- *Corresponding Author:
- R. K. Joshi
Department of Phytochemistry, Regional Medical Research Centre, Indian Council of Medical Research, Nehru Nagar, Belagavi-590 010, India
E-mail: joshirk_natprod@yahoo.com
| Date of Submission | 04 June 2016 |
| Date of Revision | 07 July 2017 |
| Date of Acceptance | 15 February 2018 |
| Indian J Pharm Sci 2018;80(2):383-390 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Zanthoxylum armatum DC. is used in traditional medicines as a therapy of many diseases including bronchial asthma. The antiasthmatic effect of Zanthoxylum armatum essential oil was studied using histamine aerosol-induced bronchoconstriction in guinea pigs and ovalbumin-sensitized albino mice. Absolute eosinophils count in blood, total immunoglobulin E, eosinophils and neutrophils in bronchoalveolar lavage fluid and histopathology of lung tissue were investigated. Treatment with Zanthoxylum armatum essential oil significantly increased the time of preconvulsive dyspnoea in histamine-induced guinea pigs. Oral treatment of Zanthoxylum armatum oil showed significant decrease in absolute eosinophils count, serum level of immunoglobulin E and the number of eosinophils, neutrophils in bronchoalveolar lavage fluid. Histopathological examination of lungs showed that essential oil rescinded bronchial asthma. Results obtained in the present investigation provided evidence that essential oil of Zanthoxylum armatum caused bronchorelaxation and showed antiasthmatic properties. The traditional use of Zanthoxylum armatum essential oil against asthma could be attributed to the bronchorelaxation and antiasthmatic activity.
Keywords
Zanthoxylum armatum, bronchorelaxation, antiasthmatic activity, essential oil
Asthma is a chronic disease involving the airways inflammation of the lungs. These airways or bronchial tubes allow air to come in and out of the lungs. Asthma can be classified as allergic asthma, caused by exposure to an allergen and non-allergic asthma caused by stress, exercise, illnesses like a cold or flu or exposure to extreme cold weather, irritants in the air or by some medications [1]. Sometimes asthma is called bronchial asthma or reactive airway disease. Asthma is characterized by inflammation of the bronchial tubes with increased production of sticky secretions inside the tubes. Common asthma symptoms include, coughing especially at night, wheezing, shortness of breath, chest tightness, pain or pressure [2]. The burden of asthma is immense, with more than 300 million individuals currently suffering from asthma worldwide, about a tenth of those living in India. The prevalence of asthma has been estimated to range 3-38 % in children and 2-12 % in adults, being the commonest chronic disorder among children [3]. Although currently available treatments for asthma include β-2 agonists, anticholinergics, methylxanthines, mast cell stabilizers, leukotriene antagonists, glucocorticoids, antiimmunoglobulin E (antiIgE) antibody like omalizumab, which are administered for long duration. Moreover, these treatments are associated with several adverse effects, like muscle tremors, restlessness, hypotension, hyperglycaemia, tachycardia, flushing, convulsions, mood changes and adrenal crisis [4]. To minimize and possibly prevent these side effects alternative and complementary medicine is being sought. Some essential oils obtained from plants have been traditionally used in respiratory tract infections. Essential oils inhalation therapy has been used to treat bronchitis as it has antiinflammatory effect on trachea [5].
