- *Corresponding Author:
- R. Uppal
Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research (NIPER), S.A.S. Nagar-160 062, Punjab
E-mail: ptiwari@niper.ac.in
| Date of Submission | 17 May 2005 |
| Date of Revision | 17 November 2005 |
| Date of Acceptance | 08 October 2006 |
| Indian J. Pharm. Sci., 2006, 68 (5): 649-653 |
Abstract
The use of fixed-dose combinations is a widespread clinical practice in the treatment of various cardiovascular disorders. These fixed-dose combinations are valuable only when they have been developed based on sound rational pharmacokinetic and pharmacodynamic criteria, and when claims for their benefits have been supported by evidence-based data and well-designed clinical studies. However, a look at the available fixed-dose combinations reveals that there are combinations which do not meet these basic criteria, and hence their clinical benefit is debatable. In this context, several stakeholders have put forward their views on the rationality of some of the fixed-dose combinations. The situation has become more complicated as there are very limited reports to assess and describe the rationality of fixed-dose combinations on an individual basis. In the present study, a thorough evaluation of 44 fixed-dose combinations used in various cardiovascular disorders using comprehensive criteria has been completed. This evaluation has demonstrated that a large number of fixed-dose combinations are rational, based on the criteria used. Finally, there seems to be enough reason to re-investigate six of them, which did not match the criteria as well as others. These six combinations could be the subject of study by the clinicians and/or pharmaceutical companies to assess their clinical benefit.
Cardiovascular disease is the world’s number one killer disease, responsible for one in every three deaths. As per a report of the WHO, an estimated 17 million people die of cardiovascular disease (CVD), particularly heart attack and stroke, every year. CVD is a group of disorders that includes heart disease (i.e., myocardial infarction and angina), stroke, hypertension, congestive heart failure (CHF), hardening of the arteries and other disorders of the circulatory system [1]. The presence of one disease is also associated with many more complications [2].
There are several reasons leading to an increase in the incidence of cardiovascular diseases. Clinical trials have demonstrated that lack of patients’ adherence [3] to therapy is a major problem. Therefore, combination therapy using fixed-dose combinations (FDCs) with agents having complementary mechanism of action represents a new innovation that increases not only the patient adherence but also the effectiveness of treatment. Moreover, the seventh report of the Joint National Committee (JNC VII) on prevention, detection, evaluation and treatment of high blood pressure [4] has made a mention of combination therapy for the management of hypertension, viz., angiotensin converting enzyme inhibitors (ACEIs) with calcium channel blockers (CCBs), ACEIs with diuretics, angiotensin receptor blockers (ARBs) with diuretics, ARBs with beta-blockers (BBs), centrally acting drugs with diuretics, and diuretics with diuretics.
The rationality of a FDC is the most controversial and debated issue in today’s clinical practice. Though there are many advantages [5] of fixed-dose combinations, like simplification of therapy, increased patient compliance, reduction of total daily dose and adverse effects, reduction of overall cost of therapy, yet there are several disadvantages too. The use of FDCs can lead to polypharmacy, dose of one ingredient alone cannot be altered, different pharmacokinetic properties can pose difficulty in frequency of administration and in case of development of an ADR, it is difficult to withdraw the suspected drug alone. Therefore, the risk-benefit assessment is essential before choosing a combination for therapy. The FDCs are of value only when they have been developed according to rational pharmacokinetic and pharmacodynamic criteria, and when claims for their benefits have been supported by evidence-based data and well-designed clinical studies.
The existing knowledge suggests that some FDCs may provide enhanced clinical benefit. Research into this area has been negligible, and there are limited reports to describe the rationality of FDCs on an individual basis. The available reports seem to have not considered all the relevant aspects of a rational FDC. The main aim of the present study was to develop comprehensive criteria for the assessment of rationality of a FDC and to evaluate cardiovascular FDCs for rationality.
