- Corresponding Author:
- E. B. Onuigbo
Department of Pharmaceutics, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, 410001, Enugu State
E-mail: ebeleonuigbo@gmail.com
| Date of Submission | 9 June 2010 |
| Date of Revision | 11 June 2011 |
| Date of Acceptance | 18 June 2011 |
| Indian J Pharm Sci, 2011, 73 (3): 323-328 |
Abstract
Span 20-based niosome was prepared by lipid film hydration technique and loaded with Newcastle disease vaccine. Three batches with Span 20, cholesterol and dicetyl phosphate in micro molar ratios of 10:10:1; 15:15:1 and 20:20:1 were prepared and evaluated for encapsulation efficiency using haemagglutination test. The morphology of the vesicles was studied by means of transmission electron microscopy. Particle size, zeta potential and polydispersity index were determined by photon correlation spectroscopy using a nanosizer. Adjuvanticity was assessed using haemagglutination inhibition test. The vesicles of Span 20-based niosomes were distinct, near spherical large unilamellar vesicles. The vesicles were of varied sizes (<1000 nm) with the entrapped Newcastle disease vaccine in the core of the vaccine. The zeta potential had a peak at -50 mV. The polydispersity index was 0.68. Haemagglutination inhibition test showed a 71% increment in immune response over that of the marketed La Sota® vaccine which had a 60% increment in immune response. The niosomal vaccine did not alter but rather enhanced the immunogenicity of the Newcastle disease vaccine.
Keywords
Adjuvanticity, multilamellar vesicles, niosome, span 20, vesicle diameter
Niosomes are vesicles composed of non-ionic surfaceactive agent bilayers, which serve as novel drug delivery systems. Niosomes are microscopic in size and their size lies in the nanometric scale. Niosomes are formed on the admixture of non-ionic surfactant of the alkyl or dialkylpolyglycerol ether class and cholesterol with subsequent hydration in aqueous media [1]. Niosomes may be unilamellar or multilamellar depending on the method used to prepare them [2]. The niosome is made of a surfactant bilayer with its hydrophilic ends exposed on the outside and inside of the vesicle while the hydrophobic chains face each other within the bilayer [3]. Hence, the vesicle holds hydrophilic drugs within the space enclosed in the vesicle while the hydrophobic drugs are embedded within the bilayer itself. The application of niosomal technology is widely varied and can be used to treat a number of diseases. One of the most useful aspects of niosomes is their ability to target vaccines and drugs to the reticulo-endothelial system. The reticulo-endothelial system (RES) preferentially takes up niosomal vesicles. The uptake of niosomes is controlled by circulating serum factors called opsonins, which mark the niosomes for clearance [4] while delivering the cargo to the antigen presenting cells. Localization of drugs encapsulated in niosomes is utilized to treat tumors known to metastasize to the liver and spleen. This localization of drugs can also be used for treating parasitic infection of the liver like leishmaniasis. Niosomes can also be utilized for targeting drugs to organs other than RES. A carrier system (such as antibodies) can be attached to niosomes (as immunoglobulins bind readily to the lipid surface of the niosomes) to target them to specific organs [5]. Many cells also possess the intrinsic ability to recognize and bind specific carbohydrate determinants and this can be exploited by niosomes to direct carrier system to particular cells. Niosomes can alter the metabolism, prolong circulation and half-life of a drug, thus decreasing the side effects of that drug. Niosomal entrapment of doxorubicin and methotrexate (in two separate studies) showed beneficial effects over the unentrapped drugs such as decreased rate of proliferation of the tumor and higher plasma levels accompanied by slower elimination [6].