Zanthoxylum armatum DC. (Rutaceae) is an important medicinal plant, which is commonly known as Indian prickly ash, Nepal pepper or toothache tree, Tejphal (Hindi), Tejowati (Sanskrit), and Mukthrubi (Manipuri and Nepal) [6]. In traditional medicine Z. armatum is used in treatment of asthma, bronchitis, colic, cough, convulsions, cardiac debility, diabetes, diarrhoea, dyspepsia, fever, goitre, difficult micturition, eye and ear disease, helminthiasis, hepatopathy, leprosy, leucoderma, paralysis, skin disease, stomach disorder, tumours, ulcers and wounds [7,8]. The Bhotiya tribes of India use this plant to treat cough and colds [9]. The major essential oil constituents such as 3-borneol, isobornyl acetate, dihydrocarveol [10], linalool, α-limonene diepoxide, α-pinene, myrcene, D-limonene [11], have been reported as the major constituent of the Z. armatum essential oil. Various biological activities viz. antioxidant activity [12], anticonvulsive, antinociceptive activity [13] and antifungal, antispasmodic, antibacterial [11] have been reported from essential oil of Z. armatum. The essential oil of Z. armatum has not been explored for its effect related to medicinal use as asthma disorder. Therefore, the aim of present study was to evaluate antiasthmatic activity of the essential oil of Z. armatum using in vivo models.
The following reagents were commercially purchased and used: ovalbumin (OVA, Sigma-Aldrich, USA), aluminium hydroxide, histamine dihydrochloride, Tween 80 (HiMedia, Mumbai, India), dexamethasone (Centaur Pharmaceuticals, Goa), Turk solution (Nice Chemical Pvt Ltd., Cochi), ethylenediaminetetraacetic acid disodium salt (EDTA-2Na) and Z. armatum oil (Kshipra Biotech Private Limited, Indore, India).
Female guinea pigs (400-600 g) purchased from J. N. Medical College, Belagavi, India and housed in standard conditions of temperature (22±2°), relative humidity (55±5 %) and light (12 h light/dark cycles) were used for bronchoconstriction activity. They were fed with vegetables, fruits, grass and water ad libitum. Female albino mice (18-25 g) were purchased from Sri Venkateshwara enterprises, Bengaluru, India and housed in standard conditions of temperature (22±2°), relative humidity (55±5 %) and light (12 h light/dark cycles) were used for bronchial asthma activity. They were fed with standard pellet diet and provided water ad libitum. The Institutional Animal Ethics Committee (IAEC) approved the experimental protocol (KLECOP/IEAC/Res.22- 10/10/2015). All experiments were conducted in strict compliance with the ethical principles and guidelines provided by Committee for the Purpose of Control and Supervision of Experiments on Animals. As per the IAEC advice, since Z. armatum is used in traditional medicine determination of toxicity of Z. armatum oil was not performed. However, as per literature available, the acute toxicity of rat and mice was determined to be safe therapeutic at a dose of 2000 mg/kg (limit dose) [13,14].
The chemical composition of the essential oil (1 % solution of essential oil in equal ratio of n-hexane:dichloromethane) was analysed using a gas chromatograph (GC; Varian 450 fitted with a fused silica capillary column TG-5, 5 % diphenyl-95 % dimethyl polysiloxane; Thermo Scientific, 30 m× 0.25 mm i.d. 0.25 μm film thickness) under the experimental conditions reported earlier [15-17]. The oven temperature was programmed from 60 to 220° at 3°/min, using nitrogen as carrier gas. The injector and the flame ionization detector temperature were set at 230 and 240°, respectively. Gas chromatography-mass spectrometer (GC-MS) analysis was employed a Thermo Scientific Trace Ultra GC interfaced with a Thermo Scientific ITQ 1100 mass spectrometer fitted with a BP-1 (100 % dimethyl polysiloxane; SGE Analytical Science) fused silica capillary column (30 m×0.25 mm; 0.25 μm film thickness). The oven temperature range was programmed from 60 to 220° at 3°/min, and helium was used as carrier gas at 1.0 ml/min for analysis. The injector temperature was set at 230°, and the injection volume was 0.1 μl in n-hexane, with a split ratio of 1:50. MS was taken at 70 eV with a mass range of m/z 40-450 and other parameters used were those reported earlier [18-20]. The major constituent of the essential oil of Z. armatum was identified and confirm (co-injection of commercial sample from Sigma-Aldrich, India (≥98 % purity).
