- *Corresponding Author:
- S. K. Mehta
Department of Chemistry, Lovely Professional University, Phagwara-144 411, India
E-mail: smchem16@gmail.com
| Date of Submission | 21 April 2017 |
| Date of Revision | 28 December 2017 |
| Date of Acceptance | 25 July 2018 |
| Indian J Pharm Sci 2018;80(5):827-836 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Synthesis of sulphanilamide-based organic nanoaggregates and their interactions with PEG-6000 were studied using absorption and emission spectroscopy. Size and morphology of sulphanilamide-based organic nanoaggregates and PEG-coated sulphanilamide organic nanoaggregates were determined using dynamic light scattering, scanning electron microscopy and transmission electron microscopy techniques. Dynamic light scattering studies suggested the formation of around 100 nm sized sulphanilamide organic nanoaggregates and 197 nm sized PEG-conjugated sulphanilamide organic nanoaggregates. The effect of pH on hydrolysis of nanoaggregates has shown maximum instability at pH 9, which was stabilized by conjugation of nanoaggregates with PEG. Further interaction studies of PEG-coated nanoaggregates were carried out in aqueous solutions of major electrolytes and organic acids present in biofluids. Results demonstrated that Mg+2 had maximum interactions with PEG-coated sulphanilamide organic nanoaggregates in aqueous solutions of chlorides and sulphates; whereas Na+ showed maximum interactions in aqueous solutions of carbonates and bicarbonates. Similarly, among organic aqueous acid solutions, L-ascorbic acid showed greater interactions than nicotinic acid with PEG-conjugated sulphanilamide organic nanoaggregates. Antibacterial activities of sulphanilamide, sulphanilamide organic nanoaggregates and PEG-coated sulphanilamide organic nanoconjugates were evaluated against four strains of Gram-positive and Gram-negative bacteria. Minimum inhibitory concentration profile suggested that sulphanilamide PEG nanoconjugates showed highest antibacterial activity against Staphylococcus aureus after 72 h of treatment. Scanning electron microscopy studies performed before and after the treatment of sulphanilamide PEG nanoconjugates, indicated accumulation of cytoplasm of bacterial cell and formation blebs, which ultimately led to cell death. Sulphanilamide PEG nanoconjugate demonstrated greater antibacterial activity compared to that of sulphanilamide.
Keywords
Sulphanilamide, organic nanoaggregates, antibacterial activity, sustained release, polymer nanoconjugate
Sulfonamides are a class of synthetic drugs, which share structural similarity with PABA (p-aminobenzoic acid) [1]. Sulfonamides and their N-derivatives are being constantly investigated to explore their diverse applications [2]. In the history of human pathology, sulfonamides were the first chemotherapeutic agents, which are effective against bacterial infections. Although penicillin and other antibiotics have diminished the status of sulfonamides [3], but still these drugs are used effectively against the infections of ophthalmic, urinary and gastrointestinal tract [4]. In humans, the sulpha drugs and their salt [5] are still of first choice along with ampicillin and gentamycin, against the infections caused by Escherichia coli. Sulpha drugs exhibit their action by competitive inhibition of the enzyme dihydropteroate synthetase towards the substrate p-aminobenzoate. Sulfonamides are antibacterial drugs, which are used both for human and animal chemotherapy to cure the infections of digestive, respiratory systems, skin and for prevention of coccidiosis of small domestic animals [6]. The dosage formation of sulpha drugs, its quality and release in the systemic circulations play vital analytical task.
Use of nanotechnology in the current scenario of research and technology is inevitable. Various applications of nanotechnology have been utilised to fabricate nanomaterials useful in the mechanical, electromagnetic and chemical fields. In the pharmaceutical field, particularly in designing dosage forms, nanotechnology is often used to improve the efficacy of various water soluble and insoluble drugs and bioactive molecules through improving solubility, retention time and bioavailability. Mostly these studies are focused on the synthesis and applications of metallic, organic and polymeric nanoparticles while small organic molecules are comparatively less explored [7]. Now a days, nanoaggregates of drugs have emerged as potential candidates for modulation of electronic and molecular properties through molecular design [8,9].
Self-assembly of drug molecules to nanoaggregates is feasible in the aqueous environment through the amphiphilic and hydrophobic interactions [10-12], which result into numerous changes at physicochemical and biochemical level. These aggregations/interactions are one of the deciding factors in determining the destiny of a pharmaceutical preparation in vivo [13]. A small change in physiological conditions might result in the coexistence of many drug aggregates, which could alter biorecognition and transport behaviour of drug candidates. Combinations of such type could become a sensation and might show prominent therapeutic effects. However, estimation of accurate drug efficacy in these cases is a tedious job. The interactions with inter and intra molecules/receptors and transport across cell membrane depended greatly on the size, structure, surface charge and hydrophobicity of the aggregates. Studies related to self-assembled drugs and their in vitro and in vivo therapeutic efficacy are lacking. Polymer-drug nanoconjugates can provide prominent insights into the self-aggregation phenomenon of drugs. This process can further alter/modify aggregation phenomenon and hence the therapeutic effect of drugs. Past years had seen the efforts regarding co-delivery of polymer-drug nanoconjugates to desired target sites [13-15]. Many polymer-drug nanoconjugates have been designed targeting site-selective drug release and many of these are under clinical trials and some have received approval [15-20]. Polymer and antimicrobial drug nanoconjugates show improved solubility and therapeutic efficacy, as well as reduced side effects and multi-drug resistance in comparisons to pure antimicrobial drug [21-23]. Polymer carrier also promotes assembly of drugs and plays vital role in modification of drug nanoaggregates.
