- Corresponding Author:
- C. S. Pundir
Biochemistry Research Laboratory, Department of Biochemistry and Genetics M. D. University, Rohtak - 124 001, India
E-mail: pundircs@rediffmail.com
| Date of Submission | 11 August 2006 |
| Date of Revision | 2 August 2007 |
| Date of Acceptance | 29 September 2007 |
| Indian J. Pharm. Sci., 2007, 69 (5): 669-673 |
Abstract
A reusable strip of oxalate oxidase was prepared by immobilizing covalently partially purified amaranthus leaf oxalate oxidase on to alkyl amine glass beads affixed on a plastic strip. The immobilized enzyme gave a conjugation yield of 48 mg/g support with 87% retention of initial activity of free enzyme. The strip showed maximum activity at pH 3.5 when incubated at 40º for 15 min. A method for discrete analysis of oxalate in urine was developed employing this enzyme strip. The method is based on measurement of hydrogen peroxide generated from urinary/plasma oxalate by strip bound oxalate oxidase using a colour reaction consisting of 4-aminophenazone, phenol and horseradish peroxidase as chomogenic system. The minimum detection limit of the method was 0.1 mM. The recovery of added oxalate was 96.5% in plasma and 98% in urine. Within and between assay coefficient of variation were <6% and <5%, respectively in plasma and urine. The method provides enormous ease in handling of immobilized enzyme during its reuse and is unaffected by chloride ions found in biological fluids.
Keywords
Oxalate, oxalate oxidase, urine, plasma, amaranthus, immobilization, enzyme strip
Measurement of oxalate in urine and plasma is required in the diagnosis and medical management of oxalate, primary and secondary hyperoxaluria, urinary stone disease and various form of lipids malabsorption e.g. sprune, insufficiency, intestinal-stasis, bowl restriction, inflammatory intestinal disease [1]. A method for discrete analysis of urinary oxalate has been reported from this laboratory using amaranthus leaf oxalate oxidase immobilized onto free alkylamine glass beads [2]. Although the method provided the reuse of enzyme for a period of 90 d and was unaffected by chloride ions found in biological fluids, the handling of free glass beads was tedious and time consuming and included the risk of their loss during transfer of reaction mixture and washing for their reuse. This problem was overcome in the present work by affixing the glass beads on a plastic strip with a non reactive fixative before immobilization of enzyme.
Materials and Methods
Zirconia coated alkylamine glass beads (pore diameter 55 nm, Corning Glass New York.; horse radish peroxidase (RZ= 1.0) and oxalic acid (Sigma Chemical Co. USA) were obtained. The non reactive fixative Araldite was purchased from the local market. All other chemicals were of AR grade.
Collection of plant material
The healthy plants of Amaranthus spinosus growing by the side of railway track of Kishan Ganj, New Delhi were identified on the basis of following characters: Erect, armed, glabrous plants up to 60 cm in height, two axillary, diverticulate sharp spines, much branched stem, green or some what reddish in colour and glabrous, leaves 3-10×1-4 cm in size, ovate or oblong from a slight decerrent base, gradually narrowed upward, apex, obtuse, rounded and glabrous, flowers dense in axillary, clusters, racemosely disposed, spikes often branched in lower regions terminals spikes often male flowers only, finally drooping perianth lobes 5, stamens 5, ovary ovoid or oblong, style 3, conical, stigma 3, timbrillate. The leaves of these plants were collected in ice bath and washed in distilled water, dried in 2 folds of dry filter paper and stored immediately at -20° until use.
Extraction and partial purification of oxalate oxidase
Crude oxalate oxidase from leaves of amaranthus was prepared3 with modification. Frozen leaves (100 g) were homogenized in potassium phosphate buffer (0.1 M, pH 7.0) in 1:4 ratio (w/v) in a chilled pestle mortar in dark. The homogenate was squeezed though a double layer of cheese cloth and the filtrate was centrifuged at 15 000 g for 15 min at 4°. The supernatant collected was subjected to 0-80% w/v (NH4)2SO4 precipitation by using 0-80% w/v aqueous solution of (NH4)2SO4 . The resulting solution was centrifuged at 10 000g for 30 min, the pellet obtained was dissolved in extraction buffer and treated as partially purified oxalate oxidase. It was stored at 4° until use.
