- *Corresponding Author:
- K. M. Mahadevan
Department of PG Studies and Research in Chemistry, School of Chemical Sciences, Kuvempu University, Jnana Sahyadri, Shankaraghatta-577 451
E-mail: mady_kmm@yahoo.co.uk
| Date of Submission | 11 October 2005 |
| Date of Revision | 07 March 2006 |
| Date of Acceptance | 24 December 2006 |
| Indian J. Pharm. Sci., 2006, 68 (6): 809-814 |
Abstract
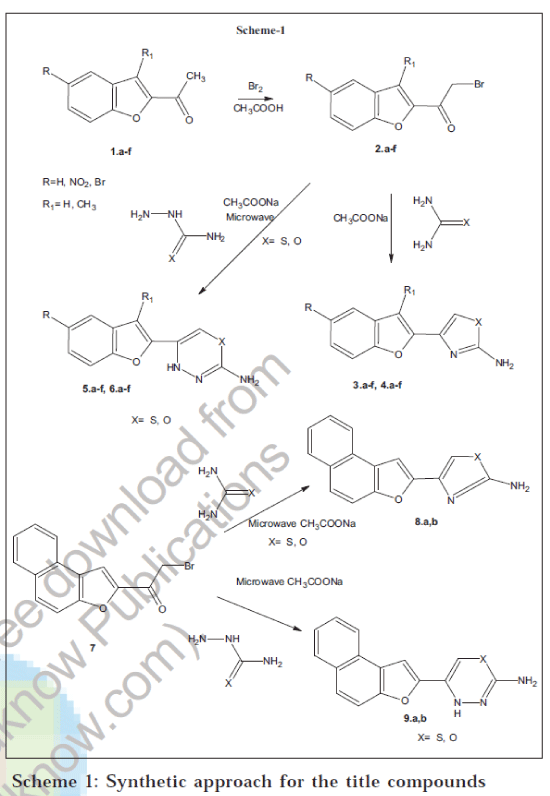
The substituted 2-acetyl benzofurans on bromination gave substituted 2-(2-bromoacetyl) benzofurans 2a-f. These on reaction with thiourea, urea, thiosemicarbazide and semicarbazide in presence of sodium acetate under microwave irradiation resulted 3a-f, 4a-f, 5a-f and 6a-f respectively. The structures of newly synthesised compounds have been established by elemental analysis, spectral data and screened for antimicrobial, anthelmintic, antiinflammatory and analgesic activities.
Keywords
Benzo[b]furan derivatives are an important class of organic compounds, which are known to be present in many natural products [1] and posses physiological activity. They find applications in agrochemicals [2,3], pharmaceuticals [4-10] and cosmetics [11,12]. Benzo[b]furans are building blocks of optical brighteners [13]. Many of the natural benzo[b]furans have physiological, pharmacological and toxic properties and as a result, there is continuing interest in their chemical synthesis [14-15]. Cyclisation reactions of various types have been used to produce substituted benzo[b]furans [16,17].
Nitrogen and sulphur containing heterocycles are compounds of biological interest and the development of new synthetic approach is a challenge for organic chemists. In the present paper, we report on the modification of the side chain in benzo[b]furans to synthesise a new series of derivatives having a new ring at the 2-position.
Thiazoles and oxazoles are generally synthesized by Hantzsch synthesis from a-halogeno ketones and thiourea and thioamides [18,19] and other workers [20,21] synthesized amino thiazoles by replacing a-halogeno ketones with ketones and halogen. Despite this modification the method still remains cumbersome (24-25 h reflux). But the synthesis of these heterocycles in presence of sodium acetate under microwave irradiation has not been reported. The advantage of this method of synthesis is that the reduction in the time of reaction, which otherwise take a long time by conventional water-bath reflux.
Keeping in view on the modification of the side chains in benzo[b]furans, to obtain new series of derivatives having a new ring at 2-position of benzo[b]furans. We report for the first time a facile microwave assisted solid phase one pot synthesis of title compounds.
