- *Corresponding Author:
- S. Saranya
Department of Animal Biotechnology, Madras Veterinary College, Tamil Nadu Veterinary and Animal Sciences University, Chennai-600 007, India
E-mail: s.saranyabiotec@gmail.com
| Date of Submission | 15 October 2016 |
| Date of Revision | 31 January 2017 |
| Date of Acceptance | 03 July 2017 |
| Indian J Pharm Sci 2017;79(5): 688-694 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Iron nanoparticles were synthesized using Musa ornata flower sheath extract. The optimum precursor salt concentration, pH of the reaction mixture, ratio between reducing agent and precursor salt and time for the synthesis of iron nanoparticles were found to be 5 mM, 9.0, 3:7 and 0th h respectively. The synthesized iron nanoparticles were characterised by UV/Vis absorption spectroscopy, Fourier transform infrared spectroscopy, X-ray diffraction spectroscopy, atomic force microscope and particle size analyser. UV/Vis absorption showed a characteristic absorption peak of iron oxide nanoparticles in the range of 250-350 nm. Fourier transform infrared spectroscopy measurement was carried out to identify the possible molecules like carbonyl, CH and OH band. From the X-ray diffraction method, it was found that the average particle size of magnetite nanoparticles was found to be 43.69 nm. The synthesized iron nanoparticles had antibacterial activity against pathogenic bacteria like Staphylococcus aureus, Streptococcus agalactiae, Escherichia coli and Salmonella enterica by well diffusion method. This biosynthesis approach has been found to be cost effective, eco-friendly and promising for applications in various fields.
Keywords
Iron nanoparticles, Musa ornata, XRD, FTIR, AFM, antibacterial activity
Nanotechnology, with a wide range of applications in biology, medicine and engineering, is a rapidly growing field [1]. Nanotechnology is concerned with the synthesis of nanoparticles of various sizes and shapes and their potential applications [2,3]. Though synthesis of nanoparticles by physical and chemical methods offers well distinct and pure nanoparticles, these nanoparticles are quite expensive and may not be environment friendly [4-6]. Use of microorganisms and plant materials could be an alternative to the physical and chemical synthesis of nanoparticles. The vision of utilizing the natural resources for the synthesis of metal nanoparticles has turned out to be a competent and environmentally safe approach. Green synthesis of metal nanoparticles using extracts of plant components is considered to be less expensive and environment friendly. During the green synthesis of metallic nanoparticles, the polyol components present in the plant extracts are responsible for the bioreduction of metal ions whereas water soluble heterocyclic components stabilize the nanoparticles formed [7,8]. Metal nanoparticles are reported to have magnetic, catalytic, optical, antiinflammatory and antimicrobial properties. Among these properties antimicrobial property is considered to be one of the important property, which might have lot of potential in animal and human medicine. Apart from being eco-friendly and less expensive, plant extract mediated nanoparticles are reported to have extensive antimicrobial activity.
In the present study, iron nanoparticles (FeNPs) were synthesized using a rapid, single step, green biosynthetic method employing aqueous extract of Musa ornata (banana) flower sheath as both the reducing and capping agent. Further, the synthesised nanoparticles were tested for antimicrobial activity against pathogenic organisms.
Materials and Methods
Ferrous sulphate (FeSO4), sodium hydroxide (NaOH) and hydrochloric acid (HCL) was purchased from Merck, India Ltd. All the chemicals were in analytical grade and used without any further purification. Streptococcus agalactiae (ATCC 13813), Staphylococcus aureus (ATCC BAA 976), Salmonella enterica (ATCC 13076), and Esherichia coli (ATCC 43888) strains were purchased from American type culture collection, USA and used in the study. Nutrient agar and MacConkey agar was purchased from Himedia Pvt. Ltd., India. M. ornata flower was purchased from a local retail shop in Chennai and utilized for the preparation of flower sheath extract.
Preparation of M. ornata flower sheath extract
M. ornata flower sheath was collected and washed thoroughly in water to remove the dust and other particulate matter. Fine pieces of sheath in water (200 g/l) were heated at 60° for 20 min followed by filtering through filter paper to separate out the extract. The extract was stored at 4° for further experiments.
