- *Corresponding Author:
- Y-H Kuan
Department of Pharmacology, School of Medicine, Chung Shan Medical University, Taichung, Taiwan, ROC
E-mail: kuanyh@csmu.edu.tw
| Date of Submission | 16 November 2016 |
| Date of Revision | 18 April 2017 |
| Date of Acceptance | 30 November 2017 |
| Indian J Pharm Sci 2018;80(1):204-210 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Amentoflavone also known as didemethyl-ginkgetin, 3’,8”-biapigenin, is a plant bioflavonoid found in serval plants, with a number of pharmacological effects including antiinflammatory, antioxidant, antiviral, neuroprotective, and anticancer. The present study revealed that secretion of prostaglandin E2 and nitric oxide were inhibited by amentoflavone in a concentration-dependent manner in the lipopolysaccharide/interferon gamma-stimulated BV2 microglial cells. Meanwhile, protein expression of inducible nitric oxide synthase and cyclooxygenase-2 induced by LPS/INF-gamma were inhibited by amentoflavone in the same concentration range. Moreover, amentoflavone not only reduced the phosphorylation of nuclear factor-kappaB but also inhibited the phosphorylation of mitogen-activated protein kinases, including ERK, JNK, and p38 mitogen-activated protein kinases induced by lipopolysaccharide. In addition, a parallel concentration-dependent manner was observed in the inhibition of secretion of prostaglandin E2 and NO, expression of inducible nitric oxide synthase and cyclooxygenase-2, and phosphorylation of mitogen-activated protein kinases and nuclear factor-kappaB pathway. These results suggested that amentoflavone possessed the potential to act against lipopolysaccharide/interferon gamma-induced secretion of prostaglandin E2 and NO via downregulation of inducible nitric oxide synthase and cyclooxygenase-2 expressions by blocking the activation of nuclear factor-kappaB pathway via phosphorylation of mitogen-activated protein kinases.
Keywords
Amentoflavone, microglial BV2 cells, MAPKs, NFκB, COX-2, iNOS
Microglia, the resident macrophages in the central nervous system, play a critical role in the innate immunological responses and constitute about 15 % of the total glial cell population in the brain [1,2]. In response to injury and infection induced by pathogens, microglia are activated immediately and secrete proinflammatory mediators such as nitric oxide (NO), prostaglandin E2 (PGE2), and proinflammatory cytokines [3,4]. Neuroinflammation is mediated by activated microglia, which lead to neuronal cell death in several neurodegenerative diseases, such as Parkinson's disease, Alzheimer's disease, multiple sclerosis, prion diseases, amyotrophic lateral sclerosis, Huntington’s disease, and Pick’s disease [5]. Therefore, microglial activation-associated neuroinflammation serves as an important target in searching potential therapeutic reagents for neurodegenerative disorders.
Lipopolysaccharide (LPS), also called endotoxin, is the major outer membrane component of Gram-negative bacteria and often applied to induce microglia activation in in vitro assay [6]. However, over-activated microglial cells induced by LPS generate proinflammatory mediators and result in dramatic neurotoxicity [7].
Nuclear factor (NF)-κB is the transcription factor for regulating expressions of numerous proinflammatory genes, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) [8]. Activation of NF- κB is regulated by mitogen-activated protein kinases (MAPK), including p38 MAPK, extracellular signalregulated kinases (ERK) 1/2, and c-Jun N-terminal kinase (JNK) subfamilies [9].
Amentoflavone, also named didemethyl-ginkgetin or 3’,8”-biapigenin, is a bioflavonoid found in several plants, such as Ginkgo biloba, Chamaecyparis obtusa, Hypericum perforatum, Selaginella tamariscina, Torreya nucifera, and Xerophyta plicata. Amentoflavone reported to possess a variety of activities that include antiinflammatory, antioxidant, antiviral, neuroprotective, and anticancer [10-12]. In macrophages, amentoflavone effectively suppresses LPS-induced NO and PGE2 production by inhibiting the activation of activator protein-1 [13]. However, the protective effect and molecular mechanism of amentoflavone on microglia activation caused by LPS has not been reported. The present study reported an antiinflammatory effect and possible molecular mechanism of amentoflavone in BV2 microglial cells after LPS administration.
