- *Corresponding Author:
- K. K. Pradhan
Department of Pharmaceutical Sciences and Technology
Birla Institute of Technology, Mesra, Ranchi-835 215, India
E-mail: kkpradhan@bitmesra.ac.in
| Date of Submission | 08 September 2016 |
| Date of Revision | 19 July 2017 |
| Date of Acceptance | 20 February 2018 |
| Indian J Pharm Sci 2018;80(2):342-349 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Granisetron, a selective 5-HT3 receptor antagonist has a good safety profile but a shorter half-life hence is only suited for a multiple dosage regimen, which increased side effects. Ion-exchange resins were extensively investigated as potential drug release modulators and the ability to get encapsulated along with drugs appeared to be a distinct advantage. Optimized conditions for drug loading using ion-exchange resins Kyron T-114 and Kyron T-134 were evaluated. The microencapsulated drug-resin complexes were incorporated into oral dispersible tablets prepared using crospovidone as a superdisintegrant. The in vitro release profile of the optimized formulation of F4 was investigated, which showed linearity of Higuchi's rate kinetic equation. The shelf life of the optimized formulation was estimated to be 6.7 y, based on accelerated stability study data obtained. The physical and solution 3-month stability studies with the three formulations, F4, F5 and F6, showed better disintegration times.
Keywords
Granisetron, Kyron T-134, Kyron T- 114, microencapsulation, oral dispersible tablet, stability study
Granisetron (C18H24N4O), endo-n-(9-methyl-9- azabicyclo [3.3.1]non-3-yl)-1-methyl-1H-indazole- 3-carboxamide, is a 5-HT3 receptor antagonist used as an antinauseant and antiemetic agent in various disease conditions [1]. Granisetron with a low sideeffect profile is used to treat the chemotherapy-induced nausea and vomiting, radiotherapy-induced nausea and vomiting, postoperative nausea and vomiting since heart rate, blood pressure were affected to a very little extent [2]. However, the plasma half-life of granisetron hydrochloride is less than 10 h, and the dosage lasts for only 4-9 h [3]. This recommended dosage regimen is to administer two to three times a day to increase its efficacy. Sustained-release dosage forms of granisetron have shown a clear advantage over other setrons [4-6]. The need for a sustained-release dosage form eventually arises in order to improve patient compliance and ease of administration.
In recent years, oral dispersible tablets (ODTs) have attained popularity due to increased patient compliance and ease of administration. Geriatric and paediatric patients with a difficulty in swallowing benefit from the ODTs [7]. The US Food and Drug Administration proposed in guidance for the definition of ODTs. “ODTs should have an in vitro disintegration time of approximately 30 s or less” [8]. ODT disintegrates in the mouth and releases the drug into the stomach for absorption. However, there has been a disadvantage in an ODT with an immediate release of drug dose into the system, which may lead to dose dumping [9]. In order to sustain the release of drug from an ODT, it may be necessary to include a multi-particulate system. The ion-exchange resin could produce a sustained release effect when it complexes with the drug [10]. This multi-particulate system is mostly encapsulated using a polymer, which would provide a barrier for drug diffusion and further prolong the release effect [11-13]. The multi-particulate system is introduced into an ODT and compacted at a shear rate not to rupture the microcapsules. This might provide a chance to avoid the dose dumping in an immediate-release dosage form. The encapsulated particle size should be ≤300 μm, at which the particulate nature is not perceived by the tongue.
In the past few years, ion-exchange resins have gained a substantial attention from pharmaceutical scientists because of their versatile properties as drug delivery vehicles. The ion-exchange resins are applicable in novel drug delivery system and in biomedical techniques. For immediate and sustained release purpose, several ion-exchange resin products have been developed for oral and per oral administration. Kyron is a derivative of cross-linked polyacrylic polymer used to mask the bitter of medicines and hence to achieve the stability of drugs. It is white to off white fine coloured powder and is easily swellable in water. Many grades of Kyron like Kyron T-104, Kyron T-114, Kyron T-134, Kyron T-154, Kyron T-159, Kyron T-123 are used. Kyron T-114 is a weak acid derivative of methyl acrylic acid cross-linked polymer having carboxylic acid functional group, which is responsible for its taste masking property. It is a white to off white free flowing, high purity polymer powder used to mask taste of many drugs specifically β-lactam antibiotics. It ionises in H+ ionic form and has moisture content less than 5 %. Kyron T-134 is also a weak acid derivative of acrylic acid cross-linked polymer having carboxylic acid functional group, which ionises in K+ ionic form. It is also a white to off white free flowing powder having moisture content less than 10 % [14].