In our approach to synthesize the title compounds as shown in the Scheme-1, we have started with 2acetylbenzofuran derivatives 1a-f and which were prepared by the reported procedure [22]. The bromination of 1a-f in glacial acetic acid gave 2a-f. Reactions of 2a-f with thiourea and urea in presence of sodium acetate produced thiazoles 3a-f and oxazoles 4a-f respectively [18,19]. The formation of the five-membered ring was confirmed by spectral studies, the absence of carbonyl stretching frequency in the IR spectrum of 3a, a broad singlet at δ 6.30 ppm corresponding to -NH2 protons (D2O exchangeable), a multiplet appeared at δ 7.30 to 7.90 ppm for six aromatic protons and the molecular ion peak at m/z 216 (M+) in its mass spectra confirms the structure assigned to 3a.
Similarly the compounds 2a-f were made to react with thiosemicarbazide and semi carbazide in presence of sodium acetate, which afforded thiadiazines 5a-f and oxadiazines 6a-f ring systems respectively. Formation of the six-membered ring system was confirmed by spectral studies. IR spectra of compounds 5a-f and 6a-f exhibit no peaks corresponding carbonyl-stretching frequency. In the 1H NMR spectrum of 5a a singlet at δ 5.50 ppm integrated for one proton at the 6 position of the thiadiazine ring, a broad singlet at δ 6.35 ppm integrating for two protons due to -NH2 protons (D2O exchangeable), a multiplet appeared at δ 7.30 to 7.90 ppm for five aromatic protons and another singlet at δ 10.20 ppm corresponds to one proton of -NH in the newly formed six-membered ring. The appearance of the molecular ion peak at m/z 231 (M+) confirms the structure of 5a. Similarly the spectral details of the remaining compounds were given in Table 1
| Compd | R | R1 | Mol. formula | Yield % | M.P° | % H | % C | N | |
|---|---|---|---|---|---|---|---|---|---|
| Found | Calcd | ||||||||
| 3a | H | H | C11H8N2SO | 65 | 214 | 3.7 | 61.11 | 12.51 | 12.96 |
| 3b | NO2 | H | C11H7N3SO3 | 68 | 254 | 3.19 | 60.27 | 14.32 | 14.61 |
| 3c | NO2 | CH3 | C12H9N3SO3 | 67 | 267 | 3.27 | 52.36 | 15 | 15.27 |
| 3d | Br | H | C11H7N2SOBr | 64 | 249 | 2.37 | 44.74 | 9.1 | 9.49 |
| 3e | H | CH | C11H10N2SO | 66 | 235 | 4.34 | 62.6 | 12.25 | 12.17 |
| 3f | Br | CH3 | C12H10N2SOBr | 69 | 261 | 3.22 | 46.45 | 9.15 | 9.03 |
| 4a | H | H | C11H8N2O2 | 72 | 216 | 4.00 | 66 | 13.65 | 14 |
| 4b | NO2 | H | C11H7N3O4 | 68 | 245 | 2.85 | 53.87 | 16.75 | 17.14 |
| 4c | NO2 | CH3 | C12H9N3O4 | 67 | 257 | 3.47 | 55.59 | 16.1 | 16.21 |
| 4d | Br | H | C11H7N2O2Br | 64 | 265 | 2.5 | 47.3 | 10.1 | 10.03 |
| 4e | H | CH3 | C12H10N2O2 | 68 | 243 | 4.67 | 67.28 | 12.8 | 13.08 |
| 4f | Br | CH3 | C12H9N2O2Br | 70 | 272 | 3.07 | 49.14 | 9.4 | 9.55 |
| 5a | H | H | C11H9N3SO | 69 | 207 | 3.89 | 57.14 | 18.1 | 18.18 |
| 5b | NO2 | H | C11H8N4SO3 | 66 | 248 | 2.96 | 48.88 | 20.35 | 20.74 |
| 5c | NO2 | CH3 | C12H10N4SO3 | 68 | 278 | 3.44 | 49.65 | 19.15 | 19.31 |
| 5d | Br | H | C11H8N3SOBr | 65 | 250 | 2.58 | 42.58 | 13.3 | 13.54 |
| 5e | H | CH3 | C12H11N3SO | 67 | 213 | 4.48 | 58.77 | 17.1 | 17.14 |
| 5f | Br | CH3 | C12H10N3SOBr | 67 | 258 | 3.08 | 44.44 | 12.75 | 12.96 |