Assay of free oxalate oxidase
The assay of oxalate oxidase was carried out with modification4. In a 15 ml test tube wrapped with black paper, the reaction mixture containing 1.9 ml of 0.05 M sodium citrate buffer (pH 3.5) and 0.1 ml partially purified enzyme was preincubated at 37° for 5 min. The reaction was started by adding 0.1 ml aqueous oxalate solution (30 mM). After incubation at 37° for 5 min, 1ml colour reagent was added. (The colour reagent consisted of 50 mg of 4-aminophenazone, 100 mg phenol and 1.0 mg horseradish peroxidase per 100 ml of 0.4 M sodium phosphate buffer, pH 7.0 and stored in amber coloured bottle at 4° and prepared fresh every week). The mixture was allowed to stand in water bath maintained at 37° for 20 min to develop colour. The blank was prepared by replacing the enzyme solution with reaction buffer. The colour was read at A520 and concentration of H2O2 was extrapolated from standard curve of H2O2. One unit of enzyme is defined as amount of enzyme required to generate 1 mol H2O2 /min. The protein content in oxalate oxidase preparation was measured by the method of Lowry et al.[5]
Preparation of reusable strip of oxalate oxidase
The strip of 15×1 cm size (length×width) was cut from a plastic sheet (A transparent plastic sheet of 0.5 mm thickness purchased from local market). One end of this strip was made round with the help of scissor. A thin layer of 0.2 mm thickness of the fixative Araldite was applied uniformly on both side of the round end of strip up to height of 2 cm with the help of a brush. Alkylamine glass (50 mg) beads were sprinkled uniformly on the fixative with the help of aluminum foil. The strip was kept in a 15 ml test tube for 24 h at room temperature for affixation of glass beads. Immobilization of oxalate oxidase onto affixed alkylamine glass beads was carried out as described by Lynn with modification6. Three milliliter glutaraldehyde (2.5% in 0.1M sodium phosphate buffer, pH 7.0) was added to the test tube. The strip containing affixed glass beads was allowed to stand for 2 h at room temperature with constant shaking. The strip was taken off glutaraldehyde solution and dipped repeatedly in to distilled water until the pH of the washing was 7.0, to ensure the complete removal of free glutaraldehyde. The beads were washed finally in 0.1 M sodium phosphate buffer (pH 7.0). The end of plastic strip containing glutaraldehyde activated glass beads was dipped in to oxalate oxidase solution (3 ml) in a 15 ml test tube and allowed to stand at 4° for 48 h with occasional shaking. After the immobilization, the strip was taken off and the remaining enzyme solution was tested for activity and protein. The strip was dipped in to distilled water 6 times to remove the unbound enzyme and tested for enzyme activity.
Assay of strip bound oxalate oxidase
It was carried out as described for assay of free oxalate oxidase except that free enzyme was replaced by plastic strip bound oxalate oxidase and reaction buffer was increased by 0.1 ml and the reaction mixture was stirred continuously during incubation. The strip was taken off the reaction mixture before addition of colour reagent. The strip was stirred in 0.05 M sodium citrate buffer pH 3.5 at 4°, when not in use.
Kinetic properties of immobilized oxalate oxidase
The following kinetic properties of immobilized oxalate oxidase were studied; optimum pH, incubation temperature, time of incubation, effect of oxalate concentration and determination of Km and Vmax from Lineweaver Burk plot.
Determination of oxalate in urine and plasma with Enzyme strip
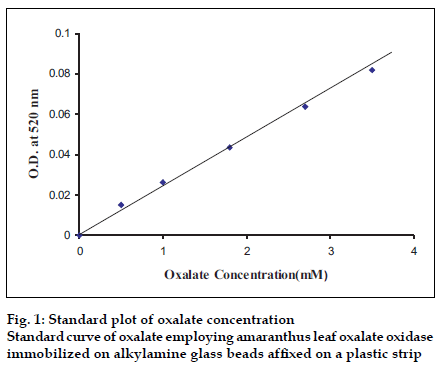
The 24 h urine samples were collected in plastic bottles containing 15 ml concentrated HCl from apparently healthy/urinary non-stone former adult males and females, as conformed by their abdominal X-ray. The final pH of acidified urine was adjusted to be with in 5.0 to 7.0 by addition of NaOH or concentrated HCl and the samples were stored at 4° until use. The urine was diluted 1:1 with 0.1 M potassium phosphate buffer. To avoid possible interference by ascorbate, 0.1 ml buffered sodium nitrite (3.5 mg/ml prepared in 0.1 M sodium phosphate buffer, pH 7.0) was added per ml of urine. The assay of urinary oxalate was carried out in the same manner as described for assay of immobilized oxalate oxidase except that oxalate solution was replaced by pretreated urine and optimal assay conditions were maintained. The concentration of oxalate in urine was extrapolated from standard curve between oxalate concentration ranging from 0.05 to 1 mM and A520 (fig.1).
