- *Corresponding Author:
- M. Sreenivasa Reddy
Department of Pharmaceutics, Manipal College of Pharmaceutical Sciences, MAHE, Manipal-576104, India.
E-mail: ms.reddy@manipal.edu
| Date of Submission | 16 May 2005 |
| Date of Revision | 22 September 2005 |
| Date of Acceptance | 08 July 2006 |
| Indian J Pharm Sci 2006, 68 (4): 432-436 |
Abstract
In the present study four minoxidil gels were prepared using carbopol, hydroxypropyl cellulose, hydroxypropyl methylcellulose and combination of hydroxypropyl cellulose, hydroxypropyl methylcellulose for the treatment of alopecia. The gels were evaluated for drug content, viscosity determination, in vitro permeation (across dialysis membrane and mouse skin), skin irritation and stability at 4, 25 and 37° tests. The drug content of the gels was found to range from 96.40±0.57 to 98.10±0.32%. The viscosity of the gels ranged between 13,780±100 and 24,950±150 cps. The drug permeation across dialysis membrane from all the formulations at the end of 24 h was almost same and ranged between 92.05±1.52 and 93.52±1.95%. Although the difference is insignificant, the percentage release of drug was found to increase in the following order of the polymer composition: HPC>Carbopol>HPMC>HPMC+HPC. All the gel formulations released almost similar amounts of drug (90.05±1.92 to 91.56±1.65%) across the mouse skin; but the cumulative amount of drug permeated across dialysis membrane was more than that of the mouse skin. The marketed topical solution was found to diffuse almost 100% of drug across dialysis membrane and mouse skin at the end of 12 h. As supported by Higuchi's equation, the drug release mechanism from all the gels was found to be diffusion dominated. The prepared gels did not produce any dermatological reactions and were well tolerated by the mice. The gels were found to be stable with respect to viscosity, drug content and physical appearance at all temperature conditions for 3 months.
Hair loss (alopecia) is seen as a normal variant of aging by most individuals; but can have both psychological and pathological consequences and these effects are taken seriously by both the patients and physicians [1]. Alopecia affects approximately 50% of men over 40 years of age and may also affect just as many women [2]. The majority of men and women (90% or more) want to reverse or halt their hair loss, feel frustrated or helpless about the condition and are self-conscious about their hair loss [1,2].
There are many drugs available to increase the hair growth on head. Minoxidil topical solution (Rogaine®2%; Pharmacia and Upjohn, USA) became the first clinically proved, safe and effective hair-growth stimulant. The 2% w/v minoxidil topical solution became available in 1986 for men and in 1991 for women. A higher and more effective concentration (5% w/v) is now available in over 20 countries including India [3]. Besides treating the alopecia, minoxidil also has the ability to stabilize hair loss or simply, maintenance of an existing head of hair. A topical formulation of minoxidil prepared with propylene glycol:water:ethanol (15:15:70) was suggested as optimal when commercial tablets are used as the source of minoxidil [4]. Minoxidil topical solution has also been used in combination with topical retinoids [5]. However, minoxidil was developed originally, as an antihypertensive drug. Hair growth was a side effect. Several other drugs like diazoxide, viprostol, tretinoin, cyclosporine-A, finasteride (a specific inhibitors of steroid type II 5∝ reductase) and RU58841 (Roussel Uclaf; a nonsteroidal topical antiandrogen) have been tested for alopecia [6]. None of these products has proved more effective than minoxidil topical solution and in some cases the risks of the treatment outweighed any possible benefits.
Presently in India, minoxidil is marketed as topical solution in aqueous vehicle for the treatment of alopecia, which offers limited contact time with the scalp. This factor has remarkable influence on the total concentration of drug available for local vasodilatation, hence the need for a suitable topical delivery system which would increase the contact time, leading to an increase in the local drug concentration. The topical gel formulations overcome the above disadvantage since the thermodynamic activity of the gel formulations may not change as quickly as the solution form. Hence in the present study, an attempt has been made to prepare and evaluate the topical gel formulations of minoxidil.