To develop a comprehensive criteria which is useful and unbiased for the evaluation of FDCs, the guidelines of WHO {“Draft guidelines for registration of fixed-dose combination medicinal products” (http://www.who.int/ medicines/organization/qsm/expert_Committee/Guidelines/ FDC-WHO-QAS04_108.doc/accessed on 13th March 2005)} and the “Note for guidance on fixed-dose combination medicinal products” by the Committee for Proprietary Medicinal Products (CPMP) (http://www.emea.eu.int/pdfs/human/ewp/024095en.pdf/accessed on 13th March 2005), Europe, were carefully studied. These are well-known guidelines, which serve as benchmark towards a rational FDC; based on these, the criteria for this study were developed. These criteria include all the dimensions of defining a rational FDC, and appropriate weighting (score) has been attached to each criterion. The total score thus obtained by a FDC will reflect its standing on the scale; however, it is to be noted that this score should not be viewed in isolation.
The first point in the seven-point criteria for evaluating the rationality of FDCs is that each active pharmaceutical ingredient (API) of the combination should preferably be in the essential medicine list (EML) of WHO or in the National List of Essential Medicines (NLEM) of India. Secondly, the dose of each API should meet the requirements for a defined population group. The dose and proportion of each API present in FDC should be appropriate for the intended use. Thirdly, the combination should have the advantage of established evidence of efficacy and safety over single compounds administered separately in terms of its therapeutic efficacy and safety. Further, the overall cost of the combination should preferably be less than the cost of the individual components. The FDC should facilitate either the reduction of the dose of individual drugs or their adverse effects. The pharmacokinetic (PK) properties of individual drugs should be similar. The PK parameters of each API should not be affected. There should be no unfavourable pharmacokinetic interaction between the APIs. In case of the PK parameters being different, the clinical benefits should be taken into consideration. Lastly, the individual drugs should have different mechanisms of action.
The Current Index of Medical Specialities [6] (CIMS) and Indian Drug Review [7] (IDR) were screened to arrive at the list of FDCs for the treatment of CVD. The rationality of each FDC was analyzed by the help of the sevenpoint criteria developed in this study. The WHO model List of EML [8] and the NLEM [9] were used for the assessment of the first criteria. The dose of the individual APIs was verified from standard textbooks [10,11] and references in pharmacology and therapeutics [12]. The published data regarding clinical evidence of safety and efficacy was collected from databases such as Pubmed, Medscape, Science Direct, and the Cochrane library. The data on reduction in dose and adverse effects was also collected from the same databases. The cost data of the individual components, as well as the FDCs, was obtained from CIMS and IDR. The detailed information about pharmacokinetic parameters was collected from Micromedex 2005 [13]. The assessment of rationality was performed by collecting evidences from published literature (in the open domain) about individual FDCs. Each FDC was assigned a score depending upon the match with the criteria.
Altogether, 44 FDCs acting on the cardiovascular system were studied. Table 1 lists all the FDCs with their marketed strengths. Their availability in variable strengths provides flexibility in the titration of dosage.
