first, to provide denser packing. Hard, brittle materials are more difficult to compress than soft ones [9]. From the Heckle plots it was found that as the compression force increased, the porosity of the compressed pellets decreased. For the granules containing 100 mg PPS, the Py was found to be the highest (3.134 psi) as compared to other granules which indicated that these granules were soft and pliable. The granules containing 100 mg PhPPS was found to have a low Py value (2.89 psi) than PPS which indicated brittle fracture which thus leads to a denser packing of the granules (Table 4). The Py was also found to decrease with increasing concentration of PhPPS.
| Drug: polymer ratio | PPS | PhPPS | HPMC K100M | |||
|---|---|---|---|---|---|---|
| He | Py | He | Py | He | Py | |
| 1:1 | 0.3189 | 3.1348 | 0.3588 | 2.8917 | 0.3406 | 2.9358 |
| 1:2 | 0.3644 | 2.7438 | 0.4002 | 2.4985 | 0.3993 | 2.5039 |
| 1:3 | 0.4145 | 2.4125 | 0.4430 | 2.2573 | 0.4408 | 2.2684 |
PPS: psyllum seed polysaccharide, PhPPS: phosphorylated psyllium seed polysaccharidem, HPMC K100M: hydroxypropyl methylcellulose K100M, He: heckel constant, Py: yield pressure in tons
Table 4: Heckel Constants Of Granules
Kawakita constants indicate the compactibility of the powder from the bulk density state to the tapped density state. The flow property of powders was determined by the following Kawakita equation. N/C=N/a+1/ab. Where ‘a’ and ‘b’ are constants. ‘a’ describes the degree of volume reduction at the limit of tapping and is called compactibility and ‘1/b’ is considered to be a constant related to cohesion and is called cohesiveness. Lower value of ‘a’ indicated less compactibility property of the particles and lower value of 1/b indicated less cohesiveness among the particles [10]. The Kawakita constants are shown in Table 5. The granules containing 100 mg PhPPS was found to have higher compactibility (a=0.694) as compared to granules containing 100 mg PPS and HPMC K100M. The granules containing 300 mg PPS had compactibility of 0.757 whereas the granules containing 300 mg PhPPS had compactibility of 0.4159. This indicated that as the concentration of PhPPS increased from 100 mg to 300 mg, the compactibility of the granules decreased. The granules containing 300 mg PhPPS had the least ‘1/b’ value (5.29) as compared to granules containing 300 mg PPS and HPMC K100M. This indicated that granules containing 300 mg PhPPS are the least cohesive and will produce better flowability than other granules.
| Drug: polymer ratio | PPS | PhPPS | HPMC K100M | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Value of “a” | Value of “b” | Value of “1/b” | Value of “a” | Value of “b” | Value of “1/b” | Value of “a” | Value of “b” | Value of “1/b” | |
| 1:1 | 0.6890 ± 0.012 | 0.0398 ± 0.011 | 25.07 ± 2.45 | 0.6949 ± 0.02 | 0.0671 ± 0.03 | 14.89 ± 1.24 | 0.767 ± 0.17 | 0.0410 ± 0.01 | 24.37 ± 1.45 |
| 1:2 | 0.5009 ± 0.1 | 0.0474 ± 0.02 | 21.07 ± 1.45 | 0.4297 ± 0.02 | 0.1006 ± 0.04 | 9.940 ± 1.24 | 0.485 ± 0.04 | 0.0446 ± 0.01 | 22.41 ± 2.68 |
| 1:3 | 0.7570 ± 0.12 | 0.0829 ± 0.02 | 12.05 ± 2.56 | 0.4159 ± 0.07 | 0.1887 ± 0.07 | 5.296 ± 1.23 | 0.386 ± 0.05 | 0.1670 ± 0.05 | 5.985 ± 1.24 |
Values are expressed as mean±SD of n=3 observations. PPS: psyllum seed polysaccharide, PhPPS: phosphorylated psyllium seed polysaccharidem, HPMC K100M: hydroxypropyl methylcellulose K100M, a: compactibility, 1/b: cohesiveness, CIP: ciprofloxacinm, SD: standard deviation
Table 5: Kawakita Constant For Cip Granules
The weight variation in all formulations was found to be in the range of 497–499 mg and hence all formulations complied with the test for weight variation (Table 6). The tablets showed hardness values between 3.22–5.28 kg/cm2. However these values alone cannot be considered as absolute indicator of their strength. Another measure of tablet’s strength is friability. Conventional compressed tablets that lose less than 1% of their weight are generally considered acceptable. In present study, the friability values for all the tablet formulations were found within prescribed limit of less than 1%. Thickness of formulations F1 to F9 varied between 1.21 to 3.36 mm. Good uniformity in drug content was found in all the tablet formulations. The value ranged from 95.31-99.21% (Table 6).