Experimental bronchial asthma was induced in guinea pigs by exposing them to 0.1 % w/v histamine aerosol under constant pressure in an aerosol chamber (24×14×24 cm3) made of perplex glass. Each animal was placed in a chamber with histamine aerosol and pre-convulsive time (PCT; time of histamine aerosol exposure to guinea pig for initiating dyspnoea leading to the appearance of convulsions) was noted. On day 0 animals were kept in the histamine chamber and PCT was recorded as a baseline value. Day 0, PCT was taken as before treatment value. As per pre-convulsion dyspnoea (PCD) was recorded, the animals were removed from the chamber and exposed to fresh air for recovery. After development of PCD, the animals were divided into 4 groups (n= 6 in each group): group-I negative control, received histamine aerosols 0.1 % w/v; group-II received intraperitoneal injection of dexamethasone 2 mg/kg once daily for 7 d, group-III and IV received orally Z. armatum oil at the dose of 200 and 400 μl/kg once daily for 7 d. On the d 7, 2 h after the last dose, the time for the onset of PCT was recorded [21]. The percent increase in the time of PCT was calculated using following formula. Percent increase in PCT = (1–T1/T2)×100, where, T1= time for PCT onset on day 0, T2= time for PCT onset on day 7.
Female mice were divided into four groups (n= 6 in each group), normal, negative control, standard and treatment group. Except normal mice other groups were sensitized by intraperitoneal injection of 50 μg OVA and 1 mg aluminium hydroxide in 200 μl phosphate buffer saline (PBS) on day 1 and day 7. Group-I was given normal saline and feed for 14 d. After sensitization, from 8 to 14 d group-II was given OVA aerosols (1 % w/v) in PBS for 30 min. Group- III was given dexamethasone (2 mg/kg) intraperitoneal injection daily for 7 d along with OVA aerosols. Z. armatum oil was given orally to group-IV at a dose of 200 μl/kg for 7 d along with OVA aerosols. On day 15, 24 h after the final allergen lung lavage was performed for preparation of bronchoalveolar lavage fluid (BALF), trachea was aspirated (three time) with PBS until 2 ml of BALF was taken. The suspension of BALF was centrifuged and the supernatant collected and stored at –80°. Blood was collected in blood collecting tube containing disodium EDTA and absolute eosinophils were determined by direct microscopic counting with a haemocytometer. Blood serum was collected to estimate IgE level; lungs were collected for histopathological examination [22]. The accumulation of inflammatory cells in BALF was examined to evaluate airway inflammation. Briefly, 24 h after the final inhalation of antigen (day 15), animals were sacrificed by over dose diethyl ether inhalation, the left bronchus was tied for histological examination. Then, the right air lumen was washed four times with 0.5 ml PBS containing. BALF from each animal was pooled in a plastic tube, cooled in ice, and centrifuged (5000 g) at 4° for 10 min. Cell pellets were re-suspended in the same buffer (1 ml). A portion of the cell suspension was mixed with Turk solution and nucleated cells were counted in a haemocytometer.
Twenty-four hours after the last OVA challenge, mice were anesthetized with diethyl ether and blood was drawn. Differential cell counts were performed after staining with a modified Giemsa stain and cells with red cytoplasmic granules were counted as eosinophil’s to calculate absolute eosinophil’s count. The serum level of OVA-specific IgE was measured using an ELISA kit with commercially available reagents, according to the manufacturer's instructions. The process of measurement was same as cytokine analysis. The detection limit was 0.1 ng/ml for IgE.
The lungs were harvested after dissection and fixed in 10 % buffered formalin for 24 h, dehydrated, embedded in paraffin, sectioned into thin slices, stained with haematoxylin-eosin and observed by light microscopy, The degree of peribronchial and perivascular inflammation was observed.
Results were expressed as mean±SEM where n= 6. Differences among data were determined using one-way ANOVA followed by Dunnet’s multiple comparison test (GraphPad Prism software, version 5.01). P≤0.05 was considered statistically significant.