Polyethylene glycol (PEG) synthesized by the polymerisation of monomer ethylene glycol and is easily available in a wide range of molecular weights. The main utilisations of PEG in pharmaceutical preparations are; orally as a laxative, excipient in dosage formulations, and coating agent in capsule preparation [24]. PEG is extremely biocompatible and not a biodegradable polymer, which is excreted unchanged by the kidney. PEG is a hydrophilic polymer and can be used to stabilize nanoparticles in aqueous media, increase solubility and avoid aggregation by steric hindrance. As PEG does not have any ionic moiety, risk is almost negligible when it came in contact with charged biological molecules like DNA [25]. Immune system does not recognise PEG easily, so its retention time and in vivo circulation increases and the chances of PEG conjugated drug nanoaggregates to reach the target site becomes high. This is known as the stealth effect and makes PEG a suitable nano-drug carrier [26].
Taking cognizance of reported studies of both antimicrobial drugs and nanoaggregates, it was decided to conjugate both sulfonamide nanoaggregates and PEG to enhance the potential applications in antimicrobial chemotherapy. Initially, sulphanilamide (SM) from the class of sulphonamide antibacterial drugs was selected for evaluating potential applications in sustained drug delivery [27,28]. This is the first attempt by our group to conjugate drug nanoaggregates with PEG to evaluate drug delivery applications against four bacterial strains.
Material and Methods
SM (>99 %), PEG-6000 (AR grade) were purchased from Sisco Research Laboratories Pvt. Ltd., India and are used without further purification. AR grade DMSO was purchased from S. D. Fine-Chem Ltd., Mumbai, India.
Preparation of SM organic nanoaggregates (SMONAs):
SM is a sulfonamide and exhibit antibacterial action against various Gram-positive and Gram-negative bacterial strains by mimicking the action of PABA. The SMONAs were synthesized by reprecipitation method [29]. In this method about 1 ml of SM solution in DMSO was injected in to 50 ml of double-distilled water keeping constant rate of addition and stirring. It was followed by sonication for about 20 min to produce SMONAs of desired size at ambient temperature. The concentration of injectable solution is the deciding factor for the size of nanoaggregates and here the concentration of SM was varied to get the desired size of SMONAs.
Preparation of PEG-coated SMONAs:
Nanoprecipitation is commonly applied to several polymeric systems. In the present case PEG was dissolved in DMSO. Under vigorous stirring this solution was poured into water already containing SMONAs, followed by sonication for 30 min. PEG acted as a stabilising agent for the SMONAs. The nucleation process of polymer immediately began when it came in contact with water and growth of nanoaggregates stopped when stability is reached. Excess organic solvent was then removed from the colloidal suspension by evaporation.
Entrapment capacity of SM with PEG:
Weighed amount of SM-loaded PEG was dissolved in methanol, sonicated for 5 min to break the PEG, diluted suitably and then analysed on a UV spectrophotometer at 260 nm [30]. The encapsulation capacity was determined by the following Eqn., entrapment capacity = actual weight of drug/theoretical weight of drug×100.
Determination of antibacterial activity:
In vitro antibacterial studies [31] of SM, SMONAs and PEG-coated SMONAs were carried out against Grampositive and Gram-negative strains by disk-diffusion assay. Minimum inhibitory concentration (MIC) values were determined using turbidimetry method [32].
Characterization:
Techniques such as UV/Vis spectroscopy, fluorescence spectroscopy, dynamic light scattering (DLS), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to characterise the SMONAs and PEG-coated SMONAs.
UV/Vis spectroscopy:
The UV-absorption studies of the SM, SMONAs and PEG-coated SMONAs were performed on a Shimadzu (UV-1800) spectrophotometer using 1 cm wide quartz cells. The range of 200-500 nm was examined and baseline correction was applied for each spectrum.
Fluorescence spectroscopy:
Steady-state fluorescence was measured using a PerkinElmer Luminescence spectrometer LS-55 using pyrene as an external fluorescent probe (2×10−6 M) at an excitation wavelength of 280 nm at 25°. Temperature was controlled using a built-in temperature controller within ±0.1° by using circulating water bath. The spectra were recorded between 290 and 450 nm using an excitation and emission slit width of 2.5 nm, each.
DLS:
DLS measurements were made using a light scattering apparatus (Nano-ZS, Malvern Instruments) equipped with a built-in temperature controller having an accuracy of ±0.1°. A quartz cuvette with a path length of 1 cm was used for measurement and average of 10 measurements was considered as experimental data. Data were analysed using the standard algorithms and is reported with an uncertainty of less than 8 %.
SEM:
SEM measurements were performed using a Zeiss Ultra 55-Limited edition SEM. Solutions of SMONAs and PEG-coated SMONAs were placed on a thoroughly cleaned glass surface and dried for 24 h in air before imaging. Silver coating was given to samples before measurement.
TEM:
TEM measurements were performed on a JEM-2100 electron microscope at a working voltage of 200 kV. A drop of freshly prepared SMONAs and PEG-coated SMONAs were placed on a carbon coated copper grid (300 mesh), and the residual solution was blotted off. The samples were dried in air at room temperature for 24 h before measurements.