| Combinations | Dose, mg | |
|---|---|---|
| Diuretics + Diuretics | Amiloride + HCTZ | (2.5+ 25), (5 + 50) |
| Frusemide + Amiloride | (40 + 5) | |
| Diuretics + BBs | Atenolol + Chlorthalidone | (25 + 12.5), (50 + 12.5), (100 +25) |
| Atenolol + Indapamide | (50 + 2.5) | |
| HCTZ + Bisoprolol | (6.25 + 5), (6.25 + 2.5) | |
| HCTZ + Metoprolol | (12.5 + 100) | |
| HCTZ + Propranolol | (25 + 40) | |
| HCTZ + Atenolol + Amiloride | (25 + 50 + 2.5) | |
| Diuretics + ACEIs | HCTZ + Captopril | (15 + 25), (25 + 25) |
| HCTZ + Enalapril | (12.5 + 5), (12.5 + 10), (25 + 10) | |
| HCTZ + Lisinopril | (12.5 + 5) | |
| HCTZ + Ramipril | (12.5 + 2.5) | |
| Indapamide + Perindopril | (2 + 0.625) | |
| Diuretics + ARBs | HCTZ + Candesartan | (16 + 12.5) |
| HCTZ + Losartan | (12.5 + 50) | |
| HCTZ + Irbesartan | (150 + 12.5) | |
| HCTZ + Telmisartan | (40 + 12.5) | |
| HCTZ + Valsartan | (80 + 12.5) | |
| Diuretics + centrally | Chlorthalidone + Clonidine | (15 + 0.1) |
| acting agents | HCTZ + Clonidine | (20 + 0.1) |
| CCBs + ACEIs | Amlodipine + Benazepril | (5 + 5), (5 + 10) |
| Amlodipine + Lisinopril | (5 + 5) | |
| Amlodipine + Ramipril | (2.5 + 2.5) | |
| Amlodipine + Enalapril | (2.5 + 2.5), (5 + 2.5), (5 + 5) | |
| CCBs + ARBs | Amlodipine + Losartan | (2.5 + 25), (5 + 50) |
| Amlodipine + Valsartan | (80 + 2.5) | |
| CCBs + BBs | Amlodipine + Atenolol | (5 + 25), (5 + 50) |
| Nifedipine + Atenolol | (10 + 50), (20 + 50) | |
| Nitrendipine + Atenolol | (10 + 50), (20 + 50) | |
| ARBs + ACEIs or BBs | Losartan + Ramipril | (50 + 1.25), (50 + 2.5), (50 + 5) |
| Losartan + Enalapril | (25 + 5), (50 + 5) | |
| Losartan + Atenolol | (50 + 50) | |
| FDCs of antiplateletagents | Aspirin + Clopidogrel | (75 + 75), (150 + 75) |
| Aspirin + Dipyridamole | (60 + 75), (100 + 75) | |
| Aspirin + Ticlopidine | (75 + 250), (100 + 250) | |
| FDCs of antilipedemics | Atorvastatin + Amlodipine | (10 + 2.5), (10 + 5) |
| Atorvastatin + Aspirin | (10 + 75) | |
| Simvastatin + Nicotinic acid | (5 + 125) | |
| Miscellaneous | Dihydralazine + Reserpine | (10 + 0.1) |
| Dihydralazine + HCTZ + Reserpine | (10 + 10 + 0.1) | |
| Hydralazine + Propranolol | (25 + 40) | |
| ISM + Aspirin | (30 + 75), (60 + 75), (60 + 150) | |
| Losartan + Ramipril + HCTZ | (50 + 25 + 12.5) | |
| Mefenamic acid + Tranexamic acid | (250 + 500) |
*BBs – Beta blockers, ACEIs – Angiotensin converting enzyme inhibitors, ARBs – Angiotensin receptor blockers, CCBs – Calcium channel blockers, HCTZ – Hydrochlorothiazide, ISM – Isosorbide-5-Mononotrate
Table 1: Marketed Fixed Dose Combinations*
The results of the assessment showed that for approximately 40% of the FDCs, the individual components were present in any one or both the EMLs. However, for over 50% of the FDCs, at least one component was absent in both the EMLs, viz., atorvastatin, lisinopril, ramipril, and clopidogrel. The doses used in the FDC matched with the recommended doses. However, there was only one instance that merits discussion. The FDC of hydrochlorothiazide (HCTZ) and bisoprolol has HCTZ at a low dose, viz., 6.125 mg, whereas the recommended dose is 12.5-50 mg/day. Several clinical trials [14,15] have demonstrated that this low dose of HCTZ is effective in controlling hypertension in combination with bisoprolol with reduced adverse effects. For 75% of the FDCs, the clinical evidence on safety and efficacy was established and documented in public domain. For one-quarter of the FDCs studied, the data on clinical safety and efficacy was not available to enable arriving at any definite conclusion. Some of the examples from this category are FDCs of hydralazine+reserpine, atorvastatin+aspirin and enalapril+amlodipine.