Materials and Methods
Minoxidil (Dr. Reddy’s Laboratories, Hyderabad), Hydroxypropyl methylcellulose (15 cps) (Emco Industries, Mumbai), Hydroxypropyl cellulose (Genuine Chemicals, Mumbai), carbopol 934 (BF Good Rich, OH, USA) and propylene glycol (S.D. Fine Chemicals, Mumbai) were used. The dialysis membrane was procured from Sigma Inc, MO, USA. Other ingredients used were of analytical grade.
Preparation of gels
The composition of different gel formulations is shown in Table 1. For formulation I, minoxidil was dissolved in a solvent mixture (ethanol:propylene glycol:water in the ratio of 50:30:20). The pH of above mixture was adjusted to 7.4 with triethanolanime. The solution was finally gelled by adding 0.5% carbopol 934 carefully with constant stirring (Remi Mechanical Stirrer, Mumbai, India) at 9001000 rpm for 15 min. After stirring, the beaker containing the gel was allowed to stand in a water bath (25°) for 30 min. For other formulations, the drug was dissolved in a solvent mixture (ethanol:propylene glycol:water in the ratio of 50:30:20). The solution was then gelled by adding 6% HPMC (Formulation II), 8% HPC (Formulation III) or 4% HPMC+4% HPC (Formulation IV) and set aside for 24 h.
| Ingredients | Gel I | Gel II | Gel III | Gel IV |
|---|---|---|---|---|
| Carbopol 934 (mg) | 50 | - | - | |
| Propylene glycol (ml) | 5 | 5 | 5 | 5 |
| Ethanol (ml) | 3 | 3 | 3 | 3 |
| Water (ml) | 2 | 2 | 2 | 2 |
| Trietahnolamine (ml) | q.s. | - | - | - |
| Minoxidil (mg) | 200 | 200 | 200 | 200 |
| HPMC (mg) | - | 600 | - | 400 |
| HPC (mg) | - | - | 800 | 400 |
Table 1: Different Gel Formulations
Drug content
Drug content was determined using a UV-Spectrophotometric method by measuring the absorbance at 288 nm [13]. The linearity curve was obtained in the concentration range of 2-20 μg/ml with a correlation coefficient of 0.9990. The accuracy of the method was carried out by recovery studies, by spiking 70-130% of assay concentration. The inter day and intra day precision was less than 1%. Minoxidil gel (500 mg) was dissolved in 50 ml of phosphate buffer (pH 7.4). The absorbance was measured after suitable dilution at 288 nm against the corresponding blank solution. The blank solution was prepared by using blank gels (containing no drug). The viscosity of the gels was measured by Brookefield Synchrolectric viscometer. The TD bar spindle of LV series was employed for the measurement of viscosity.
In vitro permeation studies through dialysis membrane [7]
The in vitro diffusion study of the gels was performed using dialysis membrane (Sigma Inc, MO, USA; Cat. No.: 250-7U; dry, unwashed, pre-cut and open ended; flat width: 35 mm; inflated diameter: 21 mm; Length: 30 mm). The membrane soaked in phosphate buffer, pH 7.4 (PB) for 6-8 h was clamped carefully to one end of the hollow glass tube of dialysis cell (2.3 cm diameter; 4.16 cm2 area). One hundred milliliters of phosphate buffer was taken in a beaker, which was used as receptor compartment. Then 500 mg of gel containing 10 mg of the minoxidil was spread uniformly on the membrane. The donor compartment was kept in contact with the receptor compartment and the temperature was maintained at 37±0.5°. The solutions on the receptor side were stirred by externally driven Teflon-coated magnetic bars. At predetermined time intervals, pipetted out 5 ml of solution from the receptor compartment and immediately replaced with the fresh 5 ml phosphate buffer. The drug concentration of the receptor fluid was determined spectrophotometrically (Shimadzu, Tokyo, Japan) against appropriate blank. The experiment was carried out in triplicate.