Results and Discussion
The conversion of SM to SMONAs was examined by recording UV/Vis and fluorescence spectra. Formation of nanoaggregates in aqueous solution resulted in broadening of absorption bands accompanied with small red shifts in maxima positions and by decrease of extinction coefficients (hypochromism) as compared to the spectra of the SM in organic solvent at the same concentration (Figure 1a). The fluorescence spectrum of SM manifested the difference in the emission profile of SMONAs in aqueous system and organic solvent system (Figure 1b). The normalized intensity emission profile clearly showed that SMONAs displayed broad featureless emission centred at 350 nm, while SM dissolved in a suitable organic solvent showed fluorescence emission at the 275 nm wavelength. The emission profile SMONAs was recorded at different dilutions of SMONAs. With increase in concentration of SM for the formation of nanoaggregates, the fluorescence intensity also increased, with no concentration-dependent shift observed in the emission profile (Figure 2). This is due to the fact that, decrease in concentration, did not affect the regular arrangement of nanoaggregates, which were uniformly dispersed in the aqueous phase.
Figure 1: Absorption spectra and emission spectra of SM and SMONAs
(a) Absorption spectra of SM (▬) and SMONAs ( ) (b) emission spectra of SM and SMONAs (c) absorption spectra of SMONAs
(
) (b) emission spectra of SM and SMONAs (c) absorption spectra of SMONAs
( ) and PEG-coated SMONAs (
) and PEG-coated SMONAs ( ) (d) emission spectra of SMONAs and PEG-coated SMONAs. SM is sulphanilamide, SMONAs
is Sulphanilamide organic nanoaggregates
) (d) emission spectra of SMONAs and PEG-coated SMONAs. SM is sulphanilamide, SMONAs
is Sulphanilamide organic nanoaggregates
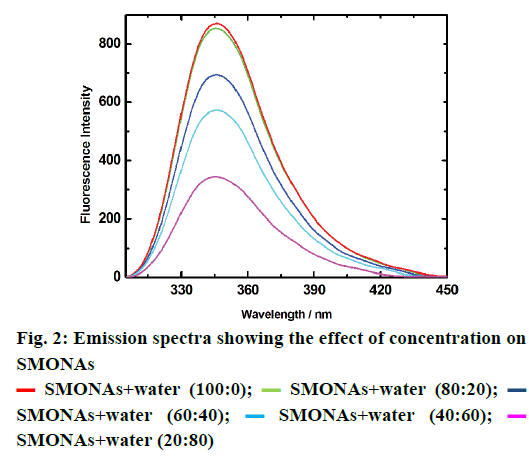
DLS studies were conducted to determine the size of the SMONAs, which was found to be around 100 nm at 25 μM concentration (Figure 3a). SEM and TEM were also conducted to determine the size of the SMONAs (Figure 3b and 3c). SEM and TEM images indicated that variable-sized SMONAs were formed with average size of 100 nm.
The particle size, polydispersity index and zeta potential of SMONAs and PEG-coated SMONAs were also calculated for more clarity (Table 1). pH of the body fluids that usually come in contact with a pharmaceutical formulation changes from acidic to alkaline, depending upon the function of the organ involved. It is necessary to have the knowledge of the effect of pH during the stability studies, transport or release of any pharmaceutical preparation. This part of study explores the stability of SMONAs at different pH, using emission spectroscopy in aqueous medium as shown in Figure 4.
| Particles | DLS diameter (nm) | PDI | Zeta potential(mV) |
|---|---|---|---|
| SMONAs | 100 | 0.1003 | -11.8 |
| PEG-SMONAs | 197 | 0.1211 | -6.76 |
Table 1: DLS, PDI and Zeta potential of SMONAs and peg-coated SMONAs
SMONAs showed an increase in emission maximum with increase in pH from 4 to 9, which indicated that greater disintegration of SMONAs occurred in alkaline media and hence would limit applications in drug delivery. This would provide rationale for the use of a capping/coating/stabilizing agent such as PEG-6000 on SMONAs.
Absorption and emission spectra confirmed the formation of PEG-coated SMONAs in an aqueous system (Figure 1). The decrease of extinction coefficients (hypochromic shift) was observed for PEG-coated SMONAs in comparisons to the spectra of the SM (Figure 1). The fluorescence spectra of PEG-coated SMONAs (Figure 1) showed the difference in the emission profile of PEG-coated SMONAs and the SMONAs.
Formation of the PEG-coated SMONAs were further confirmed using DLS, SEM and TEM studies (Figure 3). DLS analysis showed (Figure 3) the formation of the PEG-coated SMONAs resulted in an increase in the size of the nanoaggregates from 100 nm to 197 nm. Interestingly, SEM images were recorded at different optical zoom and showed fern leaf like morphology (Figure 3). TEM studies showed similar morphology with average size of 200 nm (Figure 3). Further drug PEG entrapment capacity was also calculated [30], which was observed to be 82.1±2 % suggesting that SM was effectively capped by PEG.
PEG-coated SMONAs did not show any prominent change in emission profile with change in pH (Figure 4), which in turn indicated that there was no disintegration of PEG-coated SMONAs with change in pH, as observed in the case of SMONA (Figure 4).
Keeping in view the lack of effect of pH on the disintegration of the PEG-coated SMONAs it was decided to extend studies further to determine the effect of biological ions, which might also be responsible for its disintegration. The interactions between SM and PEG-coated SMONAs with ions present in biofluids would help to understand their fate (stability and transport across biological barriers) and influence in the release of SM at the site of action.