Synthesis of FeNPs and effect of various parameters on the synthesis of FeNPs
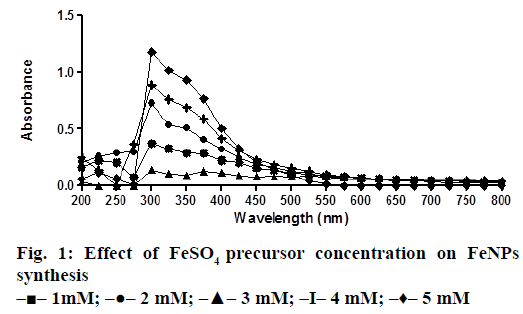
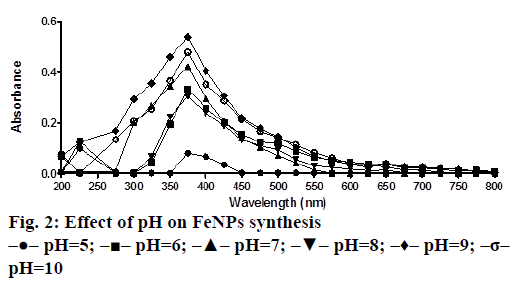
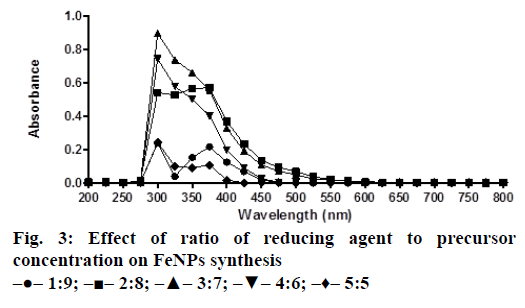
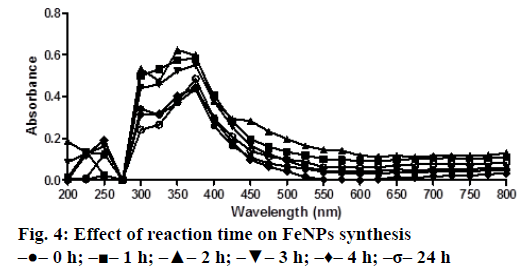
A solution of FeSO4 (1 to 5 mM) was prepared by dissolving the FeSO4 in distilled water. In a typical green synthesis protocol, M. ornata flower sheath extract was used to reduce and cap the Fe ions. 10 ml of sheath extract was added into the aqueous solution of FeSO4 with normal atmospheric pressure. The mixture was constantly stirred at 70-80° for 8 h and subjected to further stirring at room temperature overnight without heating. The effects of different concentrations of precursor salt solution (1 to 5 mM), pH and time (0, 2, 4, 6, 8, 24 h) on the stability of FeNPs were studied by UV/Vis spectrometry. The pH of reaction mixture was adjusted with 0.1 N HCL and 0.1 M NaOH solution (pH 4, 5, 6, 7, 8, 9 and 10) using calibrated pH meter. To check whether the ratio between reducing agent and precursor salt solution have any effect on the rate and shape of nanoparticles formation, the reaction mixtures were prepared in different ratios (1:9, 2:8, 3:7, 4:6 and 5:5) and the maximum absorption wavelengths of iron colloids were recorded in UV/Vis region (from 200 nm to 800 nm).
UV/Vis spectrophotometric analysis of FeNPs
Synthesis of FeNPs was preliminarily confirmed by recording the absorbance in UV/Vis spectra at a range of 200-800 nm. Change in surface plasmon resonance (SPR) of nanoparticles in the dispersion was recorded using UV/Vis spectrophotometer.
X-Ray Diffraction (XRD) and Fourier transform infra-red spectrophotometric (FTIR) analysis of FeNPs
FeNPs in the powder form were used for XRD and FTIR spectroscopy analysis. The XRD patterns were collected on Bruker AXS D8 Advanced X-ray diffractometer with Cu Kα radiation and scanning angle 2θ over the range of 10-80°. XRD patterns were calculated using X‘per Rota flex diffraction meter using Cu K radiation and λ=1.5406 Å. Crystallite size was calculated using Scherrer Eqn., CS= Kλ/β cos θ, where CS is the crystallite size, constant K=0.94, β is the full width at half maximum (FWHM)(β=FWHM x π/180), λ=1.5406×10-10 and cos θ=Bragg angle [9]. FTIR was used to characterize the nanoparticles using the powdered sample by KBr pellet technique in the range of 400-4000 cm-1.