| Formulation | Uniformity ofweight (mg) | Hardness(kg/cm2) | Friability(%) | Thickness(mm) | Drugcontent (%) |
|---|---|---|---|---|---|
| F1 | 498 ± 2.5 | 3.22 ± 0.13 | 0.370 ± 0.05 | 4.3 ± 0.02 | 96.2 ± 1.37 |
| F2 | 496 ± 3.2 | 3.66 ± 0.63 | 0.342 ± 0.07 | 4.2 ± 0.02 | 97.3 ± 1.29 |
| F3 | 499 ± 2.6 | 4.16 ± 0.30 | 0.332 ± 0.02 | 4.2 ± 0.03 | 97.4 ± 1.35 |
| F4 | 496 ± 2.2 | 4.31 ± 0.47 | 0.289 ± 0.06 | 4.3 ± 0.05 | 97.1 ± 1.25 |
| F5 | 498 ± 3.6 | 4.75 ± 0.29 | 0.236 ± 0.03 | 4.2 ± 0.06 | 96.8 ± 1.39 |
| F6 | 497 ± 2.8 | 5.18 ± 0.34 | 0.219 ± 0.07 | 4.1 ± 0.03 | 97.2 ± 1.42 |
| F7 | 497 ± 2.4 | 4.24 ± 0.13 | 0.239 ± 0.07 | 4.2 ± 0.06 | 98.4 ± 1.33 |
| F8 | 498 ± 3.1 | 4.98 ± 0.37 | 0.221 ± 0.05 | 4.3 ± 0.03 | 96.8 ± 1 0.27 |
| F9 | 497 ± 2.9 | 5.28 ± 0.51 | 0.214 ± 0.09 | 4.2 ± 0.02 | 97.9 ± 1.28 |
Values are expressed as mean±SD of n=3 observations, F1–F9 stand for formulation batches, SD: standard deviation
Table 6: Physical Evaluation Of Matrix Tablets
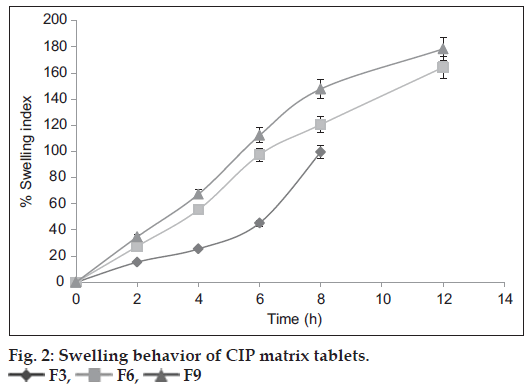
Swelling is defined as the uptake of liquid by a polymer with an increase in volume. By controlling the swelling of the polymer, the dosage form can release the drug in a controlled manner to a desired site [23]. The swelling behavior studies for F3, F6 and F9 formulations are shown in fig. 2. At the end of 8 h, the swelling of F3 formulation containing PPS was found to be 99.56%. At the end of 12 h, the swelling was found to be 164.32 and 178.4 % for F6 and F9 formulations containing PhPPS and HPMC K100M, respectively. These studies revealed that swelling of tablets containing PPS was found to be lower than that of tablets containing PhPPS and the tablets containing PhPPS had swelling comparable to that of tablets containing HPMC K100M. The maximum swelling of F6 and F9 tablets may be attributed to the higher concentration of PhPPS and HPMC K100 M, respectively. These results are in accordance with the mechanism of crosslinking regardless of the excipients of the formulation.