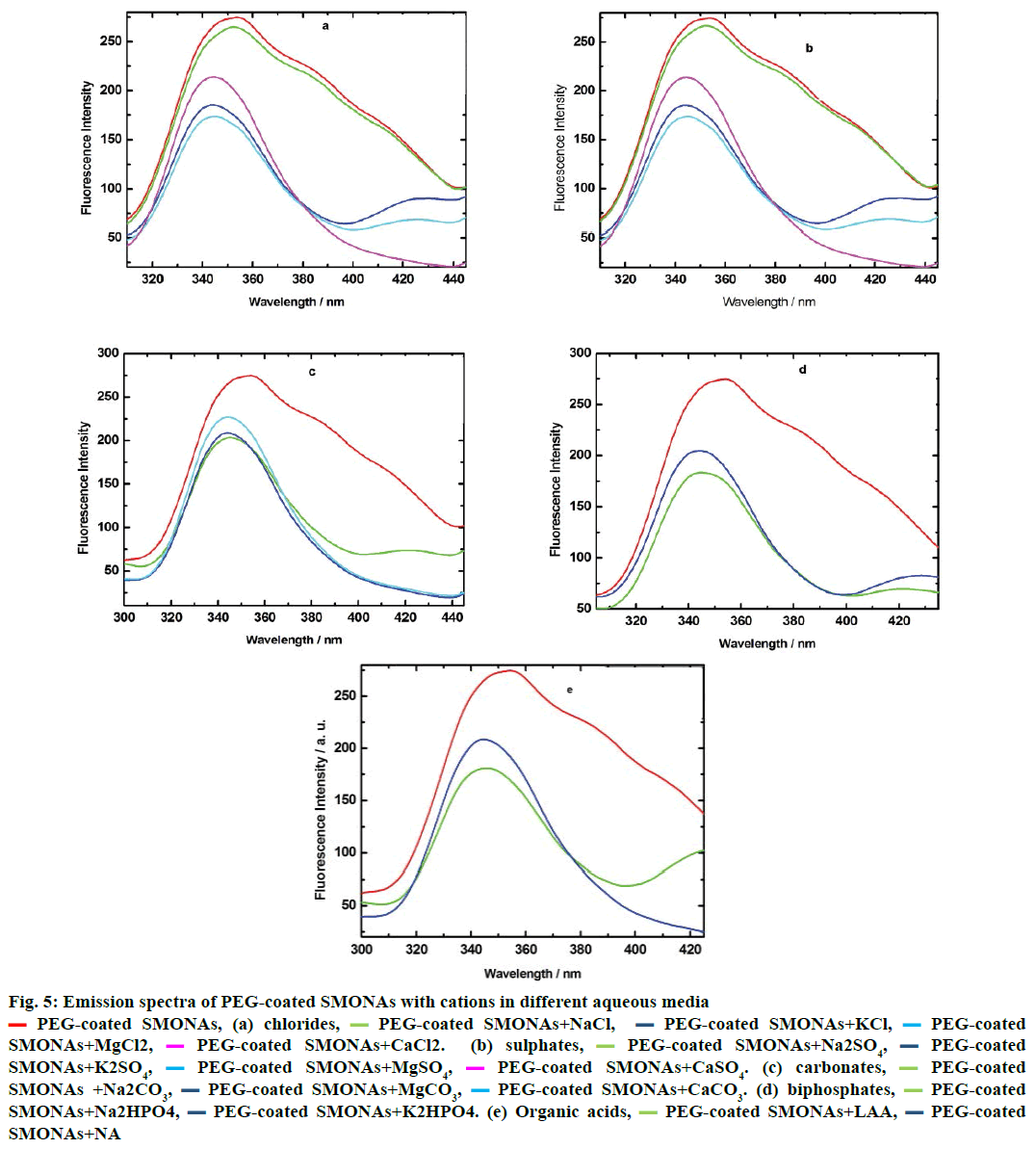
Hence, the interactions of PEG-coated SMONAs with major biological ions and few organic acids present in biofluid, investigations were performed using emisson spectroscopy at definite intervals of time. The results of the investigations revealed that different ions interacted differently in aqueous systems (Figure 5). The Mg+2 ions showed maximum interaction in aqueous solutions of chlorides and sulphates (Figure 5a and 5b) while Na+ ions exhibited maximum interactions in aqueous solutions of carbonates and biphosphates (Figure 5c and 5d) with PEG-coated SMONAs. Figure 5e suggested that L-ascorbic acid showed greater interactions with PEG-coated SMONAs than nicotinic acid in aqueous system.