Blood samples (2 ml) from apparently healthy persons of different age groups and sex was drawn intravenously with the help of sterilized syringe and needle and immediately transferred to pre chilled vial containing 15-20 IU/ml heparin. This heparinized blood was centrifuged at 2000 g for 10 min at 4°. The supernatant (plasma) was collected. To avoid possible ascorbate interference, 0.01 ml of 5 mM sodium nitrite (in 5 mM sodium phosphate buffer pH 7.0) was added to 0.1 ml of plasma and vortexed out rigorously. The assay of plasma oxalate was same as described for urinary oxalate.
Reuse of enzyme strip
The enzyme strip was dipped in the 0.05 M sodium citrate buffer, pH 3.5, five to six times before its use in the next assay. The strip was stored in the same buffer at 4°, when not in use.
Results and Discussion
An oxalate oxidase partially purified from matured leaves of Amaranthus spinosus plants was immobilized covalently onto alkylamine glass beads affixed on one end of a plastic strip with 87% retention of initial specific activity of free enzyme and conjugation yield of 48 mg/g. A comparison of immobilization of amaranthus leaf enzyme onto affixed alkylamine glass beads with those for sorghum leaf [7] and barley seedling [8] enzyme on the same support is given in Table 1, which shows better conjugation yield and higher retention of activity of amaranthus enzyme on affixed glass beads compared to other plant enzymes.
Compared to free enzyme the strip bound Amaranthus enzyme showed no change in its optimum pH i.e. 3.5, in contrast to barley and sorghum enzyme, whose pH was increased from 3.5 to 3.6 and 3.5 to 6.8, respectively, after immobilization [7,8]. The time of incubation for maximum activity of amaranthus enzyme was increased from 5 min to 15 min after immobilization. The similar procedure was also applied to sorghum and Barley enzyme7,8. Apparent Km value for oxalate was increased from 0.53×10-4M to 1.0×10-4M, after immobilization. The same effect was also observed for sorghum and barley enzyme7,8. Incubation temperature for maximum activity was increased slightly from 37 to 40° after immobilization (Table 2). However, the immobilized enzyme was unaffected by Cl- (as NaCl) up to 200 mM, which was similar to sorghum enzyme [9].
| Sources | Enzyme added to 50 mg affixed glass beads (mg) | Enzyme coupled to 50 mg affixed glass beads (mg) |
Total activity added (nmolH2O2/ min) |
% Retention | Conjugation Yield (mg / g) |
|---|---|---|---|---|---|
| Sorghum leaf7 | 4.6 | 0.83 | 338.3 | 33.9 | 5.1 |
| Barley Seedling8 | 1.725 | 0.47 | 34.3 | 84.8 | 9.5 |
| Amaranthus leaves | 4.2 | 2.4 | 240 | 87 | 48 |
Table 1: Comparison Of Immobilization Of Oxalate Oxidase From Different Sources On To Alkylamine Glass Beads.
| Kinetic parameter | Free Amaranthus enzyme | Immobilized Amaranthus enzyme | Immobilized Sorghum enzyme7 | Immobilized Barley enzyme8 |
|---|---|---|---|---|
| Optimum pH | 5.5 | 3.5 | 6.8 | 3.6 |
| Optimum temperature (°) | 37 | 40 | 37 | 25 |
| Ea (kcal/mole) | 4.5 | 8.2 | - | - |
| Incubation time (min) | 5 | 15 | 7 | 7 |
| Km for oxalate (×10-4 M) | 0.53 | 1 | 9.87 | 15.5 |
| Vmax(µmole/min) | 0.11 | 0.1 | 0.083 | 0.09 |
Table 2: Comparison Of Kinetic Properties Of Immobilized Oxalate Oxidase From Different Sources.
A simple, sensitive specific method for discrete analysis of oxalate in urine and plasma was developed using reusable strip of oxalate oxidase. The method is based on the quantification of H2O2 generated in the reaction mixture from urinary and plasma oxalate by strip bound oxalate oxidase, using a colour reaction with 4-aminophenazone, phenol and horseradish peroxidase as chromogenic system. The method has the advantage that it provides enormous ease in reuse of enzyme and unaffected by Cl- normally found in biological fluids. Further, the method avoids accumulation of dye (product of colour reaction) in the vicinity of immobilized enzyme and thus eliminates its possible interference in the assay. The following values were determined as the criteria of the method.
A linear relationship was found between oxalate concentrations ranging from 0.5 mM to 5 mM in reaction mixture up to an absorbance of 0.1. The minimum detection limit of the method is 0.4 mM oxalate/l urine, which is higher than that for the method employing sorghum [7] and barley [8] enzyme (0.1 mM).
For analytical recovery, solid oxalate was added into urine and plasma at a rate of 20 mg/l and 18µmole/l, respectively. The oxalate content of these urine and plasma samples was measured before and after addition of oxalate by the present method. The analytical recoveries of added oxalate was calculated and found to be 98% in urine and 96.5% in plasma, which is higher than that using sorghum enzyme [7] (82%) and barley enzyme [8] (85%).