Dulbecco's modified Eagle's medium (DMEM) was purchased from Invitrogen (Carlsbad, CA). Interferon-γ was purchased from R&D Systems (Minneapolis, MN). LPS (Escherichia coli, serotype 0111:B4), dimethyl sulfoxide (DMSO), foetal bovine serum (FBS), amentoflavone, and other reagents, unless specifically stated, were obtained from Sigma-Aldrich (St. Louis, MO). Antibodies against COX-2, iNOS, phospho-ERK, ERK, phospho-p38, p38, phospho-JNK, JNK, phospo-p65, p65, and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies were obtained from Jackson Immuno Research Laboratories. (Baltimore, MD). Amentoflavone was dissolved in DMSO and the final concentration of DMSO never exceeded 0.5 %.
The murine BV2 microglia cell line was cultured in DMEM supplemented with 10 % FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37° in a humidified incubator with 5 % CO2 at 37°. In all experiments, cells were treated with amentoflavone for 30 min at 0, 30, 50, 100, or 200 μM, respectively before treating with or without LPS (100 ng/ml)/gamma interferon (IFNγ; 10 U/ml) in serum-free DMEM.
Generation of NO was determined by Griess reaction [3]. BV2 cells treated with vehicle DMSO only was acted as control group, while experimental groups were treated with amentoflavone at various concentrations of either 30, 50, 100, or 200 μM for 30 min after DMSO pre-treatment. Afterwards, an additional incubation with or without LPS/INFγ for 16 h followed. The culture medium was reacted with equal volume of 0.1 % naphthylethylenediamine hydrochloride and 1 % sulfanilamide in 5 % phosphoric acid at room temperature in the dark. The absorbance at 540 nm was determined using a microplate reader.
The content of PGE2 was determined using an enzyme-linked immunosorbent assay (ELISA) [14]. After treatment, the culture medium was collected for measurement of PGE2 concentration using ELISA kit (R&D Systems, Minneapolis, MN). The concentrations were interpolated from the calibration curve generated by the recombinant PGE2 standard.
After treatment, the cells were washed with phosphatebuffered saline (PBS) and harvested in Laemmli sample buffer. Protein concentration of cellular lysates was determined by Bradford assay. Proteins were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and then incubated with 5 % nonfat milk for 1 h. The membranes were washed with PBS containing 0.1 % Tween-20, and incubated for 2 h with the indicated primary antibodies. After washing again, a 1:10 000 (v/v) dilution of horseradish peroxidaselabeled IgG was added at room temperature for 1 h and the blots were then developed using enhanced chemiluminescence Western blotting reagents [15].
Statistical analyses were performed using ANOVA followed by the Bonferroni t test for multigroup comparisons; p<0.05 was considered significant for all tests.