The cancer chemotherapy involves many side effects, which might be affected by the stability of the drug. In this case the shelf life estimation of the formulation is an important criterion along with the physical stability of the formulation. The ion-exchange resin not only imparts a sustained release property to the multiparticulate formulation but also improves the stability of the drug. Many reports include the polymeric encapsulation of the resin complex to improve the extended release property [15-19].
Materials and Methods
Kyron T-114 and Kyron T-134 were obtained from Corel PharmaChem, Ahmedabad, India, These resins were washed successively with distilled water and methanol for several times and dried at 60° for 24 h until the total moisture content was evaporated. Granisetron hydrochloride was obtained from Zhongbao Chemicals Co. Ltd., China. Eudragit polymers of grade RSPO and RLPO were gifted by Evonik Degussa India Pvt. Ltd., Mumbai, India.
Preparation of drug-resin complex
The drug-resin complexes were prepared by batch method [20]. The resin particles were dispersed in a minimum amount of water and allowed to swell for about 10 min. Then an amount of drug was added to the above solution in the ration 1:2 of resin and was stirred using a magnetic stirrer at room temperature. The drug loading onto the resin was determined by analysing the supernatant at 302 nm using a UV spectrophotometer after filtering the drug-resin complex. The filtered drugresin complex was then dried at room temperature for 24 h. The drug loading onto the resin was evaluated with varying factors such as pH, drug to the resin ratio and stirring time.
Microencapsulation of the drug-resin complex
Microencapsulation of the drug-resin complex was performed using the solvent evaporation method. About 50 mg of magnesium stearate was dispersed in 1 ml of PEG 400. Subsequently, a solution of 13.5 ml of acetone and 1.2 g of the polymer was prepared and later added to the first solution to form a suspension of milky white in colour. This suspension was slowly added to a mixture of 135 ml of liquid paraffin and 15 ml of hexane, which was continuously, stirred using motor stirrer for 4 h at an RPM of 400. Polymers of Eudragit were used during the microencapsulation procedure as shown in Table 1.
| Ingredients | EU1 | EU2 | EU3 |
|---|---|---|---|
| Eudragit RSPO | 0.6 | 1.2 | - |
| Eudragit RLPO | 0.6 | - | 1.2 |
| Acetone | 13.5 | 13.5 | 13.5 |
| Hexane | 15 | 15 | 15 |
| PEG400 | 1 | 1 | 1 |
| Magnesium stearate | 50 | 50 | 50 |
Table 1: Microencapsulation polymers used in the solvent evaporation process
The microencapsulated beads obtained were allowed to settle overnight, and vacuum filtered. The beads were washed with hexane to remove the oily layer on the beads [21]. The collected beads were dried at room temperature and stored in a plastic pouch at room temperature.
Entrapment efficiency
Entrapment efficiency of the Eudragit RSPO, RLPO, RSPO:RLPO (1:1) microcapsules was analysed in 0.1N HCl. An equivalent amount of microcapsules of the granisetron HCl (11 mg) were weighed and added to a 100 ml of 0.1 N HCl and sonicated for about 30 min and filtered. A 10 ml solution of filtrate was diluted up to 10 times and analysed using UV spectrophotometry at 302 nm.
Morphological characterization of the microcapsules
Scanning electron microscopy (SEM) analysis was done to evaluate the morphological characteristics of the microcapsules obtained. SEM was carried out using Jeol, JSM 6390 LV, Japan. Prior to examination, the samples were platinum sputter-coated to render them electrically active and studied under different magnifications to analyse the texture and appearance of the particle.
Micrometric properties
Flow property of the blends was evaluated by estimating the Carr’s index, Hausner’s ratio, tapped density and bulk density. The bulk density and tapped density were estimated by the tap density tester of ETD-1020 Electrolab.
Compression of the tablets
ODTs were prepared by direct compression method using a superdisintegrant, crospovidone at 5 % w/w of the compression weight of the tablet as shown in the Table 2. The microencapsulated beads were mixed with the bulk of the tablet except the lubricants, magnesium stearate and sodium stearyl fumarate, and blended together. To the above blend, lubricants were added to compress a 150 mg tablet using a 16-station single rotary tablet press.