Atomic force microscope (AFM), Zeta potential and particle size analysis of FeNPs
Synthesized FeNPs were characterized by AFM for their size, morphology and agglomeration. AFM image was taken with silicon cantilevers with contact mode. The mean particle size diameter and polydispersity indices of synthesized FeNPs were measured using photon correlation spectroscopy. The stability of nanoparticles in a colloidal suspension was determined based on the zeta potential value.
Antibacterial activity of FeNPs
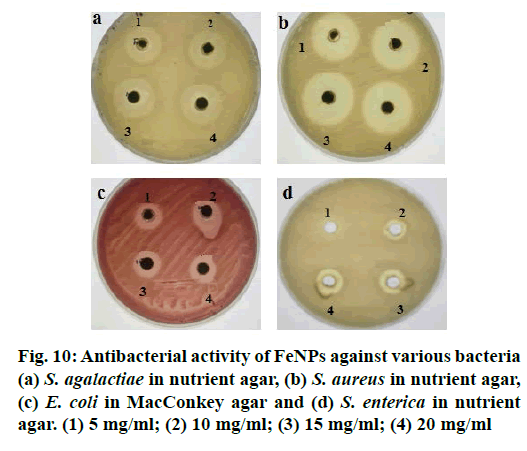
FeNPs synthesized using M. ornata flower sheath extract were tested for antibacterial activity by well diffusion method against S. agalactiae, S. aureus, S. enterica and E. coli. Twenty four hour fresh cultures of the bacterial pathogens were prepared on agar plates and the standardized (McFarland No.0.5) inoculums were used for the antibacterial assay. Powdered FeNPs were dissolved in sterile distilled water and sonicated. Four wells each of 5 mm diameter were made on each plate and the synthesized FeNPs solution, at a concentration of 1 to 4 mg/ml was loaded in each well. The plates were then incubated at 37° for 24 h and the zone of inhibition was measured.
Results and Discussion
Nanoparticulate technology has been found to have a wide range of applications since most of the biological processes occur at nanoscale levels. Chemical synthesis of nanoparticles involves the use of chemical reductants and the precursor salts in varying ratios. Establishing environment friendly nanoparticulate technology will be a welcome step in the modern scientific era. Green synthesis of metallic nanoparticles using plant extracts as reducing and capping agents is considered as a welcome step towards achieving eco-friendly and cheaper methods of generating nanoparticles. Moreover, green synthesis is gaining importance due to easy availability of plant materials and their pharmacological significance. Few metallic nanoparticles like MgO, CaO and ZnO have been reported to have antibacterial activities [10], which has prompted studies to assess the utility of metallic nanoparticles for their antibacterial activities. In present study, the flower sheath extract of M. ornata was used to synthesize FeNPs.
Synthesis of FeNPs was confirmed by the characteristic change of the sheath extract and aqueous FeSO4 mixture colour to dark yellowish brown. This indicated the reduction of aqueous iron ions to FeNPs when added to flower sheath extract of M. ornata and the colour reaction is the result of excitation of surface plasmon vibration in the metal nanoparticles [11].