| 6a | H | H | C12H9N3O2 | 66 | 210 | 4.18 | 61.39 | 19.4 | 19.53 |
| 6b | NO2 | H | C11H8N4O4 | 63 | 235 | 3.07 | 50.76 | 21.25 | 21.53 |
| 6c | NO | CH3 | C12H10N4O4 | 62 | 246 | 3.64 | 52.55 | 20.1 | 20.43 |
| 6d | Br | H | C11H8N3O2Br | 66 | 260 | 2.72 | 44.89 | 14.1 | 14.28 |
| 6e | H | CH3 | C12H11N3O2 | 65 | 218 | 4.8 | 62.88 | 18.25 | 18.34 |
| 6f | Br | CH3 | C12H10N3O2Br | 68 | 254 | 3.24 | 46.75 | 13.5 | 13.63 |
| 8a | - | - | C15H10N2SO | 66 | 276 | 3.75 | 67.66 | 10.3 | 10.52 |
| 8b | - | - | C15H10N2O2 | 69 | 268 | 4.00 | 72.00 | 16.45 | 16.8 |
| 9a | - | - | C15H11N3SO | 68 | 293 | 3.91 | 64.05 | 14.8 | 14.94 |
| 9b | - | - | C15H11N3O2 | 65 | 281 | 4.15 | 67.92 | 15.65 | 15.84 |
All compounds gave satisfactory C and H analysis
Table 1: Characterization data of compounds 3a-f, 4a-f, 5a-f, 6a-f, 8a,b and 9a,
The starting reagents were taken in the solid state and were ground to a fine powder. Melting points were determined in open capillary tubes and are uncorrected. IR spectra were recorded on a Perkin-Elmer 297 Spectrophotometer using KBr. 1H NMR spectra were recorded on a Bruker supercon FT NMR (300 MHz) instrument using TMS as internal standard. Chemical shifts are expressed in δ ppm. Purity of the compounds was checked by TLC on silica gel G as an adsorbent. Mass spectra were recorded on a Jeol JMS-D 300 Mass spectrometer operating at 70 eV. The reactions were carried out in a Whirlpool domestic microwave oven (operating between 80-800W, Model No.M-542). The reactions were carried out at power - 2, which correspond to 160W. All the animal experiments with Wistar rats and Swiss mice were carried out at National College of Pharmacy, Shimoga and permission conducting these experiments was obtained from Institutional Animals Ethics Committee (CPCSEA Regd. No.144).
Synthesis of 4-(1-benzofuran-2yl)-1,3-thiazol-2-amine (3a) was achieved by grinding thoroughly to a fine powder a mixture containing 2a (2.39 g, 0.01 mol), thiourea (0.76 g, 0.01 mol) and excess of anhydrous sodium acetate (2 g). The mixture was transferred into a 100 ml beaker covered with a watch glass. The beaker was exposed to microwave radiation for a period of eight min at an interval of 30 s to avoid the charring of the mixture. After the completion of the reaction, the mixture was extracted with chloroform and dried over anhydrous sodium sulphate. The solvent was evaporated and the compound obtained was purified by column chromatography using petroleum ether and ethyl acetate (1:9). The compounds 3b-f were synthesised following the above procedure by reacting 2b-f with thiourea.
In presence of anhydrous sodium acetate 2a was ground in a mortar with urea, semicarbazide and thio semicarbazide separately and irradiated to microwave radiations to produce 4a, 5a and 6a, respectively. Similarly in presence anhydrous sodium acetate, 7 was ground in a mortar with thiourea, urea, thiosemicarbazide and semicarbazide separately and on exposure to microwave radiation produces 8a, 8b, 9a and 9b, respectively. The physical and elemental analysis data of the newly synthesised compounds are given in Table 2.