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| EU1 | 10.5 | 10.5 | 10.5 | ||||||
| EU2 | 9.1 | 9.1 | 9.1 | ||||||
| EU3 | 9.1 | 9.1 | 9.1 | ||||||
| Crospovidone | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Accecel 200 | 100 | 100 | 100 | ||||||
| Accecel 102 | 129.5 | 131 | 131 | ||||||
| Direct compressible grade starch | 88 | 89.4 | 89.4 | ||||||
| Mannitol | 20 | 20 | 20 | 31 | 31 | 31 | |||
| Lactose | 20 | 20 | 20 | ||||||
| Magnesium stearate | 1.5 | 1.5 | 1.5 | ||||||
| Sodium stearyl fumarate | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | ||||
| Aerosil | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | |
| Total | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 |
Table 2: Formulation design of oral dispersible tablet prepared by direct compression method
Evaluation of the tablets
The tablets were evaluated for their physical properties and disintegration times. The disintegration of tablets was evaluated through disintegration tester of Electrolab. Six tablets were allowed to disintegrate in the buffer pH of 6.8 and the time interval to pass through the sieve was noted. The hardness of the tablet was tested by Monsanto hardness tester and the friability by Electrolab friabilator.
In vitro release study
An in vitro release study was performed using USP apparatus II in a dissolution medium of 0.1 N HCl of 500 ml volume for first 2 h, then a 400 ml of 0. 1 M tribasic sodium phosphate was added to the above media after 2 h, the gastric transit time. The dissolution media pH was made up to 6.5 using 1 N HCl and the volume was finalised to 1000 ml. The dissolution was performed at 37±0.5° temperature and an RPM of 100 [22]. The samples were collected at different time intervals for 10 h, by a syringe and passed through 0.45 μ filter and then a 20 μl of the filtered sample was injected into a high performance liquid chromatography (HPLC).
HPLC
A Hypersil C18 column of 4.0×150 mm with a particle size of 5 μm was used. The HPLC system of Shimadzu equipped with a LC-10AD pump, Rheodyne manual injector consisting of a 20 μl loop and SPD-10 a UV detector was used. Shimadzu LC solution software was used as an interface to record the HPLC peaks and data analysis. The mobile phase consists of monobasic sodium phosphate buffer of pH 2.0, methanol and tetrahydrofuran in the ratio of 75:24:1.
Stability study
Stability studies were performed for 3 mo at a controlled temperature and humidity conditions of 40±2°/75±5 % RH, 30±2°/65±5 % RH and 5° [23]. The formulations F4, F5 and F6 were analysed for their drug content, drug release kinetics, tablet disintegration time, tablet friability and hardness stability studies.
Drug content
Five tablets were placed in a 50 ml solution of 0.1 N HCL and sonicated for about 30 min. The solution was filtered and analysed. The data was obtained at different intervals during the stability study period of 0, 1, 2, and 3 mo. A 10 ml solution of the filtrate was diluted with the buffer solution having pH 2.0 and volume was made up to 50 ml. Then the 20 μl of the above solution was injected as test solution into the HPLC system equipped with a 300 nm detector and 4.0×150 mm, 5 μm, C18 column and the mobile phase used was a mixture of buffer solution having pH 2.0, methanol, and tetrahydrofuran in the ratio of 75:24:1. The flow rate was 1.2 ml/min. The chromatogram and peaks were analysed and theoretical plates were greater than 1200 and tailing factor was in the range of 0.8-1.5. The percent drug at different point of stability times 0, 1, 2 and 3 mo during the stability study was calculated.
Drug release profile
Drug release from the formulations was estimated using the USP dissolution apparatus 2. For dissolution medium, 0.1 N HCl was used for the first 2 h. The tablets were placed in a solution of 500 ml 0.1 N HCl at a temperature of 37±5° and 100 rpm. Then 400 ml of 0.1 N tribasic sodium phosphate was added to the above medium and pH was adjusted to 6.8 with 1 M HCl and the volume was made up to 1000 ml. The drug content was analysed spectrophotometrically at 302 nm. The drug release profile was measured at regular time intervals of 0, 1, 2 and 3 mo using the HPLC method proposed above.