Figure 5: Emission spectra of PEG-coated SMONAs with cations in different aqueous media
 PEG-coated SMONAs, (a) chlorides,
PEG-coated SMONAs, (a) chlorides,  PEG-coated SMONAs+NaCl,
PEG-coated SMONAs+NaCl,  PEG-coated SMONAs+KCl,
PEG-coated SMONAs+KCl,  PEG-coated
SMONAs+MgCl2,
PEG-coated
SMONAs+MgCl2,  PEG-coated SMONAs+CaCl2. (b) sulphates,
PEG-coated SMONAs+CaCl2. (b) sulphates,  PEG-coated SMONAs+Na2SO4,
PEG-coated SMONAs+Na2SO4, PEG-coated
SMONAs+K2SO4,
PEG-coated
SMONAs+K2SO4,  PEG-coated SMONAs+MgSO4,
PEG-coated SMONAs+MgSO4, 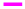 PEG-coated SMONAs+CaSO4. (c) carbonates,
PEG-coated SMONAs+CaSO4. (c) carbonates,  PEG-coated
SMONAs +Na2CO3,
PEG-coated
SMONAs +Na2CO3,  PEG-coated SMONAs+MgCO3,
PEG-coated SMONAs+MgCO3,  PEG-coated SMONAs+CaCO3. (d) biphosphates,
PEG-coated SMONAs+CaCO3. (d) biphosphates,  PEG-coated
SMONAs+Na2HPO4,
PEG-coated
SMONAs+Na2HPO4,  PEG-coated SMONAs+K2HPO4. (e) Organic acids,
PEG-coated SMONAs+K2HPO4. (e) Organic acids,  PEG-coated SMONAs+LAA,
PEG-coated SMONAs+LAA, PEG-coated
SMONAs+NA
PEG-coated
SMONAs+NA
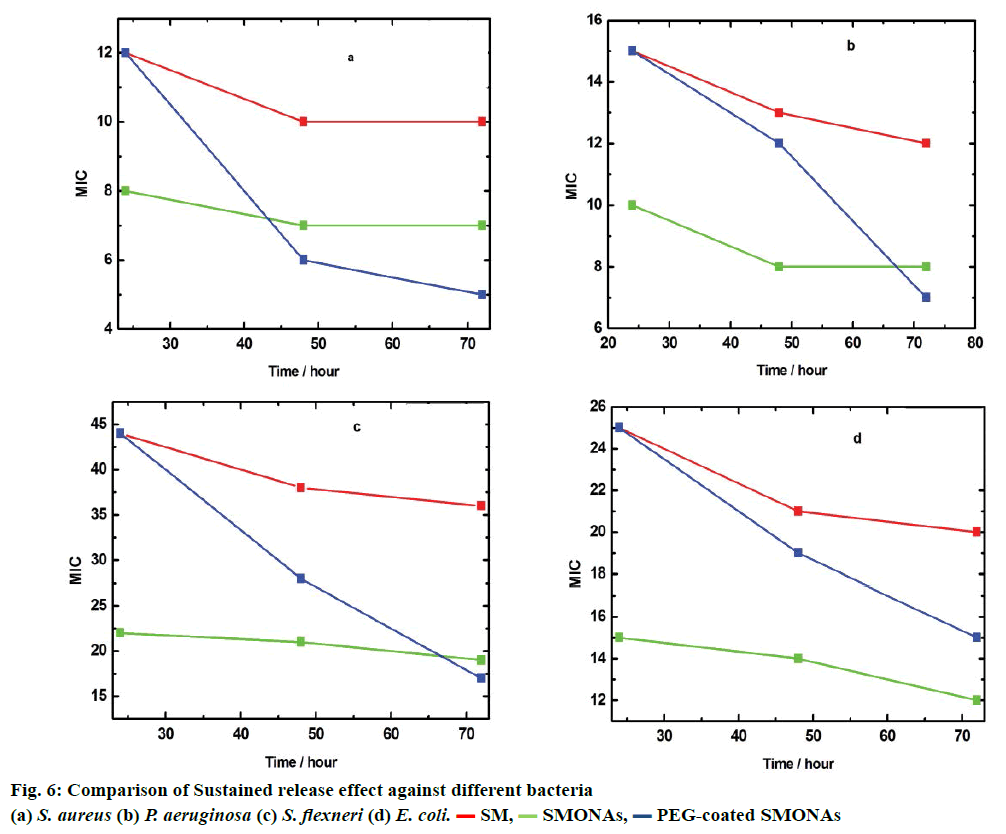
To further study the therapeutic effect of SM, SMONAs and PEG-coated SMONAs, these were screened on four different strains of Gram-positive and Gramnegative bacteria to understand antibacterial effects if any. The Gram-positive strain used was Staphylococcus aureus (MTCC-740, facultative anaerobic). The Gramnegative strains used were Escherichia coli (MTCC- 119, facultative anaerobic), Shigella flexneri (MTCC- 1457, facultative anaerobic) and Pseudomonas aeruginosa (MTCC-741, aerobic). Initially, MIC was monitored after 24 h of the treatment (Figure 6). In the case of aerobic Gram-positive bacteria S. aureus, MIC was 8 μg/ml for SMONAs followed by SM and PEGcoated SMONAs (MIC 12 μg/ml), which suggested that less concentration of SMONAs was required to inhibit the bacterial growth as compared to SM and PEG-coated SMONAs. For P. aeruginosa, MIC was 10 μg/ml for SMONAs followed by SM and PEG-coated SMONAs (MIC 15 μg/ml). In the case of S. flexneri, for SMONAs the MIC was 22 μg/ml followed by SM and PEG-coated SMONAs (MIC 44 μg/ml). Against E. coli, SMONAS showed an MIC at 15 μg/ml followed by SM and PEG-coated SMONAs (MIC 25 μg/ml, Table 2). From this discussion it could be concluded that the SMONAs exhibited more potent antibacterial effects as compared to parent SM or PEG-coated SMONAs. S. aureus was found to be most susceptible to the antibacterial effect of SMONAs. Further, to investigate the potential applications in drug delivery, MIC against different bacteria was determined at three definite intervals of time. It was observed that PEGcoated SMONAs showed promising antibacterial activities when observed after 72 h of treatment (Figure 6).