In vitro permeation studies through mouse skin [8,9]
Fresh, full thickness and hairless skin obtained from 6-8 weeks old mouse was used as permeation barrier. The hair of the mice was removed 3 days before from the date of commencement of the experiment using electrical hair clipper. The animals were housed individually for at least 7 days before an experiment to allow scratches, bites and other small skin imperfections to heal. After sacrificing the mice by cervical dislocation, abdominal and dorsal skin sections were excised with surgical scissors. Adhering subcutaneous fat on the dermal side was carefully removed from the underside of the skin. The skin section thus prepared was clamped carefully to one end of the hollow glass tube (dialysis cell) so that the stratum corneum was facing up on the receiver compartment. One hundred milliliters of PB was taken in a beaker, which was used as receptor compartment. The donor compartment was immersed into the receptor compartment so that the edge just touches the receptor fluid. For first 30 min skin washing was performed. Then the receptor fluid was replaced with fresh phosphate buffer. The known quantity (500 mg gel containing 10 mg of the minoxidil) was spread uniformly on the membrane and the experiment was continued as explained in the previous experiment.
Skin irritation test
The mice, whose hair was removed 3 days before the experiment, were divided into 6 groups (n=6) and treated as follows.
Group I – Normal; Group II – Carbopol gel (Formulation I); Group III –HPMC gel (Formulation II); Group IV – HPC gel (Formulation III); Group V – HPMC+HPC gel (Formulation IV); Group VI (marketed preparation) and Group VII – Formalin (0.8%).
The animals were treated daily upto 7 days and finally the treated skin was examined visually for erythema and edema [10]. The study protocol was approved by Institutional Animal Ethical Committee, KMC, Manipal.
Stability studies
The stability studies were carried out for all the gel formulations at different temperature conditions (4°, 25° and 37°) for 3 months. Known amounts of gels were taken out at different time intervals like 0.5, 1, 2 and 3 months and analysed for drug content, physical appearance and viscosity.
Statistical analysis
The data was analysed by Student’s t-test using Graph Pad Instat Software and P<0.05 was considered to be significant.
Results and Discussion
Gel formulations were prepared with an intention of increasing the contact time of the drug with the scalp region so that minoxidil is released in a prolonged manner for a extended period of time. Carbopol, hydroxypropylmethylcellulose, hydroxypropyl cellulose and a combination of hydroxypropylmethylcellulose and hydroxypropyl cellulose were used as gelling agents. The solvent system consisting of propylene glycol:water:ethanol has been proved to be a good system to deliver minoxidil topically [4].
The results of viscosity and drug content are shown in Table 2. The drug content of the gel preparations was found to be uniform among various batches prepared and was found to range from 96.40±0.57 to 98.10± 0.32%. The drug content determination also showed that the drug was uniformly distributed through out the gel. The viscosity of the gels was found between 13,780±100 and 24,950±150 cps.