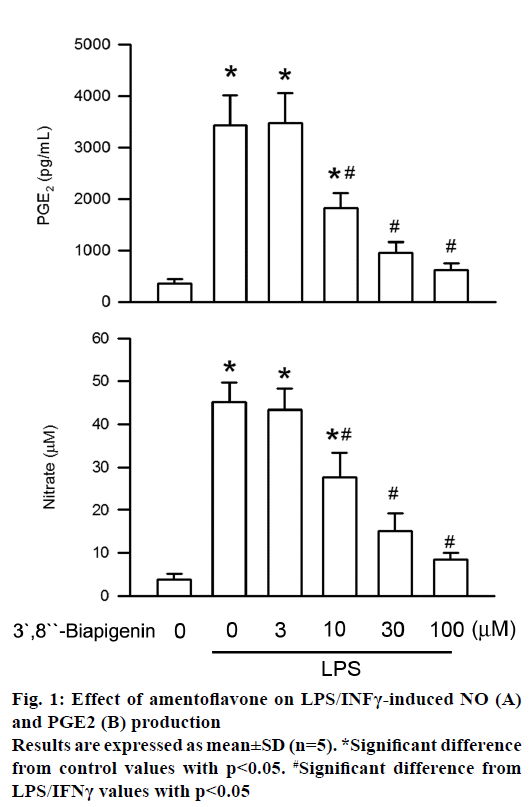
Generation of PGE2 and NO is the critical feature in LPS/INFγ-stimulated BV2 cells activation [8]. To evaluate the antiinflammatory effect of amentoflavone on BV2 cells, secretion of PGE2 and NO was measured by ELISA assay and Griess assay, respectively. Administration of LPS for 16 h significantly increased PGE2 level as compared to the control. The level of PGE2 generated was inhibited by amentoflavone in LPS/INFγ-stimulated BV2 cells in a concentrationdependent manner with an IC50 value of 15.02± 4.81 μM (Figure 1). In addition, the contents of NO secretion were increased from initial level after LPS/INFγ treatment for 16 h. Amentoflavone also concentrationdependently induced the inhibition of NO secretion with an IC50 value of 16.04±1.88 μM (Figure 1). These results suggested the LPS/INFγ-induced activation of microglia were inhibited by amentoflavone.
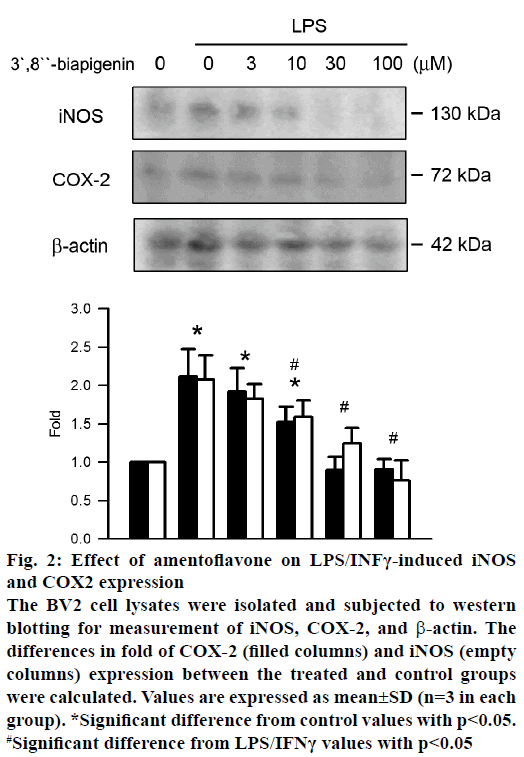
NO and PGE2 were expressed by iNOS and COX-2, respectively. The effect of amentoflavone on protein expression of iNOS and COX-2 was analysed by western blot assay. Treatment of LPS/IFNγ significantly increased the protein expression of iNOS and COX-2 (Figure 2). Amentoflavone reduced LPS/IFNγ- induced protein expression of COX-2 and iNOS in a concentration-dependent manner with an IC50 value of 11.69±5.88 and 9.31±5.19 μM, respectively.
Figure 2: Effect of amentoflavone on LPS/INFγ-induced iNOS
and COX2 expression
The BV2 cell lysates were isolated and subjected to western
blotting for measurement of iNOS, COX-2, and β-actin. The
differences in fold of COX-2 (filled columns) and iNOS (empty
columns) expression between the treated and control groups
were calculated. Values are expressed as mean±SD (n=3 in each
group). *Significant difference from control values with p<0.05.