The GC-MS analysis of essential oil of Z. armatum revealed that the main compound was identified as linalool (75.7 %). The essential oil of Z. armatum was significantly and dose-dependently increased the latent period of PCD. Percent increase in PCT in histamineinduced bronchoconstriction at the dose of 200 and 400 μl/kg body weight was found to be 61.20±0.29 and 69.48±0.99 %, respectively. Hence, the increase of PCT at a dose of 400 μl/kg was observed maximum protection compared to standard drug (dexamethasone)- treated group (Table 1).
| Grouping | Before treatment (s) |
After treatment (s) |
% Increase in pre-convulsive dyspnoea time PCD (s) |
|---|---|---|---|
| Negative control group | 108 | 120 | 6.17±2.49 |
| Dexamethasone | 90 | 180 | 50.53±3.77* |
| Z. armatum 200 µl/kg | 249 | 642 | 61.20±0.29* |
| Z. armatum 400 µl/kg | 83 | 271 | 69.48 ±0.99* |
Each value was expressed as mean±SEM; where n= 6 in each group: *p≤0.05 as compared with control by one-way analysis of variance, followed by Dunnett’s test
Table 1: Effect of Z. armatum oil on histamine-induced bronchoconstriction in guinea pigs
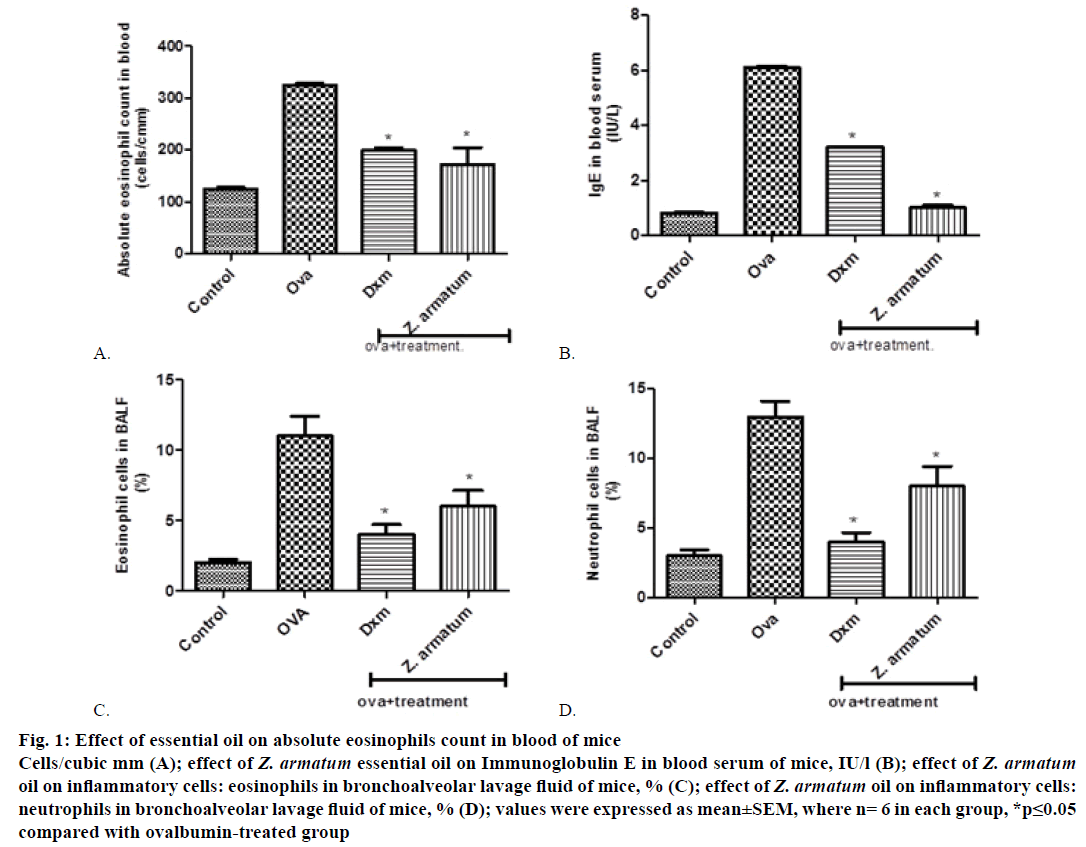
Control group showed maximum increase in eosinophil count 24 h after the final exposure of OVA blood was drown in blood collecting tube containing disodium EDTA. Z. armatum oil-treated group at the dose of 200 μl/kg significantly inhibited the increased inflammatory cell (absolute eosinophils count) in blood (Figure 1A).