| Compd. | IR (KBr) (νmax in cm-1) | PMR (δ ppm) (DMSO-d6) | Mass m/z (M+) |
|---|---|---|---|
| 3a | 3250-3365 (-NH2), 1595-1609 (C=C, C=N), 1110-1122 (C-O-C), 650-662 (C-S) |
7.30-7.90 (m, 6H, Ar-H), 6.30 (s, 2H, -NH2) |
216 |
| 4a | 3249-3364 (-NH2), 1595-1609 (C=C, C=N), 1110-1122 (C-O-C) |
7.00-7.70 (m, 6H, Ar-H), 6.31 (s, 2H, -NH2) |
200 |
| 5a | 3408 (-NH), 3253-3364 (-NH2), 1595-1609 (C=C, C=N), 1103-1122 (C-O-C) |
10.20 (s, 1H, -NH), 7.30-7.90 (m, 5H, Ar-H), 6.35 (s, 2H, -NH2), 5.50 (s, 1H, C6-H) |
231 |
| 6a | 3407 (-NH), 3253-3367 (-NH2), 1594-1605 (C=C, C=N), 1108-1120 (C-O-C) |
10.35 (s, 1H, -NH), 7.00-7.70 (m, 5H, Ar-H), 6.34 (s, 2H, NH2), 5.60 (s, 1H, C6-H) |
215 |
| 8a | 3255-3368 (-NH2), 1597-1610 (C=C, C=N), 1108-1120 (C-O-C), 650-662 (C-S) |
7.20-8.30 (m, 8H, Ar-H), 6.30 (s, 2H, -NH2) |
266 |
| 9a | 3400 (-NH), 3253-3365 (-NH2), 1597-1612 (C=C, C=N), 1110-1122 (C-O-C), 650-662 (C-S) |
10.30 (1H, -NH), 7.20-8.40 (m, 7H, Ar-H), 6.38 (s, 2H, -NH2), 5.60 (s, 1H, C6-H) |
281 |
Table 2: Spectral data of compounds
The in vitro antimicrobial activity was carried out against 24h cultures of two bacteria and one fungus. The bacteria used were Staphylococcus aureus and Klebsiella pneumoniae and the fungus used was Aspergillus niger. Pure culture of the test microorganisms was procured from the culture maintained at Biotechnology Department, Kuvempu University, Shimoga. The antimicrobial activity was performed by cup-plate method [23]. Nutrient agar and potato dextrose agars were used to culture the bacteria and fungus respectively. The compounds were tested at a concentration of 0.005 mol/ml in DMF solution. The solution of streptomycin (2 mg/ml) and griseofulvin (2 mg/ ml) were prepared in sterilized water and used as standards for comparison of antibacterial and antifungal activities respectively. Inhibition was recorded by measuring the diameter of the inhibition zone at the end of the 24h for bacteria and fungus. Each experiment was repeated thrice and the average of the three independent determinations was recorded. The results are summarized in the Table 3.
| Comp | Zone of inhibition in (mm*) | Anthelmintic activity Time in (min) | |||
|---|---|---|---|---|---|
| S. aureus | K. pneumoniae | A. niger | Paralysis (t) | Death (t) | |
| 3a | 2.9 | 35 | 26 | 123 | - |
| 3b | 7 | 23 | 28 | 105 | 95 |
| 3c | 6.5 | 29 | 23 | 90 | 115 |
| 3d | - | 26 | 21 | 80 | 120 |
| 3e | 12 | 15 | 27 | - | - |
| 3f | 8 | 21 | 18 | 80 | 120 |
| 4a | 10.5 | 18 | 17 | 90 | 120 |
| 4b | 8.9 | 18 | 26 | 100 | - |
| 4c | 5.2 | 17 | 15 | - | 130 |
| 4d | - | - | 21 | 100 | 120 |
| 4e | - | - | 10 | 90 | 140 |
| 4f | 15 | 20 | 28 | - | - |
| 5a | 13 | 24 | 18 | 110 | 125 |
| 5b | - | 17 | 25 | - | 95 |
| 5c | - | 19 | 18 | 100 | 130 |
| 5d | 12 | 16 | 24 | 100 | 120 |
| 5e | 14 | 17 | 35 | - | 110 |
| 5f | 6 | 24 | 54 | 90 | 130 |
| 6a | 4.2 | 13 | 23 | 100 | 120 |
| 6b | 14 | 42 | 26 | 130 | 165 |
| 6c | - | 25 | 15 | 160 | 180 |
| 6d | 15 | 18 | 27 | - | - |
| 6e | 17 | 19 | 32 | 120 | 150 |
| 6f | 12 | 14 | 17 | 60 | 90 |
| 8a | 13 | 12 | 15 | - | 90 |
| 8b | 15 | 14 | 12 | - | - |
| 9a | 12 | 13 | 14 | 90 | 120 |
| 9b | 16 | 15 | 13 | 120 | 90 |
| Streptomycin | 24 | 25 | - | - | - |
| Griseofulvin | - | - | 23 | - | - |
| Mebendazole | - | - | - | 90 | 90 |
*Including diameter of the well, Control (DMF), (-) - No activity
Table 3: Antimicrobial and anthelmintic activities of 3a-f, 4a-f, 5a-f, 6a-f, 7a-f, 8a, b and 9a, b.