The bio reduction of Fe ions in aqueous solutions was monitored by measuring UV/Vis spectra. UV/Vis spectral analysis was done at a wavelength range of 200-800 nm to study the absorption spectra of green synthesized FeNPs and the absorption peaks were observed at 250-350 nm ranges due to the excitation of surface plasmon vibrations in FeNPs as has been reported earlier [12]. Effect of precursor salt solution on nanoparticles synthesis revealed that 5 mM concentration of FeSO4 resulted in maximum nanoparticles synthesis with the absorption peak around 310 nm (Figure 1). The pH of the medium plays an important role in the size variability and dissolution kinetics of the nanoparticles [13]. As pH has been found to affect the stability of nanoparticles as well, in our study, the solution was adjusted to different pH and the concentration of FeSO4 was kept at 5 mM in 1:1 ratio. Maximum absorbance peak of FeNPs formation was found at pH 9.0 (Figure 2). As observed during the synthesis of silver nanoparticles [14], probably because of the availability of large number of functional groups for binding, more number of FeNPs were formed at pH 9.0. In general, alkaline pH was found to be optimum for the synthesis of metallic nanoparticles. The ratio between reducing and precursor salt solution implies the formation of nanoparticles and it varies from plant to plant. In the present study, maximum nanoparticle synthesis was achieved in the ratio of 3:7 (Figure 3). Though the reaction time is said to affect the stability of nanoparticles, we found no significant difference as far as reaction time is concerned after the addition of reducing agent (Figure 4).
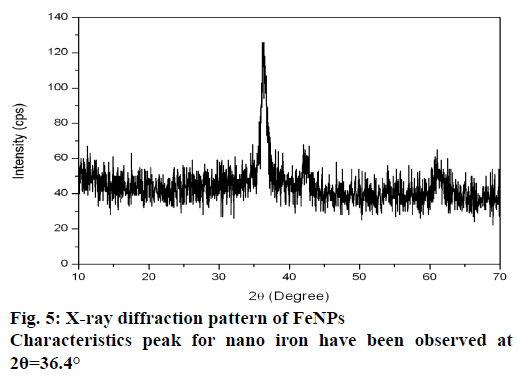
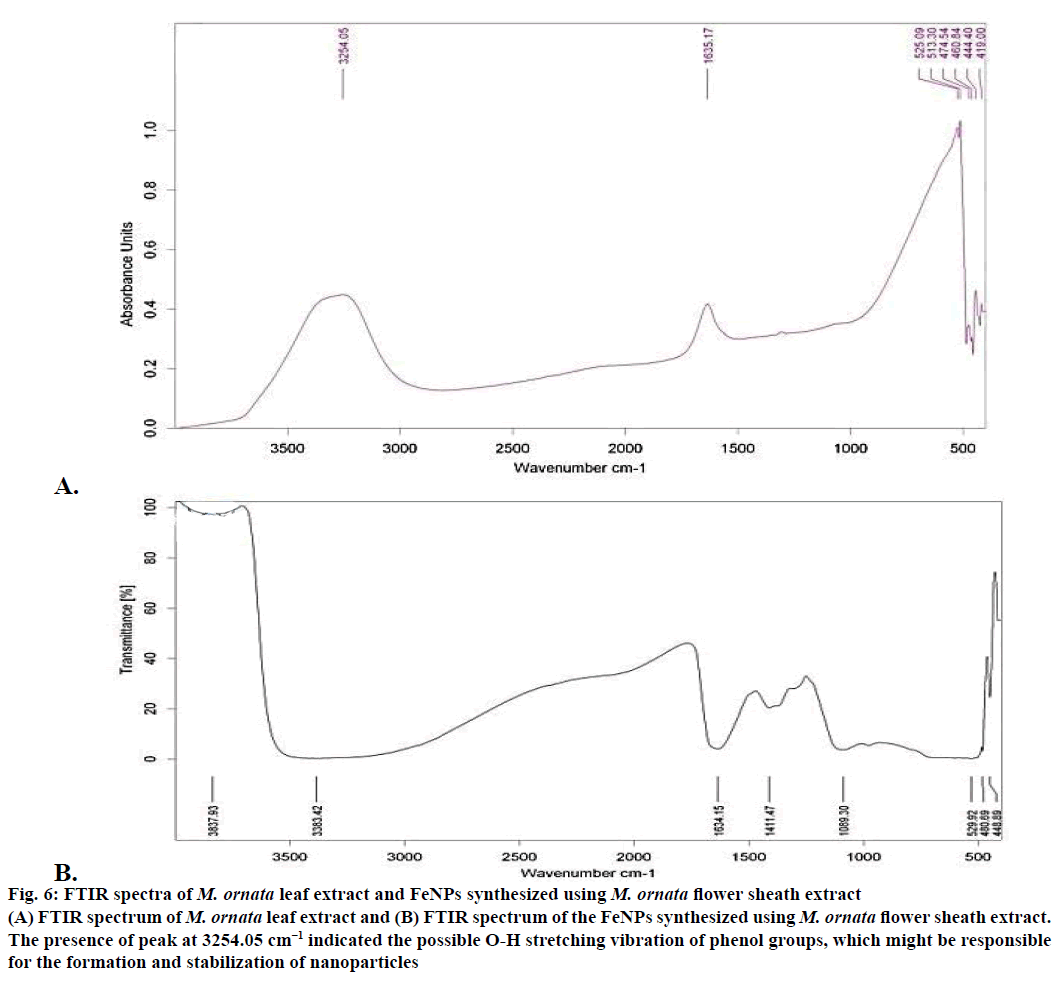
The XRD technique was used to assess the composition and phase of the nanoparticles generated. Characteristics peak for nano iron have been observed at 2θ=36.4° (Figure 5). This broadened diffraction peak suggested that the resultant nanoparticles are crystalline in nature with a size around 43.69 nm as calculated by Scherrer equation. FTIR spectra of M. ornata leaf extract are shown in Figure 6A. FTIR is used to study the surface interaction between synthesized nanoparticles with other molecules involved in the synthesis and stabilization of the nanoparticles [13]. The major absorption peaks in FTIR spectra of M. ornata leaf extract were mainly located at 3254.05, 1635.17, 525.09, 474.54 and 419.00 cm−1. The presence of peak at 3254.05 cm−1 indicate the possible O-H stretching vibration of phenol groups, which might be responsible for the formation and stabilization of nanoparticles [15].