Figure 1: Ef fect of essential oil on absolute eosinophils count in blood of mice
Cells/cubic mm (A); effect of Z. armatum essential oil on Immunoglobulin E in blood serum of mice, IU/l (B); effect of Z. armatum oil on inflammatory cells: eosinophils in bronchoalveolar lavage fluid of mice, % (C); effect of Z. armatum oil on inflammatory cells:
neutrophils in bronchoalveolar lavage fluid of mice, % (D); values were expressed as mean±SEM, where n= 6 in each group, *p≤0.05
compared with ovalbumin-treated group
Antigen-specific Th2 responses are known to induce antigen-specific IgE antibody production. Repeated OVA inhalation significantly increased the number of inflammatory cells in blood serum. On the day 15, immediately after the final OVA challenge, significantly increased the total serum level of IgE. The treatment with essential oil of Z. armatum significantly inhibited the increased level of serum IgE at a dose of 200 μl/kg body weight (Figure 1B).
After challenging with OVA, the levels of inflammatory cells including eosinophil’s and neutrophils were significantly increased in OVA-treated group as compared with normal group. But, sensitization of OVA along with the essential oil treatment, the levels of inflammatory cells were significantly decreased as compared with asthma-induced OVA group. The accumulation of inflammatory cells in BALF was significantly inhibited by 200 μl/kg body weight of essential oil (Figure 1C and 1D).
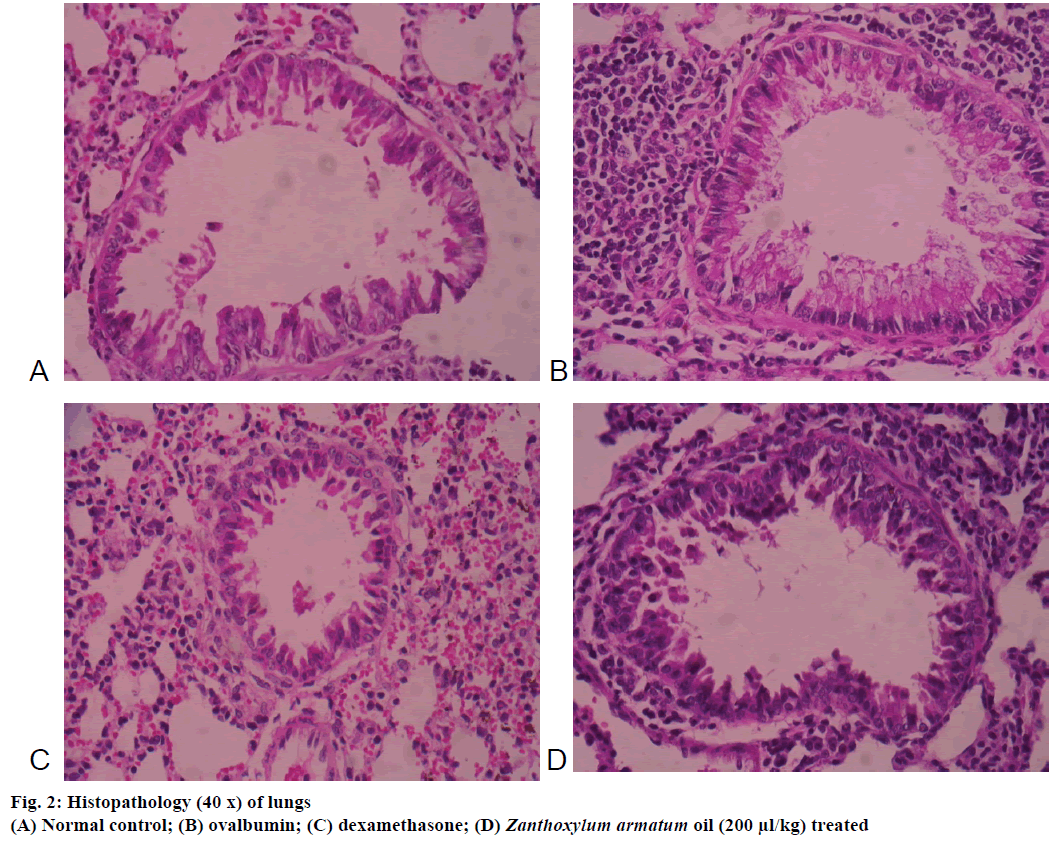
The microscopic images of tissue sections of each group stained with haematoxylin and eosin. The histopathological examination of lungs of mice exposed to OVA showed significant inflammatory alteration in peribronchial area and also increased bronchial muscles thickening, epithelial hyperplasia (Figure 2). In contrast, Z. armatum oil treatment at a dose of 200 μl/kg body weight showed significant changes (Figure 2) as compared to normal (Figure 2) and dexamethasone-treated groups (Figure 2).
Asthma is an allergic and respiratory disease commonly characterized by increased airway reactivity to different spasmogens. An initial attack of asthma was triggered by the release of inflammatory mediators like histamine, acetylcholine, leukotriene, prostaglandins or specific exposure of allergens, which reflected the signals of acute bronchoconstriction [23,24]. Histamine and other inflammatory mediators causes a host of changes in bronchial tissue by increasing the mucous secretion and simultaneous rapid constriction of bronchial smooth muscle, which narrows the bronchial tube and reduce the amount of air passes through them. Bronchodilating effect of Z. armatum oil was evaluated by observing its effects to increases the latent period of PCT in guinea pigs. The study revealed that the time of occurrence of PCT was significantly increased that suggests bronchodilating activity of Z. armatum oil against spasmogens.
Ovalbumin-induced model of allergic airway inflammation demonstrates that IgE levels in blood and eosinophilic infiltration in the lungs are markedly increased in asthmatic condition. Eosinophil count is elevated in response to the inflammation due to the OVA exposure. The pathogenesis of asthma is associated with increased infiltration of inflammatory cells and excessive mucus secretion into airway. OVAinduced asthma is recognized as a disease that results from chronic airway inflammation characteristically associated with the infiltration of lymphocytes, eosinophil’s, macrophages and neutrophils into the bronchial lumen.
An absolute eosinophil count is a blood test that measures the number of white blood cells called eosinophils. Eosinophil’s become active at a time of certain allergic diseases, infections, and other medical conditions. Treatment of the essential oil of Z. armatum significantly reduced the total absolute eosinophils in the blood. Asthma is almost always associated with some type of IgE-related reaction and therefore has an allergic basis. Numerous epidemiologic studies have shown a highly significant relationship between asthma and sensitization to various allergens as demonstrated by skin tests or the presence of specific IgE in the serum. IgE initiates the allergic response by causing mast cells to release inflammatory mediators and by recruiting eosinophils. Thus, blocking the effects of IgE is a promising strategy for preventing or ameliorating allergic symptoms [25]. This study showed significantly reduction in serum IgE level by Z. armatum oil-treated group. Eosinophils mediator secretion in asthma has been confirmed by BAL fluid analysis, which shows increased concentrations of granule-derived basic proteins [26].