To assess the reproducibility and reliability of the method, the oxalate content of two urine and plasma samples was determined six times in one run (within batch) by the present method. The oxalate value in six urine and plasma were also determined on the first day and after one week (between batch) storage at -20° by the present method. The results showed that the oxalate values in urine and plasma samples agreed with each other and within batch and between batch coefficients of variation (CV) were <6% and <5%, respectively, in plasma and urine.
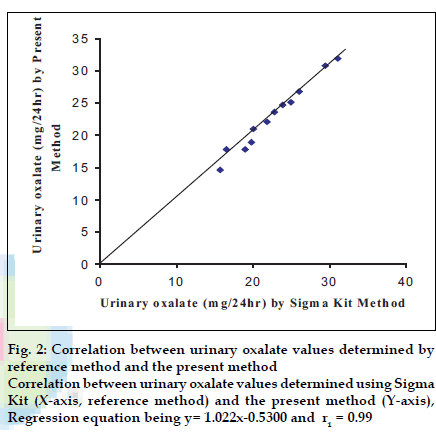
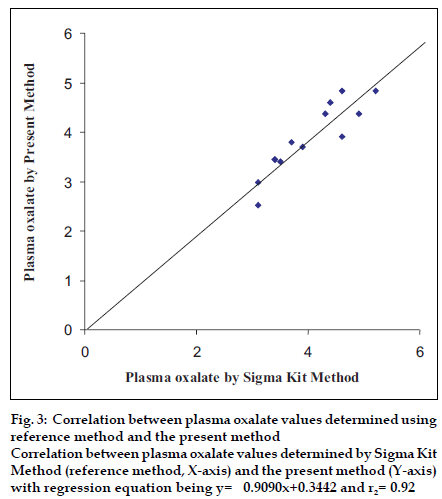
To test the accuracy of method, the oxalate value was determined in 12 urine samples and 12 plasma samples of apparently healthy persons and urinary stone formers by both the present method (y) and standard enzymatic colorimetric kit method (x). The values obtained by both methods showed a good correlation for oxalate in urine (r1= 0.99) with regression equation being y= 1.022x-0.5300 (fig. 2) and oxalate in plasma (r2= 0.92) with regression equation being y= 0.9090x+0.3442 (fig. 3).
Figure 3:Correlation between plasma oxalate values determined using reference method and the present method Correlation between plasma oxalate values determined by Sigma Kit Method (reference method, X-axis) and the present method (Y-axis) with regression equation being y= 0.9090x+0.3442 and r2= 0.92.
The oxalate value in 24 h urine samples in apparently healthy is determined by the present method and found to be in the range of 11.5 to 27.5 mg/l with a mean of 20.8 mg/l. The oxalate value in plasma samples in apparently healthy males and females as determined by the present method was in the range 2.5 to 3.8 µmol/l, with a mean of 2.94 µmol/l, which is higher than that using barley enzyme [8]. The strip bound enzyme did not show any noticeable change in its activity up to 40 d during its regular use (200 times) when stored at 4° in reaction buffer (0.05 M sodium citrate buffer pH 3.5).
References
- Decastro MDL. Determination of oxalic acid in urine-A Rev. J Pharm. Biomed 1988;6:1-14.
- Goyal L, Thakur M, Pundir CS. Quantification of urinary oxalate with alkylamine glass bound Amaranthus leaf oxalate oxidase. Anal Lett 1999;32 : 633-48.
- Goyal L, Thakur M, Pundir CS. Purification and properties of a membrane bound oxalate oxidase from Amaranthus leaves. Plant Sci 1999;142:21-8.
- Pundir CS, Chauhan DN. Detection and solubilization of a membrane bound oxalate oxidase from leaves of Amaranthusspinosus. PhysiolMolBiol Plant 1996;2:179-202.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall R. Protein measurement with the folin phenol reagent. J BiolChem 1951;193:265-75.
- Lynn M. In :Weetall HH. Editor. Immobilized enzyme, antigen antibodies and peptides. Marcel Dekker Inc: New York; 1975. p. 1-17.
- Kumari M, Pundir CS. Measurement of urinary oxalate by grain sorghum leaf oxalate oxidase immobilized to affixed alkylamine glass beads. Indian J BiochemBiophys 2004;41:102-6.
- Madanpotra S, Chaudhary R, Singh S, Pundir CS. Preparation of a reusable strip of barley oxalate oxidase for determination of urinary oxalate. Indian J ChemTechnol 2003;11:50-3.
- Thakur M, Pundir CS. Determination of urinary oxalate with alkylamine glass bound sorghum oxalate oxidase and horseradish peroxidase. Biotechnol Techniques 1999;13:227-30.