| Formulation | Drug content (%) | Viscosity (cps) |
|---|---|---|
| Gel I | 97.90 ± 0.45 | 24,950 ± 150 |
| Gel II | 98.10 ± 0.32 | 14,790 ± 125 |
| Gel III | 97.40 ± 0.71 | 13,780 ± 100 |
| Gel IV | 96.40 ± 0.57 | 15,870 ± 150 |
| Marketed | 99.01 ± 0.28 | - |
Table 2: Results Of Drug Content And Viscosity Determinations
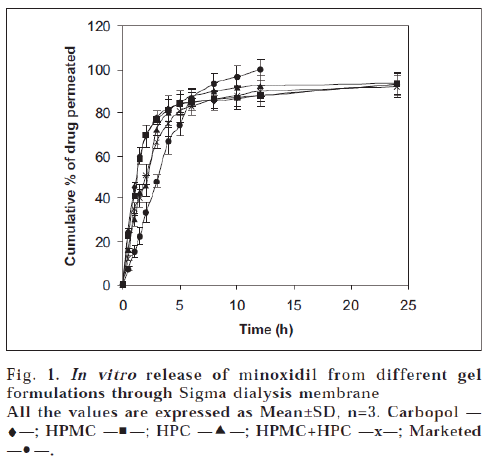
The results of in vitro permeation studies of gel formulations across dialysis membrane are depicted in fig. 1. The drug release from all the formulations at the end of 24 h was almost same and was ranged between 92.05±1.52 and 93.52±1.95%. It can be observed that carbopol gel and HPMC gel released minoxidil at a faster rate than HPC gel and HPMC+HPC gels in the initial stages of the study. Although the difference is insignificant, the percentage release of drug increased in the following order of the polymer composition: HPC>Carbopol> HPMC>HPMC+HPC. The marketed formulation showed a highest drug permeation compared to prepared gel formulations. The variations in the drug release profiles from various gels can be explained based on viscosity and swellability of the polymers. The viscosity values of the gels were increased in the following manner: Carbopol>HPMC+HPC>HPMC>HPC which do not correlate with the release profiles. Hence variations in drug release profiles could not be explained only based on viscosity. It could be explained based on the swellability of the gelling agents, viz. carbopol and HPMC possess more swellability when compared to HPC [11]. So HPC alone might have shown more drug release when compared to HPMC and carbopol.
The in vitro release data obtained was treated with Higuchi’s equation (Q=kt1/2) to understand the mechanism of drug release from the formulations. The data was plotted as cumulative percent released vs. square root of time. A close observation of the linear regression results reveals that minoxidil was released by diffusion for 6 h of the study (R2=0.8709 to 0.9643). This may be due to the formation of a thin film upon application of gel as the alcohol and water component evaporates. The thermodynamic activity of minoxidil release is likely to be modified by the non-volatile components of the formulations and minoxidil might be released by diffusion through this film [12]. At a later stage, film looses its integrity leading to a complex release pattern of the remaining drug.
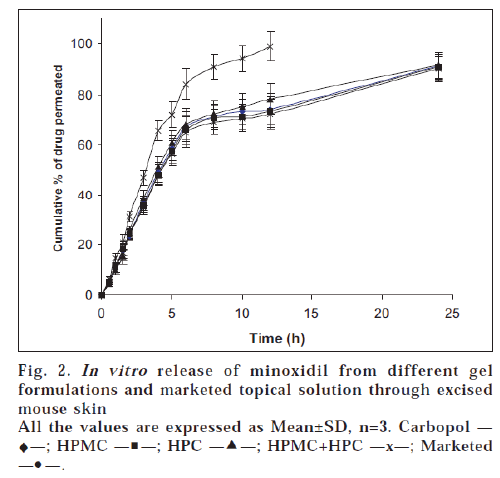
The results of in vitro permeation studies of gel formulations and marketed Minoxidil Topical Solution across mouse skin are shown in fig. 2. The release pattern of drug from marketed Topical solution was found to be curvilinear. This could be due to the formation of saturated solution due to evaporation of alcohol and water which leads to increased mass transfer to the receptor compartment. As reported earlier, propylene glycol in addition to being absorbed, also evaporates, resulting in supersaturated solution followed by precipitation of minoxidil leading to abrupt absorption pattern [8]. Drug is absorbed from the site of application as long as it remains in solution form. With an intention to keep the minoxidil in solution form, thus prolonging time of absorption, gel formulations were prepared. The comparative curvature was not seen for gel formulations.
All the gel formulations released almost similar amounts of drug (90.05±1.92 to 91.56±1.65%) when compared to those obtained by dialysis membrane. However the cumulative amount of drug release across dialysis membrane were comparatively more. In this study also marketed preparation showed a maximum drug permeation compared to prepared gels.