Disintegration time
The disintegration time of the tablets was determined using the disintegration apparatus having phosphate buffer solution of pH 6.8 as medium. The conditions maintained for stability studies were 40±2º/75±5 % RH at a temperature of 37±0.5o.
Tablet hardness and friability
The hardness and friability of tablets were tested for their stability studies at a condition of 40±2°/75±5 % RH for 0, 1, 2 and 3 mo. Hardness of the tablet was measured using Monsanto hardness tester and the results were collected as kg/cm2. The friability of the tablets was analysed by Electrolab Roche Friabilator. The percent weight loss during the friability of the tablets was not exceeded by 1 % of the initial weight.
Shelf life estimation
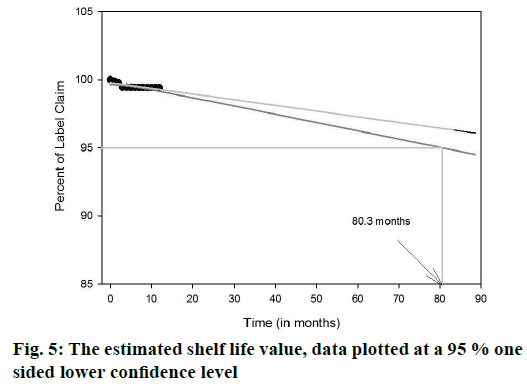
The shelf life was estimated with the help of Sigma plot software of Systat solutions. Three month mean stability data was extrapolated to 12 mo. The extrapolated data was collected and based on this extrapolated data a lower confidence interval of 95 % was estimated. Finally, the graph was plotted for the label claim of the drug remaining at the end of the stability on y-axis and time in months on x-axis with a confidence band of 95 %.
Results and Discussion
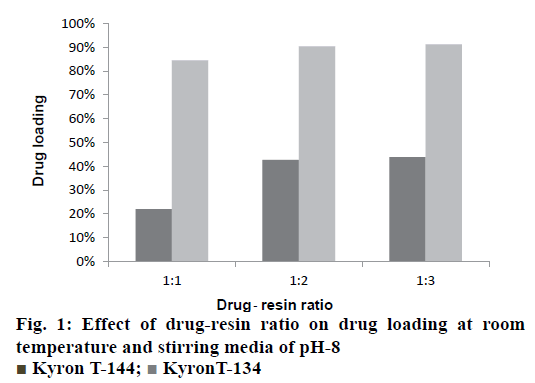
The drug-resin complex was optimized based on the different conditions of drug loading on to the resin. The optimisation of drug loading was carried out step by step based on the various factors. The Figure 1 and 2 shows the effect of drug to resin ratio and pH effect, respectively. Based on the values from Table 3, Kyron T-134 resin has shown a better drug loading in comparison with Kyron T-114 and there is a gradual increase in amount of drug loading up to 2 h of stirring time after which there is not much change in the drug loading. The conditions were chosen as a drug to the resin ratio of 1:2 with a pH of the solution as 8 and the optimum stirring time to be 2 has about 90 % of drug complex was formed in 2 h after which there is no significant increase in the drug loading. The effect of media pH was evident, during basic pH there was a gradual increase in the drug loading capacity of the resin. The ratio of drug to resin was selected as 1:2 as there was no significant increase in the drug loading at a ratio of 1:3. The microcapsules of EU3 have been with a smooth texture when compared to that of the EU1 and EU2. The EU3 microcapsules with RLPO polymer have shown a smooth texture with a mean particle size of 334 μm, whereas the EU1 and EU2 were evaluated for their texture they have comparatively a rough surface texture than EU3. The SEM images of the three microcapsules were depicted in the Figure 3. However, the mean particle size as observed was decreased in both EU1 (261 μm) and EU2 (213 μm). The particle size below 300 μm was to be chosen because this particle size gives no feel of particle presence on the tongue.
