- *Corresponding Author:
- A. P. Gadad
K.L.E S’s College of Pharmacy, Hubli-590 031, India.
E-mail: gadadap@rediffmail.com
| Date of Submission | 7 February 2005 |
| Date of Decision | 13 June 2005 |
| Date of Acceptance | 25 March 2006 |
| Indian J Pharm Sci, 2006, 68 (2): 269-273 |
Abstract
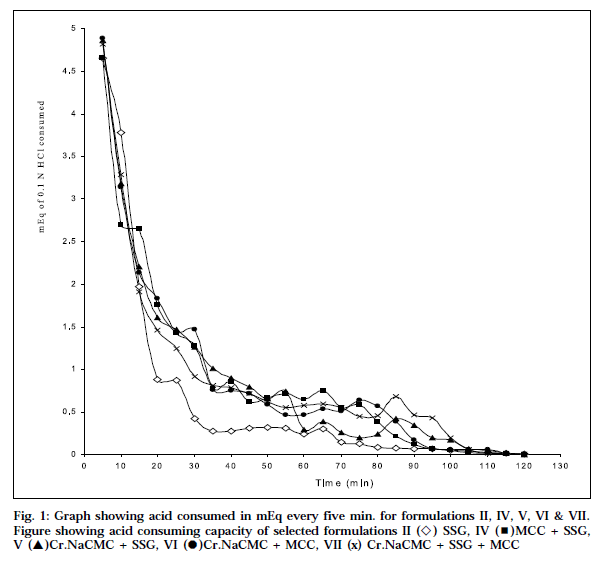
Aim of the present work is to develop non-chewable antacid tablets using different disintegrating agents viz., microcrystalline cellulose, sodium starch glycolate (Primogel®), and cross-linked sodium carboxymethylcellulose (cros-car-mellose sodium®). These agents were used alone, and in combinations, both 50% intra-granularly, and 50% extra-granularly. To cover all these variables in the formulations, seven different formulations were designed. Use of different disintegrating agents have shown varying effect on disintegration time and pattern. The disintegration time for formulation I and III did not comply with the official disintegration test in distilled water, as well as in simulated gastric fluid. All formulations, except formulation I and III, showed nearly equivalent to 30 min of Rosset-Rice time for neutralization. The graphical representation shows that when the base is available in full strength, it neutralizes the acid at a faster rate, and then the amount of base goes on reducing progressively, resulting in decrease in the rate of neutralization. Based on't' values, formulation II and VI show that the theoretical acid-consuming capacity, and the observed acid- consuming capacity values are almost equal.
Introduction
Gastric antacids are agents that neutralize acid or remove acid from the gastric contents. They are widely prescribed by physicians in the treatment of hyperchlorhydria and peptic ulcer. The antacid tablets are also used by self-medication for the treatment of a wide variety of gastric symptoms[1]. The antacid preparations are popularly used, either in the form of chewable tablets, or suspension. Chewable tablets should be masticated and swallowed at once, with a drink of water. It has been reported, that these tablets are sipped slowly for longer period of time, or otherwise, swallowed like conventional tablets. This wrong usage would either lead to reduced therapeutic efficacy, or may lead to mechanical obstruction of the ileum from impacted chewable antacid tablets[2]. Another major disadvantage with the chewable tablet, is unpleasant taste and grittiness mouth feel, leading to poor patient compliance[3,4]. Hence, to circumvent these disadvantages, the non-chewable antacid tablets (disintegrating tablets) were formulated. In the literature survey, many attempts were made to evaluate the in vitro performance of Ac-Di-sol and Primogel as adjutants, on the properties of tablets prepared with Avicel PH101 by direct compression, and also the effect of intragranular disintegrant with extragranular disintegrants, reported by M. Jovanovic[5,6].
The present study, aims to compare the microcrystalline cellulose (MCC), Primogel (SSG), and Cros-car-mellose sodium (cr-NaCMC), when used as disintegrating agents alone, and in combinations in non-chewable tablets. These disintegrating agents were used, both 50% intragranularly, and 50% extra-granularly. The effect on disintegration time and pattern was studied as a main parameter on the antacid tablets, as onset of action and rate of neutralization, which all depends on the disintegration time of tablets.