Of the 44 FDCs analyzed, 19 FDCs (43%) were found to be more cost-effective than their individual components. For a few FDCs, like aspirin+ticlopidine, clonidine+HCTZ, HCTZ+enalapril, the cost of the combination was found to be less than the added individual components’ cost. Of the remaining 25 FDCs, the cost of individual components alone was not available in 9 FDCs. Therefore, it was not possible to assess the cost advantage of the FDC. But in some, it was observed that the cost of combination was as high as three times the total added cost of individual components (e.g., atenolol+nitrendipine).
The present study noted that for 19 FDCs there was published evidence on reduction in dose or adverse effects. Of these, in 18 cases the combination reduced the adverse effects of individual components. For example, in combining HCTZ with losartan, hypokalaemia caused by HCTZ is counterbalanced by losartan [16]. In only one instance, the published evidence showed reduction in dose and adverse effects. In the FDC of HCTZ+bisoprolol, HCTZ is effective in low dose, viz., 6.125 mg, and adverse effects like hypokalaemia, hyperuricemia are reduced in the FDC [17]. However, for 25 FDCs no published report was available regarding the reduction in dose or adverse effects. In the FDC of aspirin with clopidogrel, the risk of bleeding increases but the risk-benefit analysis showed that the benefit outweighs the risk of bleeding [18].
The results of the present evaluation show similar PK properties of 37 FDCs. In 3 FDCs, even though the PK properties were different, the individual components were combined as controlled-released form to overcome this problem. For example, in atenolol+nifedipine combination, nifedipine is given in a controlled-release form to increase its duration of action. In the FDC of clonidine+chlorthalidone, despite different PK properties, the published evidence suggested that this combination is effective as once-daily dosing [19]. All the FDCs had different mechanisms of action and as a result, they produced either additive or synergistic effects. For example, the combination of lisinopril and HCTZ was found to have synergistic effects [20,21].
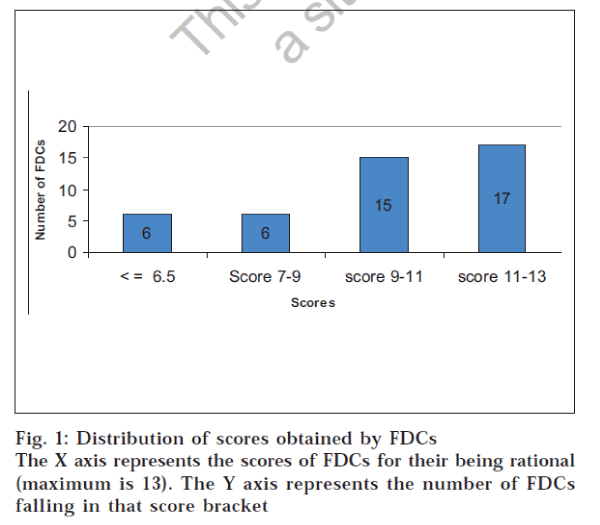
These observations were used to assign a score to the FDC. Each criterion was awarded an appropriate weighting depending upon its relative contribution to the rationality of the FDC. Therefore, the scoring criteria used in the study could award a maximum of 13 points to any FDC. It was found that 38 FDCs had scores above the median score. Hence they were considered as rational combinations. Fig. 1 shows the distribution of scores obtained by the FDCs. Two FDCs scored 13 points, which reflects a total match with the criteria for evaluating FDCs. Poor scoring of an FDC does offer scope for re-investigating the FDC for safety, efficacy,costs and adverse effects.
Evidence of safety and efficacy is of utmost importance when the two drugs are combined together as a single formulation. In the United States, an FDC is considered as a new drug, and it has to be approved by the USFDA before it can be marketed, even though the individual components are available for concurrent use [22]. The Drugs and Cosmetic Act, 1940, also takes a similar stand on this issue. The results of this study clearly demonstrate that in 33 FDCs (out of 44), the clinical evidence on safety and efficacy was established. However, for the remaining 11 combinations, no published evidence could be found. There were at least three cases in which limited numbers of trials have shown the safety and efficacy of a particular combination. For example, in the FDC of atenolol+amiloride+HCTZ, only one published evidence was found regarding the safety and efficacy of the combination in elderly patients [23]. Similar situations existed for losartan+ramipril and propranolol+HCTZ. There seems to be an urgent need for clinical trials to substantiate the safety and efficacy of FDCs.