The FTIR spectrum of synthesized FeNPs displayed three strong bands around 3383.42, 1634.15 and 480.69 cm−1 (Figure 6B). Kumar and Singhal have reported the presence of similar bands at 472, 1634 and 3438 cm−1 when colloidal β-Fe2O3 was synthesized in the presence of varying Co2+ amount [16]. Based on our study, the vibration bands could be assigned as 529.92 cm−1 (Fe), 1634.15 cm−1 (H2O bending vibration) and a broad peak at 3383.42 cm−1. Presence of organic molecule on the surface of Fe NPs has been reported to have an influence on the FTIR peaks [17] and the broad peak observed around 529.92 cm−1 instead of two sharp peaks, might be due to the organic molecule from the leaf extract on the surface of FeNPs. The weak band at 3837.93 cm−1 could well be attributed to the unsaturated nitrogen (C-N) compounds from the leaf extract.
Figure 6: FTIR spectra of M. ornata leaf extract and FeNPs synthesized using M. ornata flower sheath extract
(A) FTIR spectrum of M. ornata leaf extract and (B) FTIR spectrum of the FeNPs synthesized using M. ornata flower sheath extract.
The presence of peak at 3254.05 cm−1 indicated the possible O-H stretching vibration of phenol groups, which might be responsible for the formation and stabilization of nanoparticles
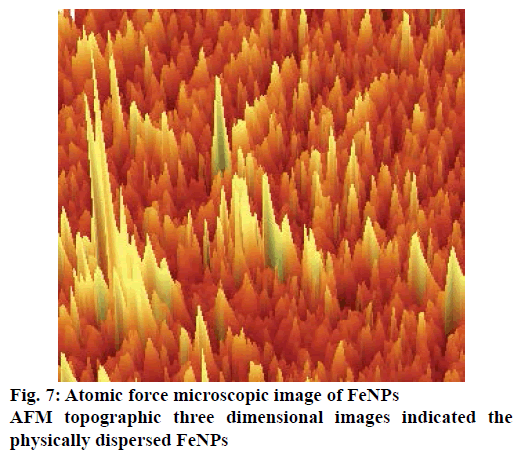
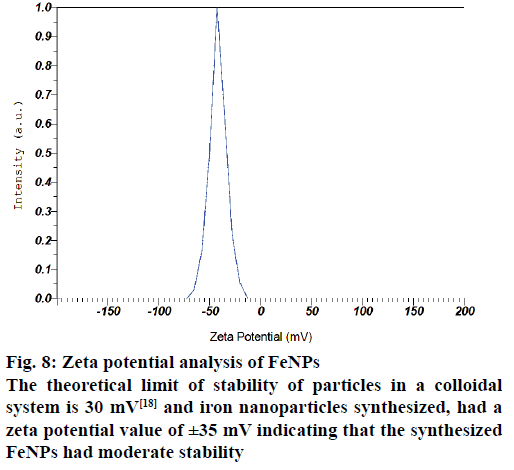
The AFM image of FeNPs has been presented as Figure 7. AFM topographic three dimensional images indicated the physically dispersed FeNPs. The magnetite nanoparticles were found to be oriented in one direction due to the magnetic properties. The surface potential of FeNPs was determined by zeta potential analyser, which is an essential characterization tool to assess the stability of the nanoparticles in aqueous solution. The theoretical limit of stability of particles in a colloidal system is 30 mV [18] and FeNPs synthesized by us had a zeta potential value of ±35 mV indicating that the synthesized FeNPs had moderate stability (Figure 8).