Dried Aluminium hydroxide IP, Magnesium hydroxide IP, Cr-NaCMC USP (gift samples from Wallace Pharmaceuticals, Dharwad.), MCC (Avicel PH101), Sodium starch glycolate IP (gift samples from Gufic Chem, Belgaum). All other chemicals are of analytical grade, and used without further purification.
The tablets were prepared by using the master formula, with varying proportions of disintegrant concentration for each formulation, as given in Table 1. In brief, accurately weighed dried aluminium hydroxide gel, magnesium hydroxide, sodium bicarbonate, lactose, and disintegrating agents, were mixed uniformly. Granules were prepared by the wet granulation process, using starch mucilage as granulating agent. The lubricated granules were compressed at a compression force of 5 kg/cm2, in a sixteen-station rotary punching machine, with flat punch face of 12 mm diameter, with a break-line. Tablet content uniformity of aluminium hydroxide and magnesium hydroxide was done as per IP procedure.
| Ingredients | Each tablet contains | ||||||
|---|---|---|---|---|---|---|---|
| I | II | III | I V | V | V I | VII | |
| Dried Aluminium hydroxide gel | 300 mg | 300 mg | 300 mg | 300 mg | 300 mg | 300 mg | 300 mg |
| Magnesium hydroxide | 150 mg | 150 mg | 150 mg | 150 mg | 150 mg | 150 mg | 150 mg |
| Sodium bicarbonate | 25 mg | 25 mg | 25 mg | 25 mg | 25 mg | 25 mg | 25 mg |
| Lactose | 30 mg | 30 mg | 30 mg | 30 mg | 30 mg | 30 mg | 30 mg |
| Sodium saccharin | 1.25 mg | 1.25 mg | 1.25 mg | 1.25 mg | 1.25 mg | 1.25 mg | 1.25 mg |
| Aerosil | 1.25 mg | 1.25 mg | 1.25 mg | 1.25 mg | 1.25 mg | 1.25 mg | 1.25 mg |
| Starch | 22 mg | 22 mg | 22 mg | 22 mg | 22 mg | 22 mg | 22 mg |
| Talc | 0.75% | 0.75% | 0.75% | 0.75% | 0.75% | 0.75% | 0.75% |
| Magnesium stearate | 0.25% | 0.25% | 0.25% | 0.25% | 0.25% | 0.25% | 0.25% |
| Disintegrating agent | |||||||
| MCC | 7.5% | -- | -- | 3.75% | -- | 3.75% | 2.5% |
| SSG | -- | 7.5% | -- | 3.75% | 3.75% | -- | 2.5% |
| Cr.NaCMC | -- | -- | 7.5% | -- | 3.75% | 3.75% | 2.5% |
Table 1: Master Formula Of Tablet With Varying Proportions Of Disintegrating Agent
The method used for the study was that of Rossett-Rice test[7,8], for the acid neutralizing capacity (ANC). The pH profile during the neutralization reaction was followed by adding 70 ml of 0.1 N HCl and 30 ml of distilled water, to a 500 ml reaction beaker. When the temperature was maintained at 37°, an equivalent weight of tablet sample was added. Simultaneously 0.1 N HCl was added at a rate of 4 ml/min, from a burette. A pH meter was attached to the reacting vessel, to record the pH during the neutralization reaction. The time taken to reach pH 3.0 and Rossett-Rice time i.e. the time during which the pH maintained between pH 3.0 and 5.0, was noted. The Rossett-Rice test attempted to stimulate the stomach, and to record the pH profile during acid neutralization.
The Rastogi and Verma[9] method was a modified version of the original method, introduced by Corrento. Two tablets were added to a 100 ml of distilled water, in a 500 ml beaker. They were mixed well, kept at 37The Rastogi and Verma9 method was a modified version of the original method, introduced by Corrento. Two tablets were added to a 100 ml of distilled water, in a 500 ml beaker. They were mixed well, kept at 37° C in water bath, stirred with a magnetic stirrer, and the pH was recorded. Continuous addition of 0.1 N HCl solution was then regulated from a burette, so that the antacid acid C in water bath, stirred with a magnetic stirrer, and the pH was recorded. Continuous addition of 0.1 N HCl solution was then regulated from a burette, so that the antacid acid mixture was always maintained at pH 3 (±0.1). The cumulative volume of acid consumed, was noted at every 5 minutes interval. Five such runs were conducted for each sample. The mEq of HCl consumed every 5 minutes versus time in minutes, was plotted.
