- *Corresponding Author:
- D. J. Singh
Department of Pharmaceutics, Bombay College of Pharmacy, Kalina, Mumbai - 400 098, India
E-mail: dipaksingh77@yahoo.com
| Date of Acceptance | 24 October, 2007 |
| Indian J Pharm Sci,2007, 69 (5): 714-715 |
Abstract
Introduction
Fluticasone propionate (FP) is a potent triß uorinated glucocorticosteroid administered by inhalation using metered dose inhalers (MDI) or dry powder inhalers (DPI) for treatment of asthma, allergic rhinitis and chronic obstructive pulmonary disease (COPD) [1]. The aerosol particles of the inhaled FP need to be in solution to facilitate their local and systemic effects. However, FP is a poorly water-soluble compound (<1 µg/ml), and hence the dissolution process is likely to be a rate-limiting step in the systemic absorption of FP [2]. Further, hydrophobic molecules are recognized and taken up by macrophages, found at all epithelial lung surfaces, and especially in the deeper regions of the lung [3]. This phenomenon can be decreased by treating these particles with hydrophilic polymer to form a protective hydration shell referred as stealth structure around the particulate [4]. The aim of present study was to evaluate in vivo lung deposition pattern and antiasthmatic efficacy of sustained release FP microparticles for improved therapy of asthma
Materials and Methods
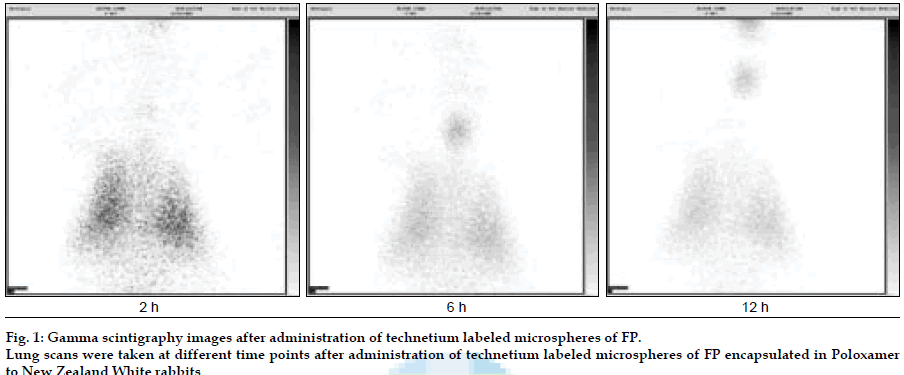
FP (Sun Pharmaceutical Industries Ltd, India) loaded Poloxamer (BASF, India) microparticles were developed using a modified spray drying method (Umang Pharmatech Pvt. Ltd, India). In vitro drug release studies were carried out using USP Type IV apparatus in simulated lung buffer (PBS pH 7.4), maintained at 37±0.50. Fine particle fraction (FPF) was evaluated using twin stage impinger (TSI). Microspheres were radiolabeled with technetium (99mTc) using SnCl2 as reducing agent. Lung deposition and retention time for the labeled microspheres were evaluated in New Zealand rabbits. The labeled microspheres (10-12 mCi) mixed with carrier (lactose monohydrate) were placed in a nebulizer cup and the rabbit, placed in nose only exposure chamber, was allowed to inhale the aerosolized powder mixture with aid of oxygen supply (0.5-2 l/min) for 5 min. Scintigraphic images of rabbit lungs were taken at periodic intervals viz., 2, 4, 8 and 12 h using a gamma camera (Millennium MPS Gamma Camera) and compared with the images obtained after free 99mTc administration. An in vivo efficacy study of microspheres of FP was carried out in ovalbumin sensitized guinea pigs.