Leishmaniasis is a disease in which a parasite of the genus leishmania invades the cells of the liver and spleen. Commonly prescribed drugs for the treatment are derivatives of antimony (antimonials), which in higher concentration can cause cardiac, liver and kidney damage. Use of niosomes in tests conducted showed that it was possible to administer higher levels of the drug without the triggering of the side effects and thus allowed greater efficacy in treatment [7]. Oral peptide drug delivery has long been faced with a challenge of bypassing the enzymes, which would breakdown the peptides and this is being investigated. In an in vitro study conducted by Yoshida et al. [8] oral delivery of a vasopressin derivative entrapped in niosomes showed that entrapment significantly increased the stability of the peptide [9]. Due to their immunological selectivity, low toxicity and greater stability, niosomes are being used to study the nature of the immune response provoked by antigens [10]. Niosomes can be used as carriers for haemoglobin within the blood. The niosomal vesicles are permeable to oxygen and hence can act as a carrier for haemoglobin in anaemic patients. One of the most useful aspects of niosomes is that they greatly enhance the uptake of drugs through the skin. Transdermal drug delivery utilizing niosomal technology is widely used in cosmetics. The low cost, greater stability and ease of preparation of non-ionic surfactant has lead to exploitation of these compounds as alternatives to phospholipids [11]. This research work evaluates the physical characterization of a niosomal based antigen delivery system.
Sorbitan monolaurate, cholesterol, dicetylphosphate were all procured from Sigma-Aldrich Inc, Germany, chloroform was purchased from Ridel-de Heen, Germany and methanol from Scharlen Chemmie SA, Spain. One day old chicks obtained from CHI farms, Nigeria were used.
Multilamellar vesicles (MLV) were prepared using a technique based on lipid hydration method [10]. Briefly, Span 20, cholesterol and dicetyl phosphate in a molar ratio of (10:10:1) were weighed and dissolved in chloroform/methanol system (2:1) in a 100 ml round bottom flask. The solvent mixture was evaporated to obtain a thin dry film. The film was hydrated with 2 ml of PBS pH 7.4 containing 0.2 ml/dose of the Newcastle disease vaccine at 4o with gentle shaking during which MLVs were formed. The vesicles were allowed to anneal for 30 min.
Different molar ratios of Span 20-based niosomes were evaluated for vaccine entrapment efficiency. Non-entrapped antigen was separated from vesicleentrapped antigen by centrifugation for 10 min at 3000 rpm. The free (unentrapped) antigen was determined in the supernatant by haemagglutination test. Where VEE=(t1-t2/t1)´100 is the percent vaccine entrapment efficiency.
The z-average vesicle diameter of the noisome was measured by dynamic light scattering using modern nanosizer (Malvern instruments, UK). Zeta potential was calculated from the mean of three runs after adjustment to a negatively charged standard. The prepared niosomes dispersions were processed by using copper grids to adsorb the particles from the suspension, then stained in 2.5% uranyl acetate for 30 s and dried. The specimens were observed under JEM–1010 Transmission electron microscope (JEOL, Japan) operated at 80 Kv at X 10500 magnification.
Haemagglutination inhibition (HI) was assessed by immunizing orally sixty 3 weeks old specific pathogen free chicken with the vaccine (0.2 ml/dose) with a 2-week interval between each immunization. All animal handling and experiments were conducted following the guidelines stipulated by University of Nigeria research ethics committee on animal handling and use. HI was read to be the highest dilution of the serum causing complete inhibition of 4HAU of antigen. By comparing the result against the negative control serum which showed no hemagglutination (zero titre), the result was validated [12].
For analyses of immune response and niosomal efficacy, data were tested by analysis of variance (more than two groups) which was performed to compare the mean values of different groups. Statistical significance was considered at P < 0.05.