The results revealed that compounds 2b, 3b, 3f, 4b, 4f and 6d were as active as standard and 5e, 5f, and 6f show high activity against A. niger. The compounds 2a,3c, 5f and 6b were found to be highly active and other compounds were moderately active against K. pneumoniae in comparison with streptomycin and griseofulvin, which were used as standard drugs.
Anthelmintic activity was evaluated on earthworms (Pheritima posthuma, Order-Annelida, Class-oligochaeta) by following the reported method [24]. The worms were collected from a local supplier at Shimoga at the time of carrying out anthelmintic activity. The worms were sorted for uniform length and size. They were washed with normal saline to remove the adhering material and were kept in 6% dextrose solution for 15 min and those with normal mobility were used for the test. The compounds were tested at a dose of 0.005 mol/ml suspension in 0.1% Tween-80 solution in saline. Mebendazole was used as standard drug at dose of (0.005 mol/ml) suspension in 0.1% Tween-80 solution in saline. Petridishes of nearly equal size were taken and two worms were placed in each petridish. Normal saline (20 ml), Tween-80 solution and mebendazole suspension were poured in to separate petridishes as a control, blank and standard respectively. The suspension of the test compounds (20 ml) was taken in different petridishes and time was noted as zero time. Time for paralysis (loss of movement) and time taken for deaths were recorded (Table 3).
Analgesic activity was determined by using a method24 based on acetic acid induced writhings in mice. Swiss mice of either sex were procured from Virus Diagnostic Laboratory, Shimoga and maintained at National College of Pharmacy and were fed with standard diet and water ad libitum. Nine groups of six mice each (22-35 g) were selected and 0.6% acetic acid (dose 10 ml/kg) was injected intraperitoneally. The number of writhes was counted for 20 min, after five min of injection of acetic acid in each mice. This reading was taken as control. Next day the same groups of mice were used for evaluating analgesic activity. Each group was administered orally with the suspension of test compound in 0.1% Tween-80 solution at the dose of 100 -mg/kg body weight of the animal 1 h before injection of acetic acid. After five minutes, the mice were observed for the number of writhes for the duration of 20 min. The mean value for each group was calculated and compared with the control. Acetyl salicylic acid was used as standard for comparison of analgesic activity and the results are recorded in Table 4.
| Compd. | Dose (mg/kg) | Mean no. of writhing | % Protection | |
|---|---|---|---|---|
| Control | Treatment | |||
| Standard | 100 | 47.0±2.50 | 11.8±1.27 | 74.9 |
| 3b | 100 | 31.5±2.08 | 15.5±2.28* | 50.8 |
| 4c | 100 | 33.2±1.87 | 14.5±1.54* | 56.3 |
| 5f | 100 | 36.5±1.41 | 16.5±0.11 | 54.8 |
| 6b | 100 | 34.8±1.51 | 16.8±2.64* | 51.7 |
| 6d | 100 | 30.8±2.76 | 12.3±1.58 | 60 |
Values are expressed as mean ± SEM, Number of animals in each group is 06: *P<0.5, % of protection = (Nc-Nt/Nc) ×100, Nc = Number of writhing in control, Nt = Number of Writhing in test compounds
Table 4: Analgesic Activity Of Some Selected Compounds
Investigation of analgesic activity reveals that the compound 4c, 5f, 6d were found to be highly active and 3b, 6b were moderately active and remaining were less active than the standard drug. When anthelmintic activity was carried out on Pheritima posthuma, the compounds 8a and 9b showed almost equipotent activity; whereas the rest of the compounds showed less activity than the standard drug.