#Significant difference from LPS/IFNγ values with p<0.05
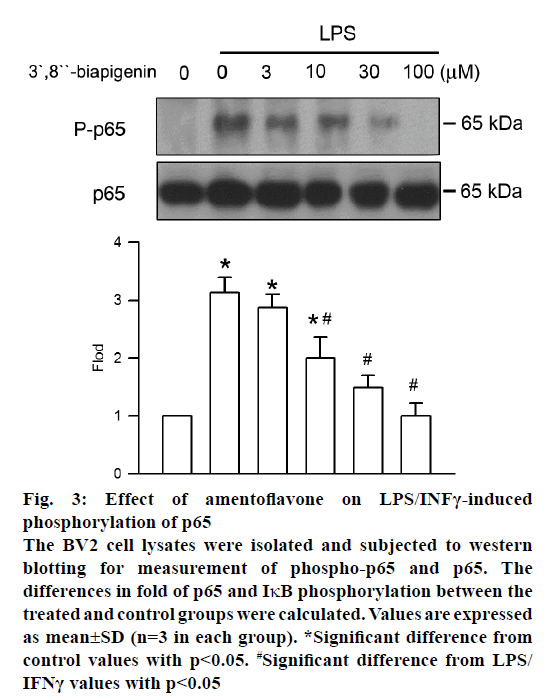
Phosphorylation of NF-κB, which prompts transcription of iNOS and COX-2, has a central role in inflammation. The effect of amentoflavone on LPS/INFγ-induced NF-κB phosphorylation was analysed by western blot assay. After LPS/INFγ administration, NF-κB phosphorylation was increased markedly. However, it was significantly suppressed by amentoflavone pretreatment in a concentration-dependent manner with an IC50 value of 11.97±4.91 μM (Figure 3).
Figure 3: Effect of amentoflavone on LPS/INFγ-induced
phosphorylation of p65
The BV2 cell lysates were isolated and subjected to western
blotting for measurement of phospho-p65 and p65. The
differences in fold of p65 and IκB phosphorylation between the
treated and control groups were calculated. Values are expressed
as mean±SD (n=3 in each group). *Significant difference from
control values with p<0.05. #Significant difference from LPS/
IFNγ values with p<0.05
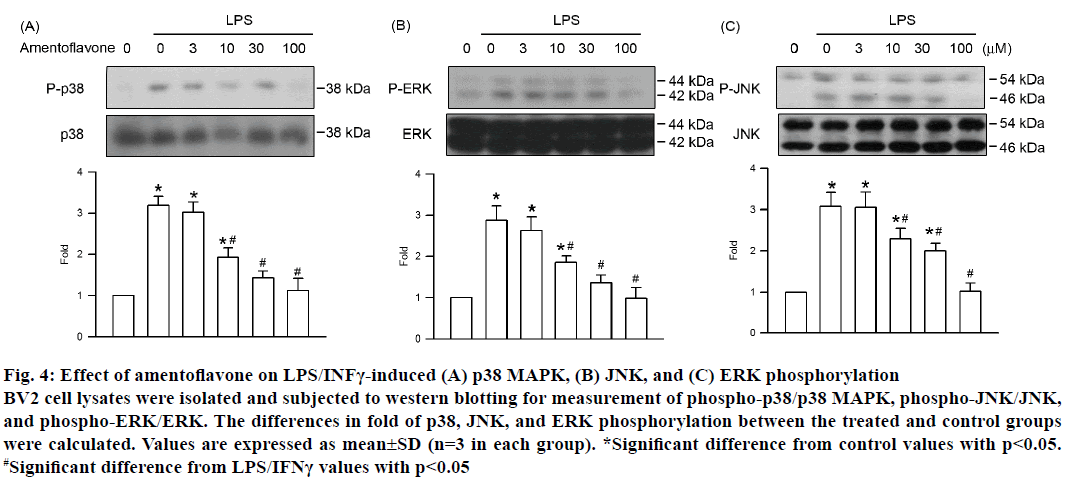
Each of the three MAPK pathways, ERK, p38 MAPK, and JNK, plays an important role in NF-κB activation induced by LPS/INFγ in neurons. Therefore, the effect of amentoflavone on MAPK activation was assessed by phosphorylation of ERK, p38 MAPK, and JNK. Activation of BV2 cells induced by LPS/ INFγ resulted in the phosphorylation of ERK, p38 MAPK, and JNK (Figure 4). Amentoflavone decreased the phosphorylation intensity of ERK, p38 MAPK, and JNK in a concentration-dependent manner with an IC50 value of 12.38±2.61, 10.79±1.14, and 15.04± 3.58 μM, respectively. In addition, there was no influence on the expression of ERK, p38 MAPK, and JNK in all treatment group (Figure 4). These results suggested that amentoflavone reduced the LPS/INFγ- stimulated inflammatory effect in BV2 cells via inhibition of the MAPK pathway.