| Compounds | Treatment (h) | MIC (µg/ml) | |||
|---|---|---|---|---|---|
| S. aureus MTCC-740 |
P. aeruginosa MTCC-741 |
S. flexneri MTCC-1457 |
E. Coli MTCC-119 |
||
| SM | 24 | 12 | 15 | 44 | 25 |
| SMONAs | 24 | 08 | 10 | 22 | 15 |
| PEG-SMONAs | 24 | 12 | 15 | 44 | 25 |
Table 2: In vitro antibacterial activities of compounds
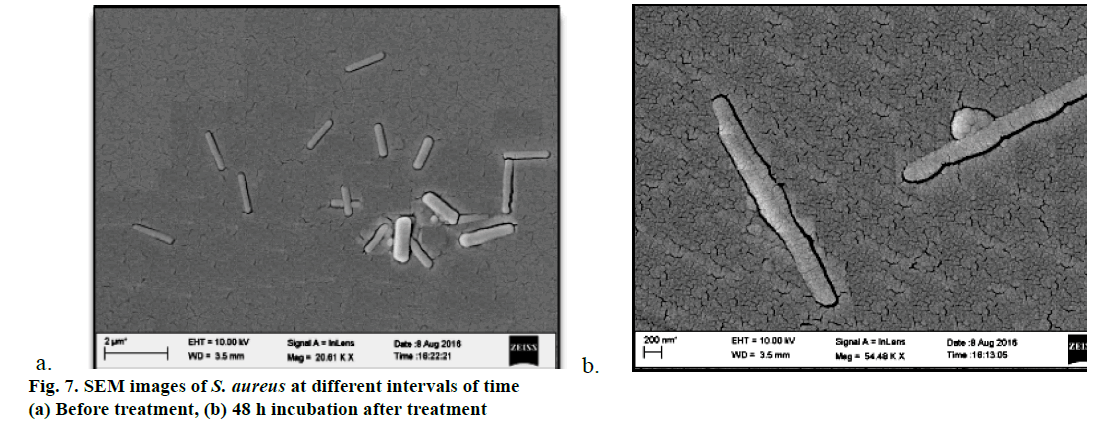
Furthermore, SEM images of S. aureus were also recorded before and after 48 h of the treatment (Figure 7a and 7b). Results of investigations revealed that before the treatment, bacterial size was found normal with no abnormalities in shape. However, after 48 h of exposure to the treatments bacterial size and shape changed with some minor bleb formation on the surface of the bacteria which might be due to the disorganisation of the bacterial cytoplasm, which could lead to death of the bacteria.
In conclusion, interactions of SM and SMONAs with PEG-6000 were investigated. DLS, SEM and TEM techniques confirmed the formation of about 100 and 197 nm sized SMONAs of SM without and with PEG, respectively. In aqueous solutions of varying pH, SMONAs showed maximum interactions at pH 9, which could explain highest dissociation under basic pH. Therefore, to make these suitable for drug delivery, SMONAs were processed to PEG-coated SMONAs and were characterized using DLS, SEM and TEM. Effect of pH and electrolytes were also studied to determine various factors responsible for sustained release. Varying pH has no effect on the sustained release of PEG-coated SMONAs. Mg+2 showed maximum interactions with PEG-conjugated SMONAs in aqueous solutions of chlorides and sulphates while Na+ showed maximum interactions in aqueous solutions of carbonates and bicarbonates. L-ascorbic acid showed more interactions than nicotinic acid with PEG-conjugated SMONAs in aqueous solutions. Antibacterial activity of SM, SMONAs and PEGcoated SMONAs were carried out against E. coli, S. flexneri, P. aeruginosa and S. aureus. MIC profile indicated that the PEG-coated SMONAs showed higher antibacterial activity as compared to SM parent drug and SMONAs. Interestingly, highest activity was observed in the case of S. aureus (MIC 5). SEM studies were also conducted on the bacteria before and after the treatment which indicated that bacterial cell death was due to the accumulation of the cytoplasm leading to bleb formation. PEG-coated SMONAs showed highest antibacterial activity than that of SM through sustained release. PEG-coated SMONAs could find potential application in the development of sustained release antibacterial preparations.
Acknowledgement
Dr. Harpreet Singh is grateful to UGC for providing Dr. D. S. Kothari Postdoctoral Fellowship (Award letter No.: F.4-2/2006(BSR)/CH/13-14/0116).
Conflicts of interest
There are no conflicts of interest.
References
- Zhang CL, Li BA, Wang Y. Solubilities of sulfadiazine in methanol, ethanol, 1-propanol, 2-propanol, acetone, and chloroform from (294.15 to 318.15) K. J Chem Eng Data 2010;55(6):2338-39.
- Prabhu AAM, Venkatesh G, Rajendiran N. Spectral characteristics of sulfa drugs: effect of solvents, pH and β-cyclodextrin. J Solution Chem 2010;39:1061-86.
- Martinez F, Gomez A. Thermodynamic study of the solubility of some sulfonamides in octanol, water, and the mutually saturated solvents. J Solution Chem2001;30:909-23.
- Delgado JN, Remers WA. Wilson and Gisvold’s Text Book of Organic Medicinal and Pharmaceutical Chemistry. 9th ed. Hagerstown, Maryland: Lippincott Williams & Wilkins; 1991.