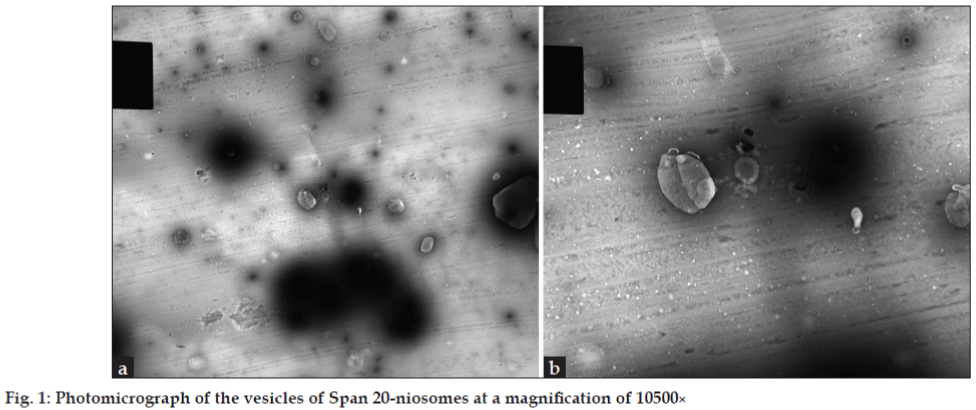
Figs. 1a and 1b show photomicrographs of the Span 20 niosomal vesicles at a magnification of 10500X. The vesicles look like self enclosed vesicles encapsulating the Newcastle disease antigen. The vesicles appear distinct and not aggregated or coalesced which in effect is caused by the negative charges of the dicetylphosphate. The sizes are not uniform and the shapes are near spherical. The niosomes had a gel like appearance and consistency when hydrated at about 60º showing better physical stability but unfortunately would destroy the viability of the vaccine. The rim of the vesicles look darker and heavier showing possibility of lamellarity of the vesicles. It has been reviewed that methods of preparation of niosomes such as hand shaking, ether injection and sonication affects vesicle size [6].
Hand shaking method forms vesicles with greater diameter compared to the ether injection method Small sized niosomes can also be produced by reverse phase evaporation (REV) method [13,14]. Microfluidization method gives greater uniformity and small size vesicles. Presence of charge tends to increase the interlamellar distance between successive bilayers in multilamellar vesicle structure and leads to greater overall entrapped volume. Inclusion of cholesterol in niosomes increases its hydrodynamic diameter and entrapment efficiency [15]. In general, the action of cholesterol is two folds; on one hand, cholesterol increases the chain order of liquid-state bilayers and on the other, cholesterol decreases the chain order of gel state bilayers. At a high cholesterol concentration, the gel state is transformed to a liquid-ordered phase[16]. An increase in cholesterol content of the bilayers results in a decrease in the release rate of encapsulated material and therefore an increase of the rigidity of the bilayers obtained [8,16,17]. The bilayers of the vesicles are either in the so-called liquid state or in gel state, depending on the temperature, the type of lipid or surfactant and the presence of other components such as cholesterol. In the gel state, alkyl chains are present in a well-ordered structure, and in the liquid state, the structure of the bilayers is more disordered. The surfactants and lipids are characterized by the gel-liquid phase transition temperature (TC). Phase transition temperature (TC) of surfactant also affects entrapment efficiency i.e. Span 60 having higher TC and provides better entrapment [15]. The electrostatic or charge stabilization of the dicetylphosphate, which has the benefits of stabilizing or flocculating the colloidal system by simply altering the concentration of ions in the system may be improved by increasing the concentration of the dicetylphosphate.