To know the mechanism of drug release, the data was plotted according to Higuchi’s equation as explained in previous section. Unlike permeation studies through Sigma dialysis membrane, Higuchi’s plots were fairly linear throughout the period of study (R2=0.9627 to 0.9899). In addition to the diffusion barrier offered by the film formed by the evaporation of alcohol and water from the gel formulations, the complex structure of excised skin might also contribute to the release, acting as rate controlling membrane for diffusion of drug to the receptor solution.
The results of skin irritation test of the gels in comparison with formalin solution, a standard irritant, are shown in Table 3. The gels produced negligible erythema and edema that were significantly less compared to formalin, which intern produced severe erythema and edema. The results indicated that the prepared gels do not produce any dermatological reaction and are well tolerated by the mice.
| Formulation | Visual observation | |
|---|---|---|
| Erythema | Edema | |
| Normal | 0.00 ± 0.0 | 0.00 ± 0.0 |
| Gel I | 0.75 ± 0.19* | 0.85 ± 0.15* |
| Gel II | 1.02 ± 0.16* | 0.92 ± 0.15* |
| Gel III | 0.99 ± 0.10* | 0.96 ± 0.11* |
| Gel IV | 0.85 ± 0.15* | 0.98 ± 0.15* |
| Marketed | 0.81 ± 0.10* | 0.88 ± 0.15* |
| Formalin (0.8% v/v) | 3.75 ± 0.31 | 3.65 ± 0.28 |
Table 3: Results Of Skin Irritation Test
The results of stability studies are shown in Table 4. There were no significant changes in the viscosity, drug content and physical appearance of the gels after storing at different temperature conditions for 3 months. However further stability studies have to be carried out for extended period of time by considering relative humidity. In the present study, all the gel formulations released minoxidil over a prolonged period of 24 h across sigma membrane and excised mouse skin when compared to marketed topical solution, the drug release from which was almost completed in 12 h. However, further preclinical and clinical studies are required to compare the effectiveness of the gels over topical solutions.
| Temperature | Gels | Time (month) | Drug content (%) | Viscosity (cps) | Appearance |
|---|---|---|---|---|---|
| 4° | Gel I | 0 | 100 ± 0.00 | 25,050 ± 125 | +++ |
| 3 | 98.51 ± 1.12 | 25,000 ± 100 | +++ | ||
| Gel II | 0 | 100 ± 0.00 | 14,850 ± 150 | +++ | |
| 3 | 98.75 ± 1.02 | 15,000 ± 100 | +++ | ||
| Gel III | 0 | 100 ± 0.00 | 13,750 ± 75 | +++ | |
| 3 | 99.00 ± 1.58 | 13,600 ± 100 | +++ | ||
| Gel IV | 0 | 100 ± 0.00 | 15,850 ± 100 | +++ | |
| 3 | 98.65 ± 1.28 | 15,250 ± 100 | +++ | ||
| 25° | Gel I | 0 | 100 ± 0.00 | 25,050 ± 125 | +++ |
| 3 | 98.00 ± 1.25 | 25,125 ± 150 | +++ | ||
| Gel II | 0 | 100 ± 0.00 | 14,850 ± 150 | +++ | |
| 3 | 97.85 ± 1.21 | 15,125 ± 50 | +++ | ||
| Gel III | 0 | 100 ± 0.00 | 13,750 ± 75 | +++ | |
| 3 | 98.50 ± 1.98 | 13,750 ± 150 | +++ | ||
| Gel IV | 0 | 100 ± 0.00 | 15,850 ± 100 | +++ | |
| 3 | 98.02 ± 1.26 | 15,750 ± 125 | +++ | ||
| 37° | Gel I | 0 | 100 ± 0.00 | 25,050 ± 125 | +++ |
| 3 | 97.95 ± 1.58 | 25,250 ± 175 | +++ | ||
| Gel II | 0 | 100 ± 0.00 | 14,850 ± 150 | +++ | |
| 3 | 97.55 ± 1.26 | 15,000 ± 75 | +++ | ||
| Gel III | 0 | 100 ± 0.00 | 13,750 ± 75 | +++ | |
| 3 | 98.01 ± 2.01 | 13,950 