Results and Discussion
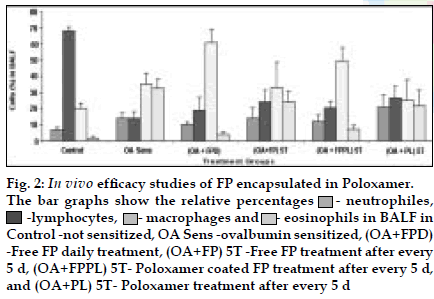
FP microparticles encapsulated in Poloxamer were successfully prepared using modified spray drying method. The microspheres showed improved dissolution profile and sustained release of FP for more than 60 h. DPI formulations prepared with lactose using FP coated with poloxamer yielded a FPF, based on emitted dose, of 34-40% as compared to blends prepared with plain drug and lactose (17- 20%). The lung deposition was calculated as ratio of activity in the lungs to activity emitted from device, which was found to be only 8-10% of the total administered dose. In case of FP loaded microspheres, the gamma scintigraphic images showed that activity in lungs was observed for 12 h (fig. 1), whereas free 99mTc cleared rapidly over a period of 6 min. More activity was deposited in peripheral region than central region of lungs indicating fine particles produced by microspheres. Furthermore, a decrease in the counts in the central region was observed over the time period resulting in a decrease in central to peripheral ratio (Table 1). In vivo efficacy study revealed that FP encapsulated in microspheres delivered every fifth day of therapy was as effective, as daily FP therapy in decreasing lung inß ammation and lowering eosinophil count as well as peripheral blood eosinophils (fig. 2).
| Time | Left Lung | Right Lung | ||||||
|---|---|---|---|---|---|---|---|---|
| C(%) | I(%) | P(%) | C/P | C(%) | I(%) | P(%) | C/P | |
| Initial (5min) | 22.99 | 42.18 | 34.83 | 0.660 | 23.19 | 41.08 | 35.73 | 0.649 |
| 2h | 21.51 | 42.01 | 36.48 | 0.590 | 22.96 | 39.92 | 37.13 | 0.618 |
| 6h | 20.71 | 41.89 | 37.40 | 0.554 | 21.13 | 39.84 | 39.03 | 0.542 |
| 12h | 19.61 | 40.38 | 40.00 | 0.490 | 17.79 | 42.03 | 40.19 | 0.443 |
C, I and P represent central, intermediate and peripheral regions of the lung, respectively
Table 1: Percent Activity In Central, Intermediate And Peripheral Region Of Lungs
Fig. 2: In vivo eficacy studies of FP encapsulated in Poloxamer.
The bar graphs show the relative percentages ![]() - neutrophiles,
- neutrophiles, ![]() -lymphocytes,
-lymphocytes,![]() - macrophages and
- macrophages and ![]() - eosinophils in BALF in
Control -not sensitized, OA Sens -ovalbumin sensitized, (OA+FPD)
-Free FP daily treatment, (OA+FP) 5T -Free FP treatment after every
5 d, (OA+FPPL) 5T- Poloxamer coated FP treatment after every 5 d,
and (OA+PL) 5T- Poloxamer treatment after every 5 d
- eosinophils in BALF in
Control -not sensitized, OA Sens -ovalbumin sensitized, (OA+FPD)
-Free FP daily treatment, (OA+FP) 5T -Free FP treatment after every
5 d, (OA+FPPL) 5T- Poloxamer coated FP treatment after every 5 d,
and (OA+PL) 5T- Poloxamer treatment after every 5 d
This indicates the lung retention of these microspheres along with sustained action. Pulmonary delivery of FP loaded in Poloxamer is an effective treatment regimen for asthma, to provide localized sustained action in the lungs with reduced local and systemic toxic effects.
Acknowledgements
The authors wish to thank BRNS-DAE (Sanction no. 2004/35/5/BRNS) for financial assistance, Burculodomo ingredients for lactose and Sun Pharmaceutical Ltd, India for FP.
References
- Ventresca GP, Mackie AE, Moss JA, McDowell JE, Bye A (1994).Absorption of oral fluticasone propionate in healthy subjects. Am J Respir Med;149:A214.
- 2.Davies NM, Feddah MR (2003). A novel method for assessing dissolution of aerosol inhaler products. Int J Pharm;255:175-87.
- Li JT, Caldwell KD(1996).Plasma protein interactions with pluronic treated colloids. Colloids Surfaces B Biointerfaces;7:9-22.
- 4.Jones BG, Dickinson PA, Gumbleton M, Kellaway IW (2002). The inhibition of phagocytosis of respirable microspheres by alveolar and peritoneal macrophages. Int J Pharm;236:65-79