Figure 8: Zeta potential analysis of FeNPs
The theoretical limit of stability of particles in a colloidal system is 30 mV [18] and iron nanoparticles synthesized, had a zeta potential value of ±35 mV indicating that the synthesized FeNPs had moderate stability
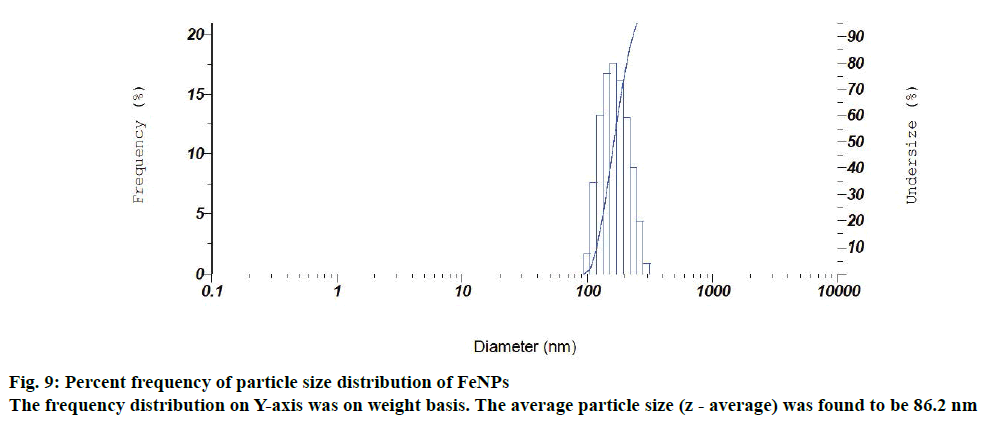
Particle size and size distribution of the FeNPs were further determined with the photon correlation spectrometer. Figure 9 depicts a normal particle size distribution (PSD) of the synthesized iron particles with respect to the diameter of the FeNPs. The frequency distribution (on Y-axis) was on weight basis. The average particle size (z - average) was found to be 86.2 nm and its polydispersity index was 0.305. The zeta potential and particle size analysis revealed that the synthesized FeNPs had moderate stability in colloidal suspension. For that reason the synthesized FeNPs had the average size of 43.69 nm (powder form) in XRD analysis. Whereas in case of particle size analysis, the synthesized FeNPs had an average particle size of 86.2 nm, which might be due to the aggregation of FeNPs in a colloidal suspension.
With ever increasing concerns over the use of antibiotics, look out for alternatives is on the rise. Molecules which do not lead to resistance are the most sought ones. Antimicrobial properties of metallic nanoparticles against certain pathogenic organisms have been reported. The antimicrobial activity of FeNPs synthesized using M. ornate flower sheath extract was investigated against most common foodborne pathogens and multi drug resistant bacteria like S. agalactiae, S. aureus, E. coli and S. enterica by well diffusion method. Though the FeNPs had a definite antibacterial activity against all the tested bacteria, no significant differences were noticed between the different pathogens except in case of S. enterica (Figure 10). The FeNPs did not have appreciable antibacterial activity at 5 mg/ml concentration compared to 10, 15 and 20 mg concentrations. The FeNPs synthesized by M. ornate flower sheath extract was found to have highest antimicrobial activity against S. aureus (32 mm) and S. agalactiae (28 mm).
