- *Corresponding Author:
- K. G. Baheti
Department of Pharmaceutical Chemistry, Y. B. Chavan College of Pharmacy, Rauza Baugh, Aurangabad-431 001, India
E-mail: nk_baheti@yahoo.com
| Date of Received | January 12, 2012 |
| Date of Revision | June 22, 2012 |
| Date of Acceptance | June 24, 2012 |
| Indian J Pharm Sci, 2012, 74 (3): 271-274 |
Abstract
A sensitive, accurate, precise and validated ion-pairing reverse-phase liquid chromatographic method for the quantitative determination of atenolol and indapamide in bulk and tablet dosage form was developed. The proposed ion-pairing reverse-phase high performance liquid chromatography method utilises C 18 column with 5 μm, 150×4.6 mm i.d. column and mobile phase consisting of 0.1% w/v solution of octane sulphonic acid, sodium salt and methanol (55:45 v/v), (pH 2.8) and ultraviolet detection at 235 nm. A linearity range of 1-250 μg/ml and 1-25 μg/ml for atenolol and indapamide, respectively, was obtained. The mean recoveries are 100.48 and 99.82% for atenolol and indapamide, respectively. The method was validated as per International Conference on Harmonization guidelines.
Keywords
Key words: Atenolol, indapamide, ion‑pairing, reverse‑phase high performance liquid chromatography
Atenolol (ATN), chemically (R,S)-4-(2-hydroxy- 3-isopropyl-aminopropoxy) phenyl acetamide, is a beta‑adrenoceptor antagonist widely used in the treatment of high blood pressure, arrhythmias and angina pectoris. It is official in the Indian Pharmacopoeia [1] and the British Pharmacopoeia [2]. Indapamide (IND), chemically (4‑chloro‑N‑(2‑methyl‑1‑indolinyl)‑3‑sulphamoyl benzamide) is a nonthiazide indole derivative of chlorosulphonamide has an antihypertensive action causing a drop in systolic, diastolic and mean blood pressure [3]. The chemical structures of ATN and IND are given in fig. 1. Analysis by various methods like ultraviolet (UV), high performance liquid chromatography (HPLC) and high performance thin layer chromatography (HPTLC) has been reported for analysis of these drugs individually or in combination [4‑7]. The literature survey reveals three reverse‑phase high performance liquid chromatography (RP‑HPLC) method for simultaneous estimation of ATN and IND [8‑10].
Sane et al. [8] reported the HPLC determination of ATN and IND by quaternary mobile phase consisting of methanol:water:glacial acetic acid:diethylamine in the ratio of 63:37:01:0.15 v/v. The retention time observed was 1.6 and 3.0 min for ATN and IND, respectively, at 241nm using a UV detector. They have used C18 (10 μm) column 300×4 mm column. Rani et al. [9] reported RP‑HPLC method for the simultaneous determination of ATN and IND from bulk and formulations. Chromatographic separation was achieved isocratically on a Waters C18 column (250×4.6 mm, 5 μm particle size) using a mobile phase, methanol and water (adjusted to pH 2.7 with 1% orthophosphoric acid) in the ratio of 80:20. The flow rate was 1 ml/min and eluent was detected at 230 nm. The retention time of ATN and IND were 1.76 min and 3.40 min, respectively. The HPLC method reported by Kher et al. [10] utilises C18, 250×4.6 mm i.d., 5 μm particle size column. The flow rate of the mobile phase was adjusted to 0.8 ml/min and the injection volume was 20 μl and detection was carried out at 241 nm. None of the reported methods have used the ion‑pairing reagent for the analysis and resolution of between ATN and IND. Hence, the proposed work aims at developing simple, precise and rapid RP‑HPLC method for better resolution between the analytes. The method uses the ion‑pairing agent for the better resolution between analytes of interest.
Working standards of ATN (purity 99.98%) and IND (purity 99.80%) were gift samples from SRS Pharmaceuticals Limited, Mumbai, India and were used without further purification. Combination drug product of ATN and IND (Label claim: ATN 50 mg and IND 2.5 mg), Aten D tablets of Zydus Cadila Ltd., India, were purchased from a local Pharmacy. Methanol (HPLC Grade, Merck Ltd., India), orthophosphoric acid (AR Grade, Loba Chemicals Ltd., Mumbai, India), octane sulphonic acid (HPLC Grade, Rankem, Faridabad, India), sodium hydroxide and hydrochloric acid (AR Grade, Qualigens Fine Chemicals, Mumbai, India) were used in analysis.