Once established, the repetitive cycle of tissue damage and inflammatory cell recruitment becomes chronic. Even in the absence of sustained allergen, the chronic inflammation persists. Eosinophil level in sputum is associated with the degree of chronic airway obstruction [26]. In the present studies eosinophil cells in BALF significantly lower in Z. armatum oil treated groups as compared to the group treated by standard drug dexamethasone. Beside eosinophils, neutrophils also have an important role in the late-phase asthmatic reaction. Neutrophil products can cause airway narrowing, increased mucus secretion and increased antigen-presenting cells responsiveness. In this studies essential oil of Z. armatum-treated group reduced the elevated neutrophils in BALF. The elevated numbers of the inflammatory cells reflects the sign of asthma. The results of this study showed that the treatment of Z. armatum oil (200 μl/kg) with OVA- sensitized mice significantly reduced the levels of eosinophils, neutrophils, and total inflammatory cells in the BALF as compared with OVA-sensitized mice. In this study linalool, a monoterpene was identified as the major constituent of Z. armatum essential oil and a number of linalool and its acetate-producing species are used in traditional medicine systems to relieve symptoms and cure a variety of ailments, both acute and chronic. Linalool was evaluated for its psychopharmacological activity in mice, revealing marked dose-dependent sedative effects on the central nervous system [27,28] as well as protection against pentylenetetrazol, picrotoxin and transcorneal electroshock-induced convulsions, hypnotic and hypothermic properties [29,30].
Moreover, linalool modulates glutamate activation expression in vitro to competitive antagonism of L- [3H] glutamate binding and in vivo model to delayed subcutaneous N-methyl-D-aspartames-induced convulsions and blockade of intra cerebroventricular quinolinic acid-induced convulsions [31-33]. Furthermore, presence of linalool in essential oil exhibited potential antiinflammatory activity in in vivo models [27]. Hence, obtained results of essential oil of Z. armatum provides evidence as bronchodilator and antiasthmatic properties in histamine and OVA-induced allergens in guinea pigs and mice. In conclusion, the essential oils obtained from the plants have been used traditionally for the treatment of respiratory tract infections. The present investigation provides evidence that essential oil of Z. armatum has bronchorelaxation and antiasthmatic properties. The traditional uses of Z. armatum against asthma could be attributed to their antiasthmatic activity as observed in present study.
Acknowledgements
The authors are grateful to Director-in-Charge Regional Medical Research Centre (ICMR), Belagavi for encouraging the study. The authors are indebted to Kshipra Biotech Private Limited, Indore, India for providing essential oil of Z. armatum.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- http://www.aaaai.org/conditions-and-treatments/asthma
- http://www.webmd.com/asthma (Accessed on June 1, 2016).
- Koul PA, Patel D. Indian guidelines for asthma: Adherence is the key. Lung India 2015;32:S1-S2.
- Boushey HA. Drugs Used in Asthma. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and Clinical Pharmacology. 12th ed. China: McGraw-Hill Companies; 2009. p. 339.
- Inouye S, Yamaguchi H, Takizawa T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J Infec Chemother 2001;7:251-4.
- Singh TP, Singh OM. Phytochemical and pharmacological profile of Zanthoxylum armatum DC. Indian J Nat Prod Res. 2011;2:275-85.
- Deshpande DJ. Handook of herbal remedies. Jodhpur: Anmol prints, 2008.
- Kapoor LD. CRC Handbook of ayurvedic medicinal plants. India: CRC press, LLC; 1990.
- Kala CP, Farooquee NA, Dhar U. Traditional uses and conservation of Timur (Zanthoxylum armatum DC.) through social institutions in Uttaranchal Himalaya, India. Conservat Soc 2005;3:224-30.
- Waheed A, Mahmud S, Akhtar M, Nazir T. Studies on the components of essential oil of Zanthoxylum armatum by GC-MS. Am J Anal Chem 2011;2:258-61.