- Delgado DR, Vargas EF, Martínez F. Apparent molar volumes of some sodium sulfonamides in water at several molalities and temperatures. J Solution Chem 2011;40:1955-63.
- Josef M. Pharmacotherapy of Internal Diseases. Prague: Grada Publishing; 1998.
- Mohammed SJ, Salih AK, Omer KM, Rashid MA. Preparation and Characterization of Organic Nanoparticles of Oxadiazole Derivative in Aqueous Media. J Nat Sci Res 2014;4:81-5.
- Nguyen TQ, Martel R, Avouris P, Bushey ML, Brus L, Nuckolls C. Molecular interactions in one-dimensional organic nanostructures. J Am Chem Soc 2004;126:5234-42.
- Fu H, Xiao D, Yao J, Yang G. Nanofibers of 1,3-diphenyl-2-pyrazoline induced by cetyltrimethylammonium bromide micelles. Angew Chem Int Ed 2003;42:2883-86.
- Fulop Z, Gref R, Loftsson T. A permeation method for detection of self-aggregation of doxorubicin in aqueous environment. Int J Pharm 2013;454:559-61.
- Nabiev I, Fleury F, Kudelina I, Pommier Y, Charton F, Riou JF, et al. Spectroscopic and biochemical characterisation of self-aggregates formed by antitumor drugs of the camptothecin family: their possible role in the unique mode of drug action. Biochem Pharmacol 1998;55:1163-74.
- Balasubramanian SV, Alderfer JL, Straubinger RM. Solvent- and concentration-dependent molecular interactions of taxol (Paclitaxel). J Pharm Sci 1994;83:1470-6.
- Sosnik A. Drug self-assembly: A phenomenon at the nanometer scale with major impact in the structure–biological properties relationship and the treatment of disease. Progr Mater Sci 2016;82:39-82.
- Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer 2006;6:668-701.
- Larson N, Ghandehari H. Polymeric conjugates for drug delivery. Chem Mater 2012;24:840-53.
- Singh J, Desai S, Yadav S, Narasimhan B, Kaur H. Polymer drug conjugates: recent advancements in various diseases. Curr Pharm Des 2016;22:2821-43.
- Pang X, Jiang Y, Xiao Q, Leung AW, Hua H, Xu C. pH-responsive polymer-drug conjugates: Design and progress. J Control Release 2016;222:116-29.
- Beretta GL, Cavalieri F. Engineering nanomedicines to overcome multidrug resistance in cancer therapy. Curr Med Chem 2016;23:3-22.
- Cavalieri F, Beretta GL, Cui J, Braunger JA, Yan Y, Richardson JJ, et al. Redox-sensitive peg-polypeptide nanoporous particles for survivin silencing in prostate cancer cells. Biomacromolecules 2015;16:2168-78.
- Markovsky E, Baabur-Cohen H, Eldar-Boock A, Omer L, Tiram G, Ferber S, et al. Administration, distribution, metabolism and elimination of polymer therapeutics.J Control Release 2012;161:446-60.
- Koziolova E, Janouškova O, Cuchalova L, Hvězdova Z, Hraběta J, Eckschlager T, et al. Overcoming multidrug resistance in Dox-resistant neuroblastoma cell lines via treatment with HPMA copolymer conjugates containing anthracyclines and P-gp inhibitors. J Control Release 2016;233:136-46.
- Camacho KM, Menegatti S, Vogus DR, Pusuluri A, Fuchs Z, Jarvis M, et al. Dafodil: A novel liposome-encapsulated synergistic combination of doxorubicin and 5FU for low dose chemotherapy. J Control Release 2016;229:154-62.
- Camacho KM, Menegatti S, Mitragotri S. Low-molecular-weight polymer-drug conjugates for synergistic anticancer activity of camptothecin and doxorubicin combinations. Nanomedicine 2016;11:1139-51.
- Cleveland MV, Flavin DP, Ruben RA, Epstein RM, Clark GE. New polyethylene glycol laxative for treatment of constipation in adults: a randomized, double-blind, placebo-controlled study. South Med J 2001;94:478-81.
- Knop K, Hoogenboom R, Fischer D. Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl 2010;49:6288-308.
- Locatelli E, Franchini MC. Biodegradable PLGA-b-PEG polymeric nanoparticles: synthesis, properties, and nanomedical applications as drug delivery system. J Nanopart Res 2012;14:1316-25.
- Kang L, Chen Y, Xiao D, Peng A, Shen F, Kuang X, et al. Organic core/diffuse-shell nanorods: fabrication, characterization and energy transfer. Chem Commun 2007;39:2695-97.
- McDonald TO, Martin P, Patterson JP, Smith D, Giardiello M, Marcello M, et al. Multicomponent organic nanoparticles for fluorescence studies in biological systems. Adv Funct Mater 2012;22:2469-78.
- Al-Kaysi RO, Müller AM, Ahn TS, Lee S, Bardeen CJ. Effects of sonication on the size and crystallinity of stable zwitterionic organic nanoparticles formed by reprecipitation in water. Langmuir 2005;21:7990-94.
- Wu F, Xu T, Liu, Chen C, Song X, Zheng Y. et al. Glycyrrhetinic Acid-Poly(ethylene glycol)-glycyrrhetinic Acid Tri-Block Conjugates Based Self-Assembled Micelles for Hepatic Targeted Delivery of Poorly Water Soluble Drug. Sci World J 2013;2013:913654.