The HPLC system JASCO make consisted of binary pump PU2080 Plus, Rheodyne universal injector and Jasco UV2075 Plus UV/Vis detector with LC‑Net BORWIN 1.2 software. The chromatographic separations were performed using Xterra® C18 column with 5 μm, 150×4.6 mm i.d. column. Mobile phase consisted of 0.1% w/v solution of octane sulphonic acid sodium salt in water: methanol (55:45 v/v). The apparent pH of mobile phase was adjusted to 2.8±0.1 with orthophosphoric acid, filtered through 0.45 μm nylon filter and degassed in ultrasonic bath for 15 min prior to use. Wavelength was selected by scanning standard solutions of both drugs over 200‑400 nm using Jasco model V‑630 double beam UV/Vis spectrophotometer with a pair of 10 mm matched quartz cells. Measurements were made with injection volume 20 μl and UV detection at 235 nm, as both the components showed reasonably good absorbance at this wavelength.
The standard stock solutions 1.0 mg/ml of ATN and IND were prepared separately by dissolving working standards in mobile phase, which was sonicated for 15 min and further diluted with the same solvent. Standard calibration solutions of ATN and IND having concentrations in the range of 1‑250 μg/ml and 1‑25 μg/ml, respectively, were prepared by diluting stock solutions with mobile phase.
Twenty tablets were weighed; powdered and mass equivalent to 50 mg of ATN and 2.5 mg of IND was taken in a 50 ml volumetric flask and dissolved in mobile phase. The solution was sonicated for 15 min and further made up to volume with mobile phase. The solution was filtered through Millipore Millex HN Nylon 0.45‑μm syringe filter and 1 ml aliquot of filtrate was diluted to 10 ml with mobile phase to obtain concentration of 100 μg/ml of ATN and 5 μg/ml IND. The method was validated for linearity, accuracy, precision, specificity and robustness as per ICH guidelines [11].
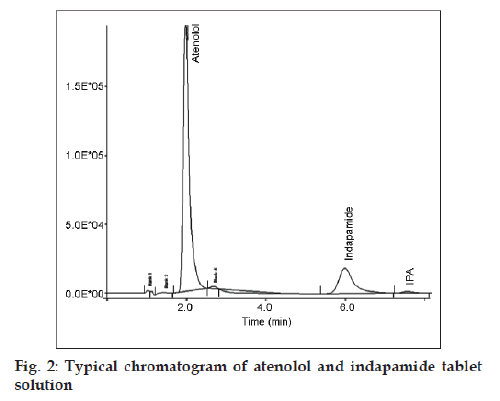
The proposed method utilises mobile phase consisting of 0.1% w/v solution of octane sulphonic acid sodium salt in water:methanol (55:45 v/v) of pH 2.8±0.1. The mobile phase was found to be ideal as it resolved the peaks of ATN (tR 2.0 min) and IND (tR 6.1 min), with clear line separation. Octane sulphonic acid was used as the ion‑pairing agent. UV detection wavelength at 235 nm, injection volume 20 μl and ambient temperature for column was found to be best for analysis. The typical chromatogram is given in fig. 2.
The assay results of ATN and IND in tablet were comparable with the value claimed by the label. The results presented in Table 1 indicate the suitability of the method for routine analysis of ATN and IND from their combination drug products. The proposed method has been validated for linearity, accuracy, precision, specificity and robustness. The results of validation study are presented in Table 2. Characteristic parameters for system suitability are given in Table 3. Repeatability of measurements of peak area was carried out using three replicates of concentration (100 and 5 μg/ml of ATN and IND, respectively). The intra‑ and inter‑day variation of the method was carried out for three concentration levels. The low % relative standard deviation (RSD) values of within a day, day‑to‑day variations and analyst‑to‑analyst variation for ATN and IND revealed that the proposed method is precise. The results of the intra‑ and inter‑day repeatability and robustness study of the methods are presented in Tables 4 and 5, respectively.