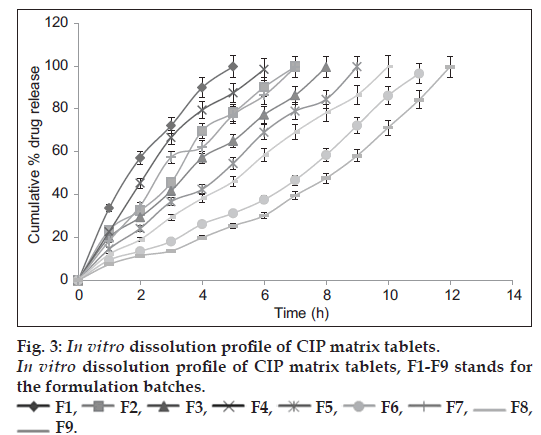
The in-vitro drug release studies (fig. 3) revealed that rapid drug release was observed from F1 i.e. the tablets containing 1:1 drug-PPS ratio and slowest from tablets containing 1:3 ratio of drug-HPMC K100M (F9). It was observed that as concentration of PPS was increased from 100 mg to 300 mg, there was decrease in drug release rate. When the PPS was replaced by PhPPS of same concentration, drug release was further controlled which may be due to the increase in swelling of PhPPS as compared to PPS. The results of in vitro drug release studies are in line with the swelling studies. The percent increase in swelling of tablets containing PhPPS was found to be 21% as compared to PPS. The percent decrease in drug release from the tablets containing 300 mg PhPPS at 5th hour was found to be 40.99 when compared with release from tablets containing 300 mg PPS. The percent release from the tablets containing 1:3 drug-PhPPS ratio was found to be 96.47% at the end of 12th hour which was comparable with the percent drug release from the tablets containing 1:3 drug-HPMC K100M ratio which was 99.64%. Increase in the content of polymer was found to decrease drug release in a span of 12 h. The in vitro dissolution rate was retarded as the concentration of PhPPS was increased from 100 mg to 300 mg. The tablets containing low concentration of all polymers under investigation had lost their matrix integrity in 5 to 7 h. Formulations containing PPS showed complete drug release in 8 h whereas those formulated with PhPPS exhibited extended drug release over the 12 h period.
Various kinetic models are used to describe the release kinetics to analyze the in vitro release data. These kinetic models indicate the mechanism of drug release through the formulations which is crucial for extended release tablets. The drug release data of the tableted formulations did not fit satisfactorily to zero-order, first-order, Higuchi model and Hixson-Crowell equation but showed good fit into the Korsmeyer equation (r2=0.9898 to 0.9981). The value of the release component ‘n’ ranged from (0.654 to 1.574). These results suggest that the drug release profile follows Korsmeyer-Peppas model. The Korsmeyer-Peppas model suggest that release of water-soluble drug occurs only after water penetrates the networks to swell the polymer and dissolve the drug, followed by diffusion along the aqueous pathways to the surface of the device.
Dissolution profiles of formulations containing PPS and PhPPS were compared and results showed that PhPPS controlled the drug release efficiently than PPS. The dissolution profile of tablets prepared using PhPPS was comparable with that of tablets prepared using HPMC K100M. This was further confirmed by the swelling studies of the formulations. This study demonstrates that PhPPS enhances matrix rigidity thereby sustaining the drug release rate when compared to PPS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Robbins SR. Gum Arabic: In a review of recent trends in selected markets for water soluble gums. Overseas Dev Nat ResourInst Bull 1988;108:18-33.
- Psyllium. Available from: http://www.truepsyllium.com. [Last cited on 2014 Jul 12].
- Desai A, Shidhaye S, Kadam V. Possible use of psyllium husk as a release retardant. Indian J Pharm Sci 2007;69:206-10.