Figure 4: Effect of amentoflavone on LPS/INFγ-induced (A) p38 MAPK, (B) JNK, and (C) ERK phosphorylation
BV2 cell lysates were isolated and subjected to western blotting for measurement of phospho-p38/p38 MAPK, phospho-JNK/JNK,
and phospho-ERK/ERK. The differences in fold of p38, JNK, and ERK phosphorylation between the treated and control groups
were calculated. Values are expressed as mean±SD (n=3 in each group). *Significant difference from control values with p<0.05.
#Significant difference from LPS/IFNγ values with p<0.05
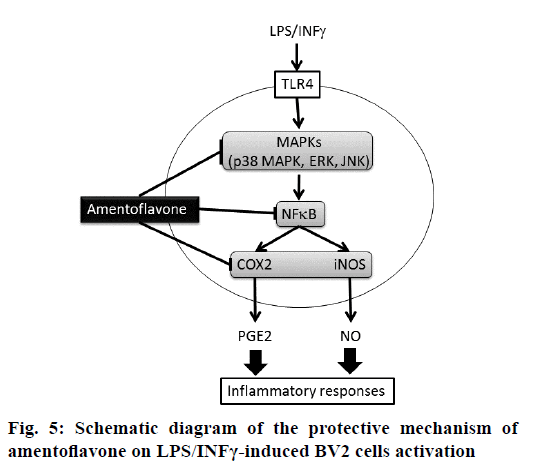
The present study was designed to explore the protective effect of amentoflavone on LPS/INFγ-induced NO and PGE2 generation via MAPK-NF-B pathway in BV2 microglial cells. Microglia is macrophage-like glial cells located throughout the brain and spinal cord with a diverse role in the innate immunity and in inflammatory neurological diseases [16]. Evidence has demonstrated that BV2 microglial cells appear to be a valid substitute for primary microglia in inflammatory experiments in in vitro assay [17]. At present study, primary neurons were simulated by BV2 microglial cells. During neuroinflammation, microglia plays the central role in several immunopathological responses involved in the production of proinflammatory mediators such as NO and PGE2. NO is one of the major proinflammatory mediators generated by iNOS, which is highly expressed in activated microglia and macrophages after treating with LPS. PGE2 is another important proinflammatory mediator converted from arachidonic acid by COX-2 [3]. According to our results, the secretion of NO and PGE2 was inhibited by amentoflavone at the similar IC50 in LPS/IFNγ-stimulated BV2 cells. In addition, amentoflavone presented parallel inhibition of COX-2 and iNOS protein expressions in LPS/IFNγ-stimulated cells. Similar observation has been proclaimed in previous study, which also shows that generation of NO and PGE2 is inhibited by amentoflavone via protein expression of iNOS and COX-2, respectively in LPStreated RAW264.7 macrophages [13]. These evidences implied that the blockade of protein expression has a critical role in the antiinflammatory activity of amentoflavone (Figure 5).