- Fromtling RA, Galgiani JN, Pfaller MA, Espinel IA, Bartizal KF, Bartlett MS, et al. Multicenter evaluation of a broth macrodilution antifungal susceptibility test for yeasts. Antimicrob Agents Chemother 1993;37:39-45.
- Mahon CR, Manuselis G. Textbook of Diagnostic Microbiology, Special Antimicrobial Susceptibility Tests. Philadelphia: W.B. Saunders Company; 1995.
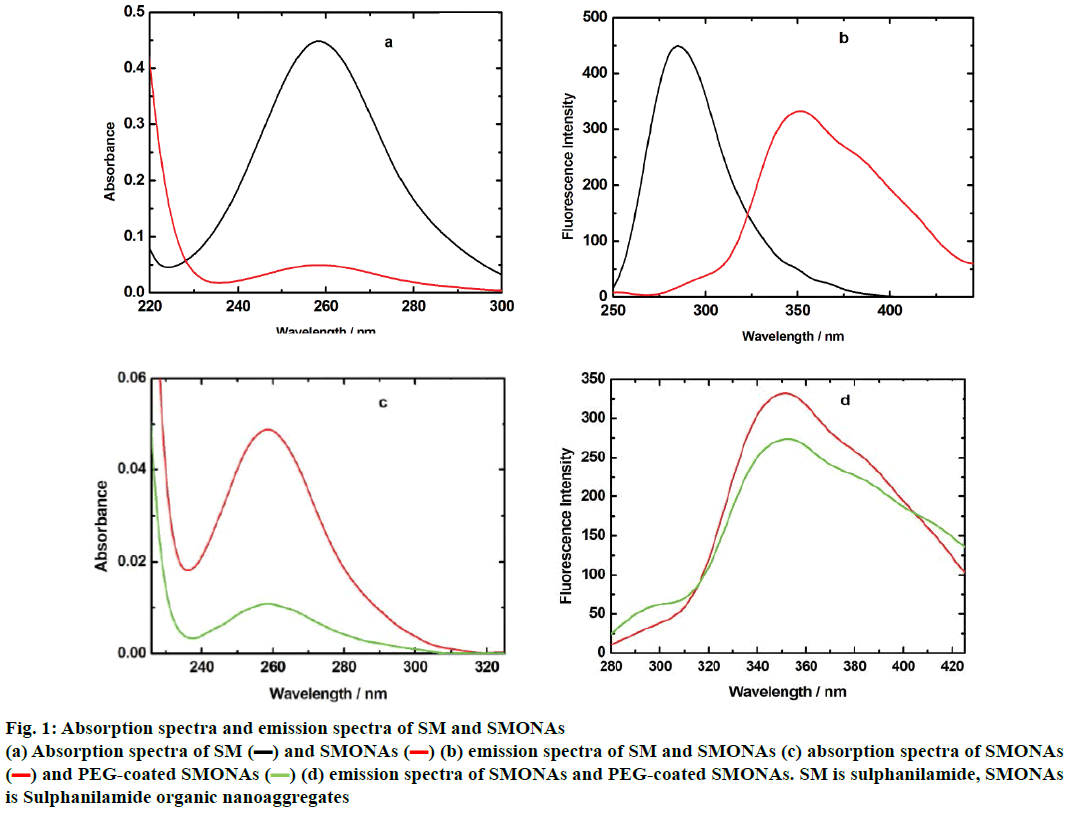
 SMONAs+water (100:0);
SMONAs+water (100:0);  SMONAs+water (80:20);
SMONAs+water (80:20);  SMONAs+water (60:40);
SMONAs+water (60:40);  SMONAs+water (40:60);
SMONAs+water (40:60);  SMONAs+water (20:80)
SMONAs+water (20:80)
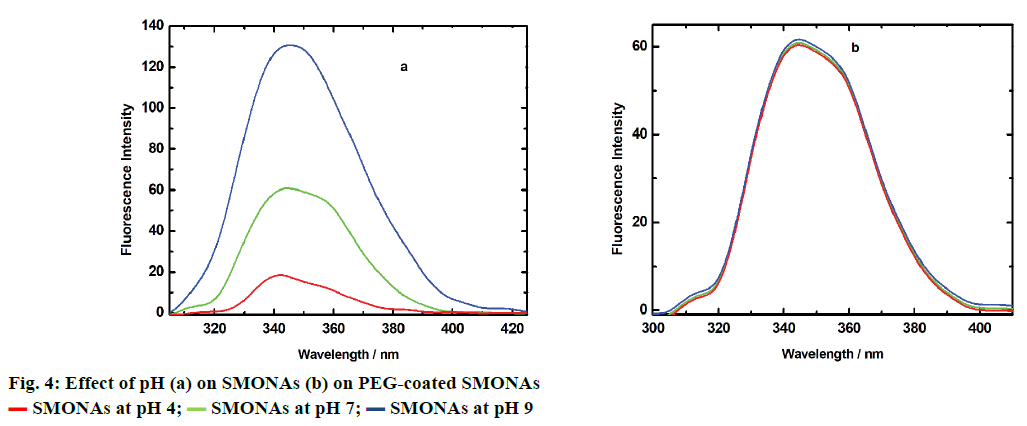
 SMONAs at pH 4;
SMONAs at pH 4;  SMONAs at pH 7;
SMONAs at pH 7;  SMONAs at pH 9
SMONAs at pH 9