| Component | Label claim | Amount found | % Label claim* ± RSD |
|---|---|---|---|
| ATN | 50 mg | 50.1 mg | 100.2 ± 0.25 |
| IND | 2.50 mg | 2.48 mg | 99.2 ± 0.60 |
Table 1: Analysis of atenolol and indapamide By ion-pairing reverse-phase high Performance liquid chromatography method
| Parameters | Atenolol | Indapamide |
|---|---|---|
| Linearity | 1-250 µg/ml | 1-25 µg/ml |
| Equation of line Y | 28644.1X | 63437.98X |
| r2 | 0.999 | 0.998 |
| Limit of detection | 0.4 µg/ml | 0.33 µg/ml |
| Limit of quantitation | 1.0 µg/ml | 1.0 µg/ml |
Table 2: High performance liquid Chromatographymethod validation of Atenolol and indapamide
| Parameter | Atenolol | Indapamide |
|---|---|---|
| Retention time (min) | 2.0 | 6.1 |
| Tailing factor | 0.9 | 1.1 |
| Theoretical plates | 3298 | 2740 |
| Resolution | - | 7.8 |
| % RSD | 0.08 | 0.55 |
Table 3: System Suitability Parameters
| % ATN | % RSD* | % IND | % RSD* | |
|---|---|---|---|---|
| Intra-day precision | 102.4 | 0.59 | 101.3 | 0.81 |
| Inter-day precision* | 102.6 | 0.81 | 103.8 | 0.76 |
Table 4: Intra-And inter-day repeatability Of developed method for atenolol and Indapamide
| Factor | Level | % ATN | % RSD* | % IND | %RSD* |
|---|---|---|---|---|---|
| Flow rate (ml/min) (±0.1 ml) | 0.9 | 99.1 | 0.87 | 99.9 | 0.06 |
| 1.1 | 98.8 | 0.20 | 100.1 | 0.22 | |
| Methanol (±2%) | 44.1 ml | 100.7 | 0.44 | 100.2 | 0.25 |
| 45.9 ml | 98.7 | 0.58 | 99.8 | 0.87 | |
| Wavelength (±2 nm) | 233 nm | 99.4 | 0.53 | 100.0 | 0.77 |
| 237 nm | 99.8 | 0.47 | 99.3 | 0.28 | |
| pH (±0.2) | 2.6 | 99.9 | 0.78 | 101.1 | 0.96 |
| 3.0 | 100.3 | 0.18 | 100.0 | 0.22 |
Table 5: Robustness Results
Accuracy of the method was checked by recovery study using standard addition method, at three different concentration levels. The preanalysed samples were spiked with standard ATN and standard IND and the mixtures were analysed by the proposed method. Recovery of standard drugs added was found to be 99.32‑102.62% for ATN and 99.07‑100.39% for IND with the value of % RSD less than 2 indicating that the proposed method is precise for the simultaneous estimation of ATN and IND in combined drug products. Results of recovery study are shown in Table 6.
| Component | % Spiked | % Recovered ± RSD* |
|---|---|---|
| ATN | 80 | 99.32 ± 0.28 |
| ATN | 100 | 99.50 ± 0.19 |
| ATN | 120 | 102.62 ± 0.62 |
| IND | 80 | 99.07 ± 0.15 |
| IND | 100 | 100.39 ± 0.23 |
| IND | 120 | 100.01 ± 0.85 |
Table 6: Recovery Data
The proposed method was found to be advantageous over the reported methods because of better resolution of 7.78 of peaks of ATN and IND. The precision and accuracy of method was within the expected pharmacopoeial limits with % RSD less than 2. From the above study, it can be concluded that the proposed method is novel, accurate and precise. The better resolution between ATN and IND and validation of the method indicates that it can be adopted for routine dissolution testing and purity determination of tablets containing these Active Pharmaceutical Ingredients.
Acknowledgements
Authors are heartily grateful to Chairman, Padmashree Mrs. Fatma Rafiq Zakaria and Principal, Y.B. Chavan College of Pharmacy, Aurangabad, for providing research facilities and authors are grateful to SRS Pharmaceuticals, Mumbai, India, for providing ATN and IND as a generous gift samples.
References
- The Indian Pharmacopoeia. Atenolol. Delhi: Government of India: Ministry of Health and Family Welfare. The Controller of Publication; 1996. p. 72.
- The British Pharmacopoeia. Atenolol. British High Commission. London: Her Majestys Stationary Office; 1993, p. 55.
- Gilman AG. Treatment of myocardial ischemia and hypertension. Goodman and Gilman?s The Pharmacological Basis of Therapeutics. 8th ed., Vol. 1. Singapore: Pergamon Press; 1990, p. 718-9.
- Florey K. Atenolol. Analytical Profile of Drug Substances. Vol. 13. London: Academic Press; 2005, p. 21-2.
- Brittain HG. Indapamide. Analytical Profile of Drug Substances and Excipients. Vol. 23. California: Academic Press; 2005, p. 259-60.
- Bhaskara BL, Kumar SA, Kumar UR. A facile and rapid HPLC method for the determination of atenolol in pharmaceutical formulations. Asian J ApplSci 2011;49:306-13.
- Yadav SS, Rao JR. Simultaneous HPTLC analysis of atenolol and indapamide in tablet formulation. Int J Compr Pharm 2011;9:1-4.
- Sane RJ, Joshi L, Tendolkar RV, Gangal DP, Ladage KD, Kothurkar RM. High performance liquid chromatographic determination of atenolol and indapamide in pharmaceutical formulations. Indian J Pharm Sci 1990;52:204-6.
- Rani GT, Sankar DG, Kadgapathi P, Satyanarayana B. A validated RP-HPLC method for simultaneous estimation of atenolol and indapamide in pharmaceutical formulations. E-J Chem 2011;8:1238-45.
- Kher GJ, Ram VR, Bhavesh LD, Joshi HS. HPLC method development and validation of combined dosage form of atenolol and indapamide in tablets. Int J Pharm Technol 2011;3:3277-98.
- ICH. Q2B validation of analytical procedure: Methodology International Conference on Harmonization, Geneva, November 1996, 1-15.