- Lalwani AN, Parikh JR. Preparation and evaluation of an ispaghulabased directly compressible matrixing agent for controlled release. Acta Pharm 2008;58:309-16.
- Reddy MM, Reddy JD, Afrasim M, Shivakumar HG. Formulation of sustained-release matrix tablets using cross-linked karaya gum. Trop J Pharm Res 2012;11:28-35.
- Khandelwal KR. Practical Pharmacognosy: Techniques and Experiments. 19th ed. Pune (MH): NiraliPrakashan; 2008. p. 159.
- Krishnarajan D, Gowthaman R, Narayana Rao BL, Pavan OC, Sainudheen PM, Badarudheen M. Effect of cellulose and non-cellulose polymers on ciprofloxacin extended release tablets. J Chem Pharm Res 2012;4:3617-23.
- Martin A, Swarbrick J, Cammarata A. Physical Pharmacy: Physical Chemical Principles in the Pharmaceutical Sciences. 3rd ed. Bombay: Varghese Publishing House; 2005. p. 503, 513-4.
- Lachman L, Lieberman A, Kanig JL. The Theory and Practice of Industrial Pharmacy. 3rd ed. Bombay: Varghese Publishing House; 1987. p. 67.
- Yamashiro M, Yuasa Y, Kawakita K. An experimental study on the relationship between compressibility, fluidity and cohesion of powder solids at small tapping numbers. Powder Technol 1983;34:225-31.
- Government of India, Ministry of Health and Family Welfare. Indian Pharmacopoeia. Vol. 1. New Delhi: Indian Pharmacopoeia Commission; 2010. p. 437.
- Chowdary KP, Mohapatra P, Murali Krishna MN. Evaluation of olibanum and its resin as rate controlling matrix for controlled release of diclofenac. Indian J Pharm Sci 2006;68:497-500.
- Vidyadhara S, Rama Rao P, Prasad JA. Development and in vitrokinetics of propranolol hydrochloride controlled release matrix tablets. Indian Pharm 2006;1:66-70.
- Vasantha P, Lakshmi PE, Ashok A. In vitroevaluation of controlledrelease tablets of nifedipine. Indian Pharm 2005;1:71-3.
- Shaikh AA, Pawar YD, Kumbhar ST. Preformulation and rheological characterization of various polymers for mucoadhesive drug delivery. Indian J Pharm Res Dev 2011;4:1-9.
- Seetharaman S, Balya H, Abdul H. Formulation and evaluation of sustained release matrix tablets of ciprofloxacin HCL using gum kondagogu and chitosan as matrix forming polymers. Int J Pharm Sci Rev Res 2014;24:115-9.
- Muhammad K, Hussin F, Ghazali YC, Kennedy JF. Effect of pH on phosphorylation of sago starch. CarbohydrPolym 2000;42:85-90.
- Woo K, Seib PA. Crosslinking of wheat starch and hydroxypropylated wheat starch in alkaline slurry with sodium trimetaphosphate. CarbohydrPolym 1997;3:263-71.
- Gliko-Kabir I, Yagen B, Penhasi A, Rubinstein A. Phosphatedcrosslinked guar for colon-specific drug delivery. I. Preparation and physicochemical characterization. J Control Release 2000;63:121-7.
- Tran T, Piyachomkwan K, Sriroth K. Gelatinization and thermal properties of modified cassava starches. Starch Stärke 2007;59:46-55.
- Bruni G, Amici L, Berbenni V, Marini A, Orlandi A. Drug-excipient compatibility studies. Search of interaction indicators. J Therm Anal Calorim 2004;68:561-73.
- Khullar P, Khar RK, Agarwal SP. Evaluation of guar gum in the preparation of sustained-release matrix tablets. Drug Dev Ind Pharm 1998;24:1095-9.
- Martin A, Swarbrick J, Cammarata A. Physical Pharmacy: Physical Chemical Principles in the Pharmaceutical Sciences. 3rd ed. Bombay: Varghese Publishing House; 2001. p. 503, 513-4.