Acknowledgements
The authors are thankful to Professor and Chairman, Department of Chemistry, Kuvempu University for providing laboratory facilities and are also thankful to Principal National College of Pharmacy, Shimoga, Chairman, Department of Bio-technology, Kuvempu University and Director, Virus diagnostic laboratory, Shimoga for providing animals for the experiments.
References
- Simpson, T.J., In; Thomson, R.H., Eds., The Chemistry of Natural Products, Blackie, London, 1985.
- Keay, B.A. and Dibble, P.W., In; Review of the Literature 1982-1995, Comprehensive Heterocyclic Chemistry, II-A, Pergamon, New York, 1996, 2, 413.
- Bos, M., Stadler, H. and Wichmann., J.U.S. US Patent No., US5, 955, 495; through Chem. Abstr, 199, 131, 214186n.
- Powers, L.J., J. Med. Chem., 1976, 19, 57.
- Nagahara, T., Yokoyama, Y., Inamura, K., Katakura, S., Komoriya, S., Yamaguchi, H., Hara, T. and Iwamoto, M., J. Med. Chem., 1994, 37, 1200.
- Ohemeng, K.A., Appollina, M.A., Nguyen, V.N., Schwender, C.F., Singer, M., Steber, M., Ansell, J., Argentieri, D. and Hageman, W., J.Med. Chem., 1994, 37, 3663.
- Gubin, J., Vogelaer, H., Inion, H., Houben, C., Lucchetti, J., Mahaux, J., Rosseels, G., Peiren, M., Clinet, M., Polster, P. and Chatelain, P., J. Med. Chem., 1993, 36, 1425.
- Zawadowski, T., Suski, S., Rajchaman, J., Bogdal, M. and Szafranski B., Acta. Pol. Pharm., 1993, 50, 457.
- Yang, Z., Liu, H.B., Lee, C.M., Chang, H.M. and Wong, H.N.C., Org. Chem., 1992, 57, 7248.
- Gilchrist, T., In: Heterocycl Chemistry, 3rd Edn, Longman, Singapore.,1997.
- Chatterjee, R. and Kapoor, R., US Patent No., 5118707, through Chem. Abstr., 117, 97119r, 1992
- Leung, A.Y. and Foster, S., In; Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics, Wiley, New York., 1996.
- Schmidt, E., In; Ullmann’s Encyclopedia, 6th Edn, Optical Brightners,Electronic Release, 1999.
- Katrizky, A.R., Fali, C.N. and Li, J., J. Org. Chem., 1997, 62, 8205.
- Kappe, C., Murphree, S. and Padwa, A., Tetrahedron, 1997, 53,14179.
- Cagniant, P. and Cagniat, D., In; Adv. Hetercycl. Chem., 1975, 18, 337.
- Mustafa, A., In; Benzofurans, Wiley, New York, 1974.
- Wawzonek, O., In; Heterocyclic Compounds, John Wiley and Sons, New York, 1975.
- Dean, F.M., In; Naturally Occurring Oxygen Ring Compounds, 2ndEdn, Butterworths, London, 1963, 176.
- Livingtone, R. Rod’s., In; Chemistry of Carbon Compounds, 2nd Edn, Elisveir, Amsterdam, 1997, 4, 96.
- Satuton, J., In; Comprehensive Organic Chemistry, Edt, by Barton, D.H. R., and Olis, W. D., Oxford, 1979, 4, 629.
- Elliot, E.D., J. Amer. Chem. Soc., 73, 1951, 754.
- Mahadevan, K.M., Basavaraj, P and Vaidya, V.P., Indian J.Heterocycl. Chem., 2001, 11, 15.
- Satyanarayana, K. and Rao, M.N.A., Indian J. Pharm. Sci.,1998, 60, 379.