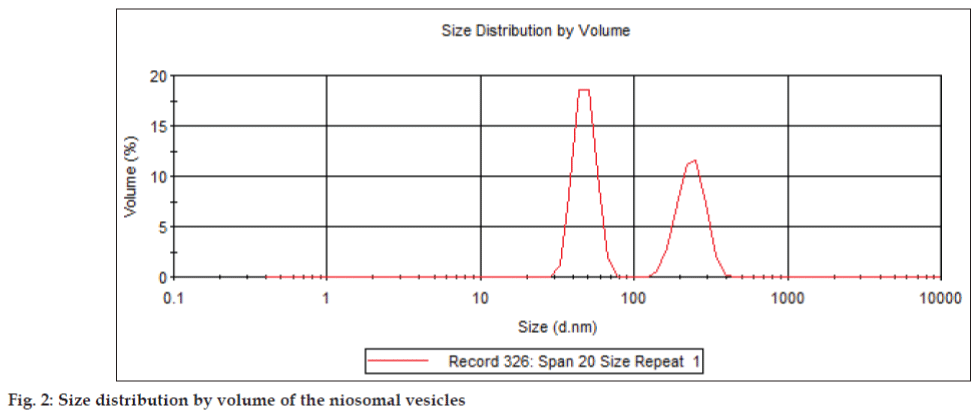
A theory was developed which suggests that the stability of a particle in solution is dependent upon its total potential energy function VT. This theory recognizes that VT is the balance of several competing contributions: VT=VA+VR+VS. VS is the potential energy due to the solvent, it usually only makes a marginal contribution to the total potential energy over the last few nanometers of separation. Much more important is the balance between VA and VR, these are the attractive and repulsive contributions [18]. They potentially are much larger and operate over a much larger distance. DVLO theory suggests that the stability of a colloidal system is determined by the sum of these van der Waals attractive (VA) and electrical double layer repulsive (VR) forces that exist between particles as they approach each other due to the Brownian motion they are undergoing. This theory proposes that an energy barrier resulting from the repulsive force prevents two particles approaching one another and adhering together. Fig. 2 shows the size distribution of the Span 20 niosomal vesicles, which range from 100–1000 nm. The adjuvanticity of a niosome depends on structural characteristics such as vesicle size, surface charge, lipid to antigen ratio, the number of lamellae and the rigidity of the bilayer [19,20]. It is also affected by the fatty acid composition of the surfactant hydrophobe [21,22]. Addition of a hypertonic salt solution to a suspension of niosomes brings about reduction in diameter [23]. The mean size of niosomes also increases proportionally with increase in the HLB of surfactants like Span 85 (HLB 1.8) to Span 20 (HLB 8.6) because the surface free energy decreases with an increase in hydrophobicity of surfactant [15].
Niosomes having a composition of cholesterol:Span 20:dicetylphosphate (10:10:1, 15:15:1 and 20:20:1) were prepared and evaluated for vaccine encapsultion efficiency using HI test. The values obtained of the different ratios were similar and the highest encapsulation was 50%. The method used did not give a clear and distinct result for the vaccine encapsulation efficiency.
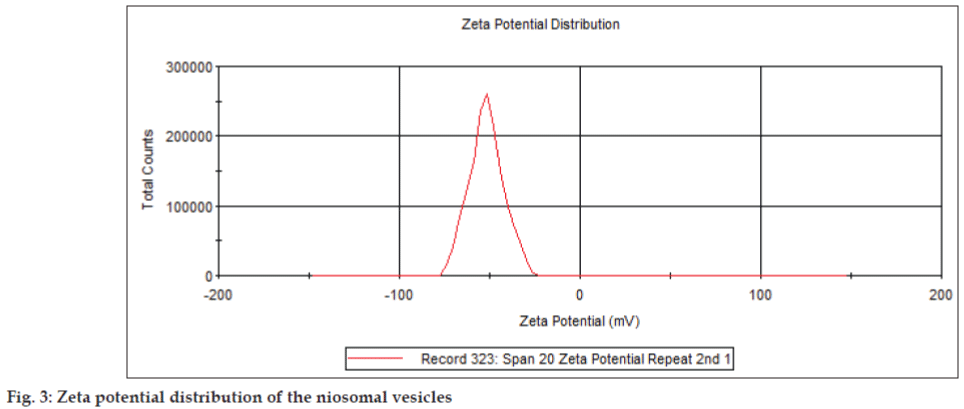
It has long been recognised that the zeta potential is a very good index of the magnitude of the interaction between colloidal particles. Dissociation of acidic groups on the surface of a particle will give rise to a negatively charged surface. Conversely, a basic surface will take on a positive charge. In both cases, the magnitude of the surface charge depends on the acidic or basic strengths of the surface groups and on the pH of the solution. The surface charge can be reduced to zero by suppressing the surface ionisation or by decreasing the pH in case of negatively charged particles or by increasing the pH in the case of positively charged particles. The zeta potential of the noisome under study ranged from -100 to 0 mV (fig. 3). The negative charge is contributed by the dicetylphosphate present in the dispersion. The presence of a net charge, whether negative or positive, can increase water uptake within the double layer. Formulation of niosome with molar ratio of 1:1 is most beneficial for the efficient encapsulation, and extra cholesterol is unfavorable. It implies that equal molarity of non ionic surfactant and cholesterol can make the membrane compact and well organised[24]. The magnitude of the zeta potential gives an indication of the potential stability of the colloidal system. If all the particles in suspension have a large negative or positive zeta potential then they will tend to repel each other and there will be no tendency for the particles to come together. However, if the particles have low zeta potential values then there will be no force to prevent the particles coming together and flocculating. The general dividing line between stable and unstable dispersions is generally taken at either +30 or -30 mV. Particles with zeta potentials more positive than +30 mV or more negative than -30 mV are normally considered stable. However, if the particles have a density different from the dispersant, they will eventually sediment forming a close packed bed (i.e. a hard cake) [18].