| Time (h) | Drug loading | |
|---|---|---|
| Kyron T-134 resin | Kyron T-114 resin | |
| 1 | 75.60 % | 19.50 % |
| 2 | 90.37 % | 32 % |
| 3 | 91.70 % | 38.90 % |
| 4 | 93.90 % | 40 % |
Table 3: Effect of stirring time on drug loading
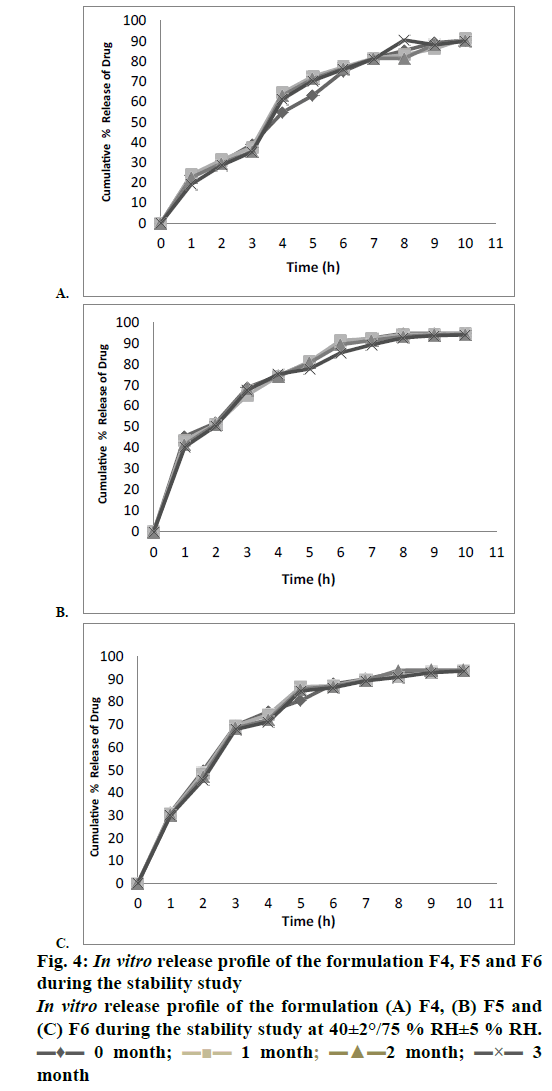
The entrapment efficiency of the microspheres was observed to increase when the polymers in combination were used; EU1 has shown a better yield than EU2 and EU3. The EU2 and EU3 have shown no significant difference. The entrapment efficiency of the microspheres was evaluated with a recovery of 97.3, 98.4 and 98.8 % for EU1, EU2, and EU3, respectively. Crospovidone was chosen to be the super disintegrant in a 5 % w/w of the tablet weight. The formulations F1, F2 and F3 magnesium stearate was used as a lubricant showing a disintegration time of >1 min. Sodium stearyl fumarate was used in the formulations F4-F9, which eventually decreased the disintegration time. The blend properties such as Carr’s index and Hausner’s ratio, of the formulations F1 to F9 were shown in the Table 4. The formulations were optimized based upon the disintegration times, and in vitro release profile. The disintegration times of F1, F2 and F3 formulations were found to be >1 min, where there is a requirement of disintegration times of <30 s for an ODT. However, the formulations F4, F5, F6, F7, F8 and F9 show desirable amount disintegration in less than <20 s. The hardness and friability of the formulations provide a good handling capacity of the ODTs; the values of disintegration, hardness and friability are tabulated in Table 5. The friability of the F7, F8 and F9 formulations was around 0.7 % and the formulations F4, F5 and F6 showed a relatively less friability than the prior ones. For the consideration of optimized formulations, F4, F5 and F6 were selected based upon the disintegration times, which are further evaluated for the in vitro release profile, Table 6 shows the release profile of the three formulations F4, F5 and F6 formulations. The F4 formulation was proved to show a more sustained release effect than its counterparts. The release kinetics performed for the F4 formulation has shown an r2 value close to unity for Higuchi rate kinetic Eqn. The assay was performed during the stability study period to estimate the percent recovery of drug from the formulation. For the stability study, three formulations were selected F4, F5 and F6, which differ in the polymer of the microcapsules. The percent recovery of the drug during the time interval of 1, 2 and 3 mo kept under the conditions of 40±5°/ 75±5 %, there was no significant change in the percent recovery of the drug from the formulations. The percent drug recovery at 30±2°/65±5 % RH and at 5° is also tabulated in Table 7, which showed no notable change in the drug content. This might prove that complexation of ion-exchange resin to drug increase the stability of the drug molecule. The tablets were evaluated for their physical change and no significant change in the colour or appearance of the tablet was observed. The friability and hardness were tested during the stability study of the samples kept under the conditions of 40±2°/ 75±5 % RH. There was an insignificant change in the friability and hardness as shown in the Table 8 of the formulations. The change in the friability and hardness of the formulations would show worst effect during the tablet handling and administration of the ODT. The stability study for this formulation was mainly performed to analyse the changes of drug release profile. As in a multi-particulate system of drug-resin complex encapsulated in a microsphere, there is an increased amount of stability of the drug. The only limiting factor in the stability study was the degradation of the polymer, which might affect the release rate of the drug from the microcapsule [24-26]. However, there was no significant change in the release profile of the three polymer formulations during the stability study as observed in the Figures 4A, B and C for the F4, F5 and F6 formulations, respectively. The shelf life of the optimized ODT formulation (F4) was estimated to be 80.3 mo (around 6.5 y). The percent drug remaining was plotted against time in months at a lower confidence interval of 95 %. Figure 5 shows the estimated shelf life of the formulation F4.