NF-κB is the essential transcription factor for regulating expression of a number of proinflammatory genes, such as NO and PGE2, in the LPS-stimulated BV2 microglial cells [8]. In mammalian cells, the NF-κB family contains five structurally related proteins, which are p50, p52, p65 (RelA), RelB, and c-Rel. There are two forms, homodimer and heterodimer, of NF-κB. The NF-κB heterodimer is composed of p50 and p65, which is the most abundant and functionally most important form of NF-κB. Under normal condition, NF-κB dimers keep the inactive form associated with the IκB, which is the inhibitory protein in the cytosol. After cellular activation with different stimulants, phosphorylation of IκB is induced by IκB kinase. Subsequently, 26S proteasome degrades the IκB phosphorylation via ubiquitination leading to NF-κB dimers activation and is phosphorylated at p65. The active form of NF-κB translocates to the nucleus, binds to transcription site, and induces the array of proinflammatory genes [18]. Pervious study has demonstrated amentoflavone inhibits LPS-induced NF-κB activity in RAW264.7 macrophages [19]. In murine model of ulcerative colitis, amentoflavone inhibits expression of iNOS, COX-2, and proinflammatory cytokines via NF-κB pathway [20]. At present, LPS-induced p65 phosphorylation was inhibited by amentoflavone in BV2 microglial cells in a concentration-dependent manner. In addition, our data had shown parallel concentration-dependent inhibition of NF-κB phosphorylation and iNOS and COX-2 expression. Taken together, amentoflavone reduced the LPS/IFNγ-induced expression of iNOS and COX-2 via NF-κB pathway.
Activation of NF-κB pathway is mediated by the phosphorylation of MAPKs in LPS/IFNγ-stimulated BV2 cells [3]. Up to now, at least three distinct subfamilies of MAPKs, ERK, p38 MAPK, and JNK, have been clearly characterized in mammals. Dual phosphorylation of a tyrosine and a neighbouring threonine residue within the conserved tripeptide motif Thr-X-Tyr located in the activation loop of the kinase domain are required for MAPK activation [21]. The current study demonstrated that LPS/INFγ-induced phosphorylation of ERK, p38 MAPK, and JNK were inhibited by amentoflavone in a concentrationdependent manner. Amentoflavone exhibited parallel inhibition of LPS/INFγ-induced phosphorylation of MAPK and NF-κB p65 with similar IC50 value. These results suggested that the generation of NO and PGE2 was inhibited by amentoflavone through MAPKs, including ERK, p38 MAPK, and JNK, in LPS/INFγ- stimulated BV2 microglial cells.
Neuroinflammation leads to not only generation of reactive oxygen species and proinflammatory cytokines but also formation of activated microglia and astrocytes; and at the same time, contributes to neuronal and oligodendrocytic death in neurodegenerative patients [22,23]. MAPKs-NF-κB activation, especially the constitutively activated NF-κB, plays an important role in neuroinflammation, has been found to possess a critical linkage with a wide variety of neurodegenerative patients [24-26]. At present, we found pre-treatment with amentoflavone significantly reduced the secretion of PGE2 and NO via protein expression of iNOS and COX-2 in BV2 microglial cells stimulated by LPS/INFγ. The mechanism of inhibition was attributed mainly to the down-regulation of IκBNFκB pathway activation by blocking the upstream activator MAPKs, which included ERK, p38 MAPK, and JNK. Experimental results supported the use of amentoflavone as a potential preventive reagent for neuroinflammtory diseases associated with direct infection caused by Gram-negative bacteria.
Acknowledgements
The authors would like to thank the Ministry of Science and Technology of the Republic of China, Taiwan, for financially supporting this research under Contract No. MOST 104-2320-B-040 -006 -, 105-2320-B-040 -022-, and 106-2320-B-040 -022 -MY3.
Conflict of interest
All authors declare no conflict of interest.
Financial support and sponsorship
Nil.
References
- Carson MJ, Thrash JC, Walter B. The cellular response in neuroinflammation: The role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin Neurosci Res 2006;6:237-45.
- Nayak D, Roth TL, McGavern DB. Microglia development and function. Ann Rev Immunol 2014;32:367-402.
- Yeh CH, Yang ML, Lee CY, Yang CP, Li YC, Chen CJ, et al. Wogonin attenuates endotoxin-induced prostaglandin E2 and nitric oxide production via Src-ERK1/2-NFκB pathway in BV-2 microglial cells. Environ Toxicol 2014;29:1162-70
- Yeh CH, Shih HC, Hong HM, Lee SS, Yang ML, Chen CJ, et al. Protective effect of wogonin on proinflammatory cytokine generation via Jak1/3-STAT1/3 pathway in lipopolysaccharide stimulated BV2 microglial cells. Toxicol Ind Health 2015;31:960-66.
- Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem 2007;14:1189-97.
- Sun Y, Shang D. Inhibitory Effects of Antimicrobial Peptides on Lipopolysaccharide-Induced Inflammation. Mediators Inflamm 2015;2015:167572.
- Moniruzzaman M, Lee G, Bose S, Choi M, Jung JK, Lee H, et al. Antioxidant and Antiinflammatory activities of N-((3,4-Dihydro-2H-benzo[h]chromene-2-yl)methyl)-4- methoxyaniline in LPS-Induced BV2 microglial cells. Biol Pharm Bull 2015;38:1831-5.
- Jeong JW, Jin CY, Kim GY, Lee JD, Park C, Kim GD, et al. Antiinflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. Int Immunopharmacol 2010;10:1580-6.
- Madrid LV, Mayo MW, Reuther JY, Baldwin Jr AS. Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem 2001;276:18934-40.
- Park NH, Lee CW, Bae JH, Na YJ. Protective effects of amentoflavone on Lamin A-dependent UVB-induced nuclear aberration in normal human fibroblasts. Bioorg Med Chem Lett 2011;21:6482-4.
- Zhang Z, Sun T, Niu JG, He ZQ, Liu Y, Wang F. Amentoflavone protects hippocampal neurons: anti-inflammatory, antioxidative, and antiapoptotic effects. Neural Regen Res 2015;10:1125-33.
- Tarallo V, Lepore L, Marcellini M, Dal Piaz F, Tudisco L, Ponticelli S, et al. The bioflavonoid amentoflavone inhibits neovascularization preventing the activity of proangiogenic vascular endothelial growth factors. J Biol Chem 286;19641-51.
- Oh J, Rho HS, Yang Y, Yoon JY, Lee J, Hong YD, et al. Extracellular signal-regulated kinase is a direct target of the antiinflammatory compound amentoflavone derived from Torreya nucifera. Mediators Inflamm 2013;2013:761506.
- Kuan YH, Huang FM, Lee SS, Li YC, Chang YC. Bisgma stimulates prostaglandin E2 production in macrophages via cyclooxygenase-2, cytosolic phospholipase A2, and mitogen-activated protein kinases family. PLoS One 2013;8:e82942.
- Yeh YC, Yang CP, Lee SS, Horng CT, Chen HY, Cho TH, et al. Acute lung injury induced by lipopolysaccharide is inhibited by wogonin in mice via reduction of Akt phosphorylation and RhoA activation. J Pharm Pharmacol 2016;68:257-63.
- Henn A, Lund S, Hedtjärn M, Schrattenholz A, Pörzgen P, Leist M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 2009;26:83-94.
- Mariani MM, Kielian T. Microglia in infectious diseases of the central nervous system. J Neuroimmune Pharmacol 2009;4:448-61.
- Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta 2010;1799:775-87.
- Woo ER, Lee JY, Cho IJ, Kim SG, Kang KW. Amentoflavone inhibits the induction of nitric oxide synthase by inhibiting NF-kappaB activation in macrophages. Pharmacol Res 2005;51:539-46.
- Sakthivel KM, Guruvayoorappan C. Amentoflavone inhibits iNOS, COX-2 expression and modulates cytokine profile, NF-κB signal transduction pathways in rats with ulcerative colitis. Int Immunopharmacol 2001;17:907-16.
- Rauch N, Rukhlenko OS, Kolch W, Kholodenko BN. MAPK kinase signalling dynamics regulate cell fate decisions and drug resistance. Curr Opin Struct Biol 2016;41:151-8.
- Hsieh HL, Yang CM. Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res Int 2013;2013:484613.
- Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H, et al. Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine 2016;1:2:1003.
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 2009;27:693-733.
- Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev 2010;29:405-34.
- Rahimifard M, Maqbool F, Moeini-Nodeh S, Niaz K, Abdollahi M, Braidy N, et al. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res Rev 2017;36:11-9.