The mucosa-associated lymphoid tissues represent a highly compartmentalized immunological system. The primary reason for using a mucosal route of vaccination is that most infections affect or start from mucosal surfaces, and that in these infections, topical application of a vaccine is often required to induce a protective immune response at the site of pathogen entry [25]. Presently, most vaccines are administered via the parentral route or via other invasive routes. Invasive mode of vaccine administration can trigger the systemic immune response, but may not essentially provide adequate mucosal immune protection. On the other hand, effective mucosal vaccines will not only elicit superior local immune protection, but has been shown to trigger systemic response analogous to that of parenterally-delivered vaccine. When given orally, the vaccines are degraded by enzymes from the gastric and intestinal juices rich in proteases such as trypsin or chymotrypsin. Therefore, they do not reach intact the site of absorption, namely the enterocytes [26]. Furthermore, the brush border and the cytosol of the absorptive cells are full of peptidases that will degrade the vaccines. There is an enormous challenge for development of vaccines that can reduce or even better avoid enzyme degradation, protecting the antigen and allowing the uptake of antigen by the enterocytes [27]. Table 1 shows the HI test of the immune response of the birds to niosome-entrapped vaccine and commercial vaccine. The commercial vaccine had a higher and rapid stimulation of the lymphoid cells but the niosomal vaccine had a sustained and higher overall stimulation of the antibodies than the commercial vaccine. The encapsulation of the vaccine in the niosome did not alter the antibody response but rather protected the antigen and enhanced immune response.
| Treatment group | Primary immunization *(log2) | Secondary immunization *(log2) |
|---|---|---|
| Negative control | 0.00 | 0.00 |
| Positive control | 5.50 ± 0.67 | 6.00 ± 0.63 |
| Span 20 niosome | 3.20 ± 0.85 | 7.10 ± 0.18 |
*The antibody titres are expressed in log base 2
Table 1: Immune response of the birds to the vaccine formulations
References
- Buckton G. Interfacial phenomena in Drug Delivery and Targeting. Switzerland: Academic Publishers; 1995. p. 154-5.
- Handjani-Vila RM, Ribier A, Rondot B, Vanlerberghe G. Dispersions of lamellar phases of non-ionic lipids in cosmetic products. Int J Cosmetic Sci 2003;1:303-14.
- Azmin MN, Florence AT, Handjani-Vila RM, Stuart JF, Vanlerberghe G, Whittaker JS. The effect of non-ionic surfactant vesicle (niosome) entrapment on the absorption and distribution of methotrexate in mice. J Pharm Pharmacol 2005;37:237-42.
- Weissman G, Bloomgarden D, Kaplan R, Cohen C, Hoffstein S, Collins T, et al. A general method for the introduction of enzymes, by means of immunoglobulin-coated liposomes, into lysosomes of deficient cells. ProcNatlAcadSci 1975;72:88-92.