| Property | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Carr's index | 16.2 | 20.3 | 19.2 | 7.14 | 18.7 | 15.4 | 8.2 | 10.5 | 9.2 |
| Hausner’s ratio | 1.193 | 1.2547 | 1.273 | 1.076 | 1.23 | 1.182 | 1.09 | 1.1173 | 1.103 |
Table 4: Carr’s index and Hausner’s ratio of the formulations F1 to F9
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Disintegration Hardness Friability |
155 5.2 0.26 |
135 4.9 0.254 |
129 5.4 0.26 |
15 3.8 0.54 |
15 4 0.62 |
15 4.1 0.65 |
12 3.50 0.72 |
12 3.6 0.75 |
12 3.6 0.75 |
Table 5: Disintegration, hardness and friability of the oral dispersible tablet formulations
| Time (h) | F4 | F5 | F6 |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 1 | 23.65 | 31.2 | 45.62 |
| 2 | 30.23 | 49.72 | 52.065 |
| 3 | 38.97 | 69.65 | 68.75 |
| 4 | 54.8 | 75.68 | 74.52 |
| 5 | 63.2 | 80.63 | 80.82 |
| 6 | 75.09 | 87.92 | 90.54 |
| 7 | 81.54 | 90.15 | 92.32 |
| 8 | 85.32 | 93.19 | 94.58 |
| 9 | 89.2 | 93.4 | 94.62 |
| 10 | 90.41 | 93.89 | 94.65 |
Table 6: In vitro release profile of the optimised formulations f4, f5, f6oral dispersible tablets
| Stability conditions | Formulation | Drug content | |||
|---|---|---|---|---|---|
| 40±5°/75±2 % RH | F4 | 93.26 | 91.4 | 91.94 | 91.23 |
| F5 | 92.5 | 93.2 | 93.68 | 92.1 | |
| F6 | 97.3 | 97.15 | 97.89 | 96.7 | |
| 30±5°/65±2 % RH | F4 | 94.23 | 94.1 | 94.12 | 93.2 |
| F5 | 93.2 | 93.2 | 93.68 | 92.4 | |
| F6 | 98.2 | 98.1 | 97.9 | 97.35 | |
| 5° | F4 | 94.89 | 94.72 | 94.68 | 94.5 |
| F5 | 94.5 | 94.34 | 94.23 | 94.4 | |
| F6 | 97.6 | 97.54 | 98.2 | 97.4 | |
Table 7: Assay of the drug content during the stability period of 3 months
| Time (months) | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Disintegration timea | 15.34 | 15 | 14.67 | 14 |
| 15.67 | 16 | 16.34 | 15.34 | |
| 15.34 | 14.67 | 14 | 13 | |
| Friability | 0.54 | 0.58 | 0.56 | 0.54 |
| 0.62 | 0.59 | 0.59 | 0.57 | |
| 0.65 | 0.62 | 0.63 | 0.68 | |
| Hardness | 3.8±0.1 | 3.8±0.2 | 3.7±0.1 | 3.5±0.4 |
| 4±0.1 | 4±0.2 | 4±0.3 | 4±0.4 | |
| 4.1±0.1 | 4.1±0.2 | 4.1±0.3 | 4.1±0.4 |
aAll disintegration values were the average of three readings
Table 8: Disintegraton, hardness and friability values during the stability study at conditions of 40±2°/75 % RH±5 %
The study has revealed sustained release ODTs prepared by the solvent evaporation technique of microencapsulation of the drug-resin complex. The Kyron T-134 with 90 % as drug loading was the optimized resin for the microencapsulation. Sodium stearyl fumarate used as lubricant provided a better compressibility of microspheres without rupturing during the direct compression method. The in vitro release profile has shown a linear relationship when the Higuchi rate model was considered, this shows the sustained release profile of the formulation. In vitro release study conducted during the stability study showed no significant change in the release profile, where the microcapsule was considered stable under stress in the provided conditions. The formulations showed an insignificant amount of change in the disintegration times, friability and hardness. However, the ODTs need better packaging to improve the patient compliance and administration in spite the estimated shelf life of the product being greater than 6 y.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Aapro M. Granisetron: an update on its clinical use in the management of nausea and vomiting. Oncologist 2004;9:673-86.