In the present investigation USP ANC10 was studied for antacid formulations, and the results were expressed in mEq of HCl consumed, as IP doesn’t specify ANC in terms of mEq of HCl consumed. The present investigation was undertaken to evaluate the effect of different disintegrating agents on their acid neutralization properties. Evaluation parameters like weight variation, hardness, thickness, and friability of all the tablet formulations, were found to be satisfactory and within IP limits. Values observed for tablet properties for all formulations I to VII are in the range, weight variation (0.573 to 0.578 g), friability (0.20 to 0.40), thickness (3.85 to 3.90 cm), and hardness (6.0 to 7.5 kg/cm2).
Disintegration time for Formulation I and III didn’t comply with the official disintegration test in distilled water, as well as in the simulated gastric fluid. Formulation VII failed to comply with the official disintegration test, in simulated gastric fluid. All other formulations disintegrated quickly well within the limits of official disintegration time, both in distilled water, as well as in simulated gastric fluid, as shown in Table 2.
| Formulations | Disintegration time | Formulation contains | Onset to pH 3.0 | Time (min) | Theoretical acid consuming capacity (mEq) | Observed acid consuming capacity (mEq) | % Base available for neutralization | t-value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Distilled water | 0.1N HCl | Dried Al(OH)2 (mg) | Mg(OH)2 (mg) | ||||||||
| I | 458 min 8 s | 365 min | 600.8 | 300.2 | 91 min 33 s | 126 | 21.73 | 15.36 | 70.68 | +236.75 | |
| II | 3 min 27 s | 4 min 21 s | 600.4 | 300.0 | 6 min 02 s | 134 | 21.72 | 21.70 | 99.91 | +0.08* | |
| III | 182 min 0 s | 249 min | 600.0 | 300.0 | 44 min 38 s | 122 | 21.70 | 16.41 | 75.61 | +118.01 | |
| IV | 2 min 14 s | 4 min 22 s | 600.0 | 299.8 | 5 min 42 s | 120 | 21.70 | 21.57 | 99.39 | +0.86* | |
| V | 33 s | 8 min 54 s | 598.8 | 299.2 | 3 min 15 s | 125 | 21.68 | 21.44 | 98.86 | +2.28* | |
| VI | 25 s | 7 min 48 s | 599.0 | 299.4 | 4 min 44 s | 125 | 21.67 | 21.67 | 100.00 | +0.42* | |
| VII | 25 s | 27 min 02 s | 600.6 | 300.0 | 5 min 12 s | 122 | 21.77 | 21.65 | 99.42 | +4.01* | |
| M | -- | -- | 400.0 | 400.0 | 360 min 35 s | 120 | 17.75 | 16.85 | 94.92 | +28.67 | |
Table 2: Significance Of Chemical Composition With Disintegration Time And Acid Neutralizing Capacity Of Non Chewable Antacid Formulations
Formulation I did not show the breakdown of tablet, either in distilled water, or in simulated gastric fluid and remained intact, the reason being that the microcrystalline cellulose doesn’t take water inside the tablet actively, but it rather goes into solution very slowly[11]. Formulation III swells quickly in contact with distilled water or in simulated gastric fluid, but there was no breakdown of tablets (swells and floats). This shows that formulation alone with Ac-Di-sol doesn’t gives effective disintegration time; and this may be attributed to the hydrogen bonds between adjacent cellulose particles that are brought closely together by plastic deformation[12]. Formulation VII showed a good disintegration time within seconds in distilled water, but disintegration time is prolonged to 27’02" in simulated gastric fluid, which may be due to interaction between the antacid bases and acid, resulting in formation of more porous tablets, leading to more space for the particles to swell without immediately breaking the tablet[12].