It is concluded that, green synthesis of FeNPs using M. ornate flower sheath extract was successful at a higher pH of 9.0 without using any toxic chemicals as reducing and capping agents. The average crystal of FeNPs was found to be 43.69 nm and these nanoparticles had antibacterial activity.
Acknowledgements
The authors thank the Government of India and Government of Tamil Nadu for supporting this work under the National Agricultural Development Programme (RKVY). The authors also thank the Director of Research and Dean, Faculty of Basic Sciences, Tamil Nadu Veterinary and Animal Sciences University, Dean, Madras Veterinary College, Chennai, Professor and Head, Department of Animal Biotechnology, Madras Veterinary College and the Professor and Head, University Research Farm, Madhavaram for the facilities provided.
Conflict of interest
Nil.
Financial support and sponsorship
Nil.
References
- Albrecht MA, Evans CW, Raston CL. Green chemistry and the health implications of nanoparticles. Green Chem 2006;8:417-32.
- De D, Mandal MM, Gauri SS, Bhattacharya R, Ram S, Roy SK. Antibacterial effect of lanthanum calcium manganite (La0.67Ca0.33MnO3) nanoparticles against Pseudomonas aeruginosaATCC 27853. J Biomed Nanotechnol 2010;6:138-44.
- Dixon MB, Falconet C, Ho L, Christopher WK, O’Neill BK, Newcombe G. Removal of cyanobacterial metabolites by nano filtration from two treated waters. J Hazard Mater 2011;1882:88-95.
- Sastry M, Ahmad A, Khan MI, Kumar R. Microbial nanoparticle production. In: Niemeyer CM, Mirkin CA, editors. Nanobiotechnology: Concepts, Applications and Perspectives. Weinheim: Wiley-VCH Verlag GmbH and Co. KGaA; 2004. p. 126-35.
- Bhattacharya D, Rajinder G. Nanotechnology and potential of microorganisms. Crit Rev Biotechnol 2005;25:199-204.
- Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nano Part Res 2008;10:507-17.
- Rosi NL, Giljohann DA, Thaxton, CS, Lytton-Jean AKR, Han MS, Mirkin CA. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 2006;312:1027.
- Shchukin DG, Schattka JH, Antonietti M, Caruso RA. Synthesis of nano-sized magnetic ferrite particles inside hollow polyelectrolyte capsules. J PhysChem B 2003;107:952.
- Shobha G, Vinutha M, Ananda S. Biological synthesis of copper nanoparticles and its impact - a Review. Int J Pharm Sci Invent 2014;38:28-38.
- Sawai J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J Microbiol Methods 2003;54:177-82.
- Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effects of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureusand Escherichia coli. J Nanomedicine 2007;3:168-17.
- Pattanayak M, Nayak PL. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Azadirachtaindica(neem). World J Nano Sci Tech 2013;2:6-9.
- Sarkar B, Bhattacharjee S, Daware A, Tribedi P, Krishnan KK, Minhas PS. Selenium nanoparticles for stress resilient fish and livestock. Nanoscale Res Lett 2015;10:371.
- Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS. Cinnamon zeylanicumbark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf B Biointerfaces 2009;73:332-8.
- Latha N, Gowri M. Bio synthesis and characterization of Fe3O4 nanoparticles using Caricaya papayaleaves extract. Int J Sci Res 2014;3:1551-6.
- Kumar A, Singhal A. Synthesis of colloidal β-Fe2O3 nanostructures-influence of addition of Co2+ on their morphology and magnetic behaviour. J Nanotech 2007;18:475703.
- Lee J, Isobe T, Senna M. Preparation of ultrafine Fe3O4 particles by precipitation in the presence of PVA at high pH. J Colloid Interface Sci 1996;177:490.
- Duman O, Tunc S. Electrokinetic and rheological properties of Na-bentonite in some electrolyte solutions. MicroporMesopor Mat 2009;117:331-8.