- Barkatullah, Ibrar M, Muhammad N, Rehman I, Rehman M, Khan A. Chemical composition and biological screening of essential oils of Zanthoxylum armatum DC leaves. J Clin Toxicol 2013;3:1-6.
- Negi JS, Bisht VK, Bhandari AK, Bisht R, Negi SK. Major constituents, antioxidant and antibacterial activities of Zanthoxylum armatum DC essential oil. Iranian J Pharmacol Ther 2012;11:68-72.
- Ibrar M, Muhammad N, Barkatullah, Khan H, Jahan F, Ashraf N. Antinociceptive and anticonvulsant activities of essential oils of Zanthoxylum armatum. Phytopharmacology 2012;3:191-8.
- Verma N, Khosa RL. Hepatoprotective activity of leaves of Zanthoxylum armatum DC in CCl4 induced hepatotoxicity in rats. Indian J Biochem Biophys 2010;47:124-7.
- Joshi RK. Chemical composition of the essential oil of Baccharoides lilacina from India. Nat Prod Commun 2013;8:401-2.
- Joshi RK. Essential oil of flowers of Anaphalis contorta, an aromatic and medicinal plant from India. Nat Prod Commun 2013;8:225-6.
- Joshi RK. Chemical composition of the essential oil of Croton bonplandianus from India. Nat Prod Commun 2014;9:269-70.
- Joshi RK. Essential oil of Senecio bombayensis from Western Ghats region of India. Chem Nat Compd 2014;50:382-83.
- Joshi RK. GC/MS analysis of the essential oil of Leucas indica from India.Nat Prod Commun 2014;9:1607-08.
- Joshi RK. Chemical composition In vitro antimicrobial and antioxidant activities of the essential oils of Ocimum gratissimum, O. sanctum and their major constituents. Indian J Pharm Sci 2013;75:457-62.
- Parmar NS. Screening methods in pharmacology. New Delhi: Narosa Publishing House; 2006. p. 200.
- Bates JHT, Rincon M, Irvin CG. Animal models of asthma. Am J Physiol Lung Cell Mol Physiol 2009;L401-L10.
- Nelson HS. Prospects for antihistamines in the treatment of asthma. JAllergy Clin Immunol 2003;112:96-100.
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma from bronchoconstriction to airway inflammation and remodelling. Am J Respir Crit Care Med 2000;1720-45.
- Platts-Mills TAE. The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med 2001;164:1-5.
- Bloemen K, Verstraelen S, Heuvel RVD, Witters H, Nelissen I, Schoeters G. The allergic cascade: Review of the most important molecules in the asthmatic lung. Immunol Lett 2007;113:6-18.
- Peana AT, Aquila PS, Panin F, Serra G, Pippia P, Moretti MD. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002;9:721-6.
- Jirovetz L, Jager W, Buchbauer G, Nikiforov A, Raverdino V. Investigations of animal blood samples after fragrance drug inhalation by gas chromatography/mass spectrometry with chemical ionization and selected ion monitoring. Biol Mass Spectrom 1991;20:801-03.
- Buchbauer G, Jirovetz L, Jager W, Dietrich H, Plank C. Aromatherapy: evidence for sedative effects of the essential oil of lavender after inhalation. Z Naturforsch 1991;46:1067-72.
- Elisabetsky E, Coelho De Souza GP, Dos Santos MAC, Siquieira IR, Amador TA, Nunes DS. Sedative properties of linalool. Fitoterapia 1995; 66(5):407-14.
- Elisabetsky E, Brum LF, Souza DO. Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine 1999;6:107-13.
- Silva Brum LF, Elisabetsky E, Souza D. Effects of linalool on [3H] MK801 and [3H] muscimol binding in mouse cortical membranes. Phytother Res 2001;15:422-5.
- Brum LFS, Emanuelli T, Souza DO, Elisabetisky E. Effects of linalool on glutamate release and uptake in mouse cortical synaptosomes. Neurochem Res 2001;26:191-4.