The USP ANC measures the milliliters of 0.1 HCl neutralized by 1.0 g of tablet in 1.0 h at 37°±1° C, whereas Rossett-Rice test measures the milliliters of 0.1 N HCl neutralized by 1.0 g of tablet in 30 min at 37°±1° C (Note! volume of acid consumed was expressed in mEq). The mean content of the tablet expressed in mEq/g by USP ANC for formulations I, II, III, IV, V, VI and VII, are 21.86, 24.59, 20.45, 26.58, 26.43, 25.10 and 24.30, respectively. The mean content of the tablet expressed in mEq/g by Rossett-Rice ANC with Rossett-Rice time for formulations I, II, III, IV, V, VI and VII, are 17.07 (19 min and 30 s), 24.13 (27 min and 15 s), 16.96 (19 min and 15 s), 24.18 (27 min and 30 s), 23.59 (26 min and 45 s), 23.95 (27 min and 25 s), and 23. 59 (26 min and 50 s), respectively. The Rossett-Rice test attempts to simulate the stomach conditions, and record the pH profile during acid neutralization. The Rossett-Rice time (time during which pH is maintained between 3.0 and 5.0) taken to neutralize the mean content of the tablet was expressed in mEq, and the Rossett-rice time. All the formulations except formulation I & III showed nearly equivalent to 30 min of Rossett-Rice time for neutralization. The mEq/g of acid consumed from the USP and Rossett- Rice test were almost same.
The Rastogi and Verma method was undertaken to evaluate in vitro ANC, onset of action to pH 3.0, rate of neutralization, and duration of action. The labeled composition, pH of antacid acid mixture, theoretical and observed acid consuming capacity (in mEq of HCl), the time (min) taken by the antacid to be fully neutralized by the acid, and the percentage of the antacid available for neutralization at a reasonable rate for the formulations under study, are shown in the Table 2. *Marketed chewable tablet was used for comparative study with formulated Non-chewable tablets without crushing, to measure these parameters. Chewable tablets if swallowed as such, may not always lead to intestinal obstruction, but being non-disintegrating, doesn’t disintegrate readily in the stomach, thereby delaying the onset of action (time taken to reach pH 3.0), and the percentage of base available for neutralization also reduces.
The initial pH of the antacid acid mixture varies between 7.79 and 7.80 for all formulations. It is evident from the table, that the percentage of antacid base available for neutralization varied from 70.68 (formulation I) to 100.0 (formulation VI). The time (min) required to neutralize the antacids varied between 120 and 125. The onset of action for marketed chewable tablet, formulation I and III showed 360min 35s, 91min 33s and 44min 38s, respectively whereas formulations II, IV, V, VI & VII showed a quicker onset of action 6 min 02 s, 5 min 42 s, 3 min 15 s, 4 min 44 s and 5 min 12 s, respectively, which is correlated with the disintegration time in distilled water, and simulated gastric fluid. The graphical representation shows the mEq of acid consumed every 5 min, versus time (Fig. 1). The graph reveals that at the initial stages, when the base is present in its full strength,it neutralizes the acid at a faster rate. As the neutralization progresses, the amount of base goes on reducing progressively, resulting in corresponding decrease in the rate of neutralization.
According to the statistics, the test for significance i.e. ‘t’ values were found to be acceptable at 0.05% level of significance for formulations II, IV, V and VI, and at 0.01% level of significance for formulation VII.
Acknowledgements
Authors thank the Wallace Pharmaceuticals and Gufic Chem Pvt. Ltd for providing the gift samples and KLES College of Pharmacy, Belgaum for providing necessary facilities to carry out the work.
References
- Gilman, A.R., In; Goodman, L.S., Rall T.W., Murad F., Eds., The Pharmacological Basis of Therapeutics, 5thEdn., MacMillan Publishing company, New York.,1975, 960.
- David Potyk, M.D.; N. Eng. J. Med., 1970, 134, 283.
- Roth, H.P., and Berger, G.G.; Gastroenterology, 1960, 38, 630.
- Klein, K. and Libermann, D., N. Eng. J. Med., 1982, 17, 1492.
- Jovanovic, M. Pharmazie, 1988, 43, 213.
- Pattani, S.P. Pharm. J., 1981, 19, 311.
- Rossett, N. E. and Rice, J. Gastroenterology, 1954, 26, 490.
- Kerkhof, N.J. J. Pharm. Sci., 1977, 66, 1529.
- Verma, R.K., Sharma, M. and Bindal, M.C. Indian. J. Pharm. Sci., 1994, 11-12, 125.
- The United States Pharmacopoeia. The National Formulary, USP 22, NF 17, United States Pharmacopoeial Convention, Inc., Rockville, M.D., 1990, 1528.
- Paronen, P., Juslin, M. and Karnanen, K. Drug Develop. Ind.Pharm., 1985, 11(2 and 3), 425.
- Jovanovic, M. Pharmazie, 1987, 42, 488.