- Ho KY, Gan TJ. Pharmacology, pharmacogenetics, and clinical efficacy of 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Curr Opin Anaesthesiol 2006;19:606-11.
- Blower P. A pharmacologic profile of oral granisetron (Kytril tablets). Semin Oncol 1995;22(4):3-5.
- Blower P, Aapro M. Granisetron vs ondansetron: is it a question of duration of 5-HT3 receptor blockade? Br J Cancer 2002;86:1662.
- Gebbia V, Cannata G, Testa A, Curto G, Valenza R, Cipolla C, et al. Ondasetron versus granisetron in the prevention of chemotherapy‐induced nausea and vomiting. Results of a prospective randomized trial. Cancer 1994;74:1945-52.
- Raftopoulos H, Cooper W, O’Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer 2015;23(3):723-32.
- Parkash V, Maan S, Yadav SK, Jogpal V. Fast disintegrating tablets: Opportunity in drug delivery system. J Adv Pharm Technol Res 2011;2:223.
- Guidance for Industry: Orally Disintegrating Tablets. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); 2008.
- Dey NS, Majumdar S, Rao ME. Multiparticulate drug delivery systems for controlled release. Trop J Pharm Res 2008;7:1067-75.
- Bhaskar R, Murthy RS, Miglani BD, Viswanathan K. Novel method to evaluate diffusion controlled release of drug from resinate. Int J Pharm1986;28:59-66.
- Motycka S, Nairn JG. Preparation and evaluation of microencapsulated ion‐exchange resin beads. J Pharm Sci 1979;68:211-5.
- Sriwongjanya M, Bodmeier R. Entrapment of drug-loaded ion-exchange particles within polymeric microparticles. Int J Pharm 1997;158:29-38.
- Torres D, Boado L, Blanco D, Vila-Jato JL. Comparison between aqueous and non-aqueous solvent evaporation methods for microencapsulation of drug-resin complexes. Int J Pharm 1998;173:171-82.
- Jha SK, Sharma RU, Surendra V. Taste masking in pharmaceuticals: an update. J Pharm Res 2008;1:126-30.
- Bhise MR, Narkhede MB, Mohod SP, Mohade AH. Development and evaluation of extended release ambroxol HCl and guaifenesin suspension. Res J Pharm Technol 2012;5:98-102.
- Huang RG, Schwartz JB, Ofner CM. Microencapsulation of chlorpheniramine maleate-resin particles with crosslinked chitosan for sustained release. Pharm Dev Technol 1999;4:107-15.
- Jeong SH, Berhane NH, Haghighi K, Park K. Drug release properties of polymer coated ion‐exchange resin complexes: Experimental and theoretical evaluation. J Pharm Sci 2007;96:618-32.
- Zeng HX, Wang M, Jia F, Lin SJ, Cheng G, Pan WS. Preparation and in vitro release of dual-drug resinate complexes containing codeine and chlorpheniramine. Drug Dev Ind Pharm 2011;37:201-7.
- Zeng HX, Cheng G, Pan WS, Zhong GP, Huang M. Preparation of codeine-resinate and chlorpheniramine-resinate sustained-release suspension and its pharmacokinetic evaluation in beagle dogs. Drug Dev Ind Pharm 2007;33:649-65.
- Anand V, Kandarapu R, Garg S. Ion-exchange resins: carrying drug delivery forward. Drug Discov Today 2001;6:905-14.
- Sahil K, Akanksha M, Premjeet S, Bilandi A, Kapoor B. Microsphere: A review. Int J Res Pharm Chem 2011;1:1184-98.