This study has made a systematic point-by-point evaluation of fixed-dose combinations acting on the cardiovascular system, on the basis of the comprehensive criteria. An attempt has been made to use a system of scoring in relation to each FDC satisfying the criteria. A large majority of FDCs were found to comply with the criteria developed for the assessment of rationality. However, those that did not comply with the criteria could be the subject of study by the clinicians and/or pharmaceutical companies for assessing their clinical benefit.
References
- Ara, S., J. Manag. Care Pharm., 2004, 10, 326.
- The sixth report of the Joint National Committee on Prevention,Detection, Evaluation, and Treatment of High Blood Pressure, Arch.Intern. Med., 1997, 157, 2413.
- Neutel, J.M. and Smith, D.H., J. Clin. Hypertens., 2003, 5, 127.
- The Seventh Report of the Joint National Committee on Prevention,Detection, Evaluation, and Treatment of High Blood Pressure, J.Amer. Med. Assoc., 2003, 289, 2560.
- Oster, J.R. and Epstein. M., J. Clin. Hypertens., 1987, 3, 278.
- Current Index of Medical Specialties Updated Prescribers’ Hand – Book, July 2004, Atmedica Private Limited, Bangalore, India.
- Indian Drug Review (IDR), JulyAug 2004, Mediworld Publication
- 13th WHO Model List of Essential Medicines. Available at: http:// mednet3.who.int/Eml/medicines_alphabetical_order.as/accessed on 5th Aug 2004).
- National List of Essential Medicines 2003. Pharma Times, 2003, 35, 37.
- British Medical Association., Royal Pharmaceutical Society of Great Britain., British National Formulary., BMA., London, 2003. (No. 45)
- Sweetman. S.C., Eds., In; Martindale: The complete drug reference, 33rd Edn., Pharmaceutical Press., London, 2002.
- Gilman, A.G., Hardman, J.G., Limbird, L.E., Molinoff, P.B. and Ruddon, R.W., Eds., In; Goodman and Gilman: The Pharmacological Basis of Therapeutics, 10th Edn., McGraw Hill, New York, 2001.
- USP drug Information. Thomsons Micromedex. Canada: Updated Software, 19742005.
- Frishman, W.H., Burris, J.F., Mroczek, W.J., Weir, M.R., Alemayehu, D., Simon, J.S., Chen, S.Y. and Bryzinski, B.S., J. Clin. Pharmacol., 1995, 35, 182.
- Benetos, A., Adamopoulos, C., Argyriadis, P., Bean, K., Consoli, S. and Safar, M., J. Hypertens., 2002, 20, S21.
- Owens, P., Kelly, L., Nallen, R., Ryan, D., Fitzgerald, D. and O’Brien,E., J. Hypertens., 2000, 18, 339.
- Zachariah, P.K., Messerli, F.H. and Mroczek, W., Clin. Ther., 1993,354. 15, 779.
- Diener, H.C., Bogousslavsky, J., Brass, L.M., Cimminiello, C., Csiba, L., Kaste, M., Leys, D., MatiasGuiu, J. and Rupprecht, H.J. Lancet, 2004, 24, 331.
- Grossman, S.H. and Gunnells, J.C., J. Clin. Pharmacol., 1980, 20, 193.
- Leduc, J.J., Madonna, O. and Gressin, V., Therapie., 1994, 49, 17.
- Ishimitsu, T., Yagi, S., Ebihara, A., Doi, Y., Domae, A., Shibata, A.,Kimura, M., Sugishita, Y., Sagara, E., Sakamaki, T. and Murata, K.,Jpn. Heart J., 1997, 38, 831.
- Reid, J.L., J. Hum. Hypertens., 1995, l 4, S19
- Duckett, G.K. and Cheadle, B., Brit. J. Clin. Pract., 1990, 44, 354