- Hunter CA, Dolan TF, Coombs G, Baillie AJ. Vesicular systems (niosomes and liposomes) for delivery of sodium stibogluconate in experimental murine visceral leishmaniasis. J Pharm Pharmacol 1988;40:161-5.
- Khandare JN, Madhavi G, Tamhankar BM. Niosomes novel drug delivery system. East Pharm 1994;37:61-4.
- Gayatri Devi S, Venkatesh P, Udupa N. Niosomalsumatriptan succinate for nasal administration. Int J Pharm Sci 2000;62:479-81.
- Yoshida H, Lehr CM, Kok W, Junginger HE, Verhoef JC, Bouwistra JA. Niosomes for oral delivery of peptide drugs. J Control Rel 1992;21:145-53.
- Sheena IP, Singh UV, Kamath R, Uma Devi P, Udupa N. Niosomalwithaferin A, with better tumor efficiency. Indian J Pharm Sci 1998;60:45-8.
- Azmin MN, Florence AT, Handjani-villa RM, Stuart JEB, Valerberghe G, Wittaker JS. The effect of non ionic surfactant vesicle (noisome) entrapment on the absorption and distribution of methotrexate in mice. J Pharm Pharmacol 1985;37:237-42.
- Schreier H. Liposomes and niosomes as topical drug carriers: Dermal and transdermal delivery. J Controlled Rel 1985;30:863-8.
- Office International Des Epizooties (OIE) (World Organization of Animal Health) OIE Manual of Standards of Diagnostics Test and Vaccines; 2004.
- Raja Naresh RA, Chandrashekhar G, Pillai GK, Udupa N. Antiinflammatory activity of Niosome encapsulated diclofenacsodiumwith Tween-85 in Arthritic rats. Ind J Pharmacol 1994;26:46-8.
- Parthasarathi G, Udupa N, Umadevi P, Pillai GK. Niosome encapsulated of vincristine sulfate: Improved anticancer activity with reduced toxicity in mice. J Drug Target 1994;2:173-82.
- Yoshioka T, Stermberg B, Florence AT. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60, and 80) and a sorbitantriester (Span 85). Int J Pharm 1994;105:1-6.
- Silver BL, editors, The Physical Chemistry of Membranes. New York: Alan and Unwin and Soloman Press; 1985. p. 209-30.
- Yoshioka T, Sternberg B, Moody M, Florence AT. Niosomes from Span surfactants: Relations between structure and form. J Pharm PharmcolSupp 1992;44:1044.
- Derjaguin BV, Landau L. Zeta potential: An introduction in 30 minutes. ActaPhysiochim. URSS 1941;14:633.
- Gregoriadis G. Immunological adjuvants: A role for liposomes. Immunol Today 1990;11:89-97.
- Gupta RK, Relyveld EH. Adjuvants - A balance between toxicity and adjuvanticity. Vaccine 1993;11:293-306.
- Gall D. The adjuvant activity of aliphatic nitrogenous bases. Immunol 1966;11:369-86.
- Bomford, R. Adjuvants for anti-parasite vaccines. Parasitol Today 1989;5:41-6.
- Malhotra M, Jain NK. Niosomes as Drug Carriers. Indian Drugs 1994;31:81-6.
- Aliasgar S, Ambikanandan M. Studies in topical application of niosomally entrapped nimesulide. J Pharm PharmSci 2002;5:220-5.
- Aliasgar S, Tushar KV, Mansoor MA. Nanocarriers for systemic and mucosal vaccine delivery. Recent Pat Drug DelivFormul 2007;1:1-91.
- Lee VH, Robinson JR. Enzymatic Barriers to Peptide and Protein Absorption. CRC Crit Rev Ther Drug Carrier Syst 1998;5:69-97.
- Alleman E, Leroux JC, Gurny R. Polymeric Nano-and Microparticles for the oral delivery of peptides and peptidomimetics. Adv Drug Del Rev 1998;34:171-89.