- Kumbar SG, Kulkarni AR, Aminabhavi TM. Crosslinked chitosan microspheres for encapsulation of diclofenac sodium: effect of crosslinking agent. J Microencapsul 2002;19:173-80.
- https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Q uality/Q1A_R2/Step4/Q1A_R2__Guideline.pdf.
- Gonzalez Vidal NL, Zubata PD, Simionato LD, Pizzorno MT. Dissolution stability study of cefadroxil extemporaneous suspensions. Dissolution Technol 2008;15:29-36.
- Zeng HX, Cheng G, Pan WS, Zhong GP, Huang M. Preparation of codeine-resinate and chlorpheniramine-resinate sustained-release suspension and its pharmacokinetic evaluation in beagle dogs. Drug Dev Ind Pharm 2007;33:649-65.
- Khamanga SM, Parfitt N, Nyamuzhiwa T, Haidula H, Walker RB. The evaluation of eudragit microcapsules manufactured by solvent evaporation using USP apparatus 1. Dissolution Technol 2009;16:15-22.
 Kyron T-144;
Kyron T-144;  KyronT-134
KyronT-134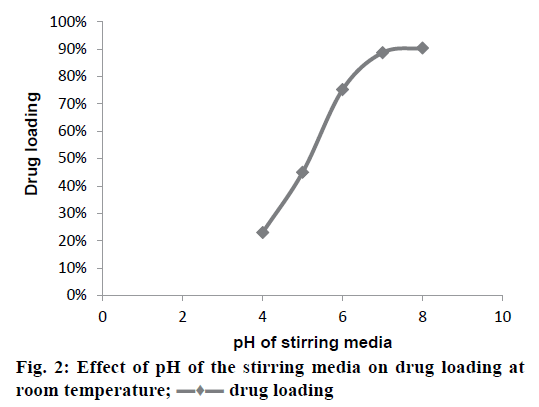
 drug loading
drug loading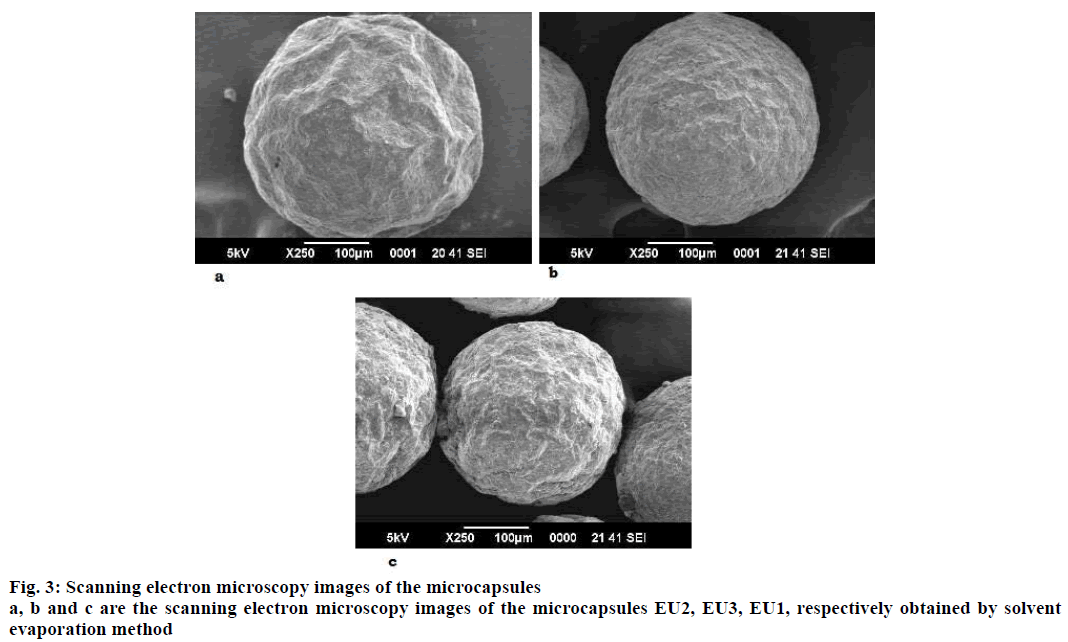
 1 month;
1 month;  2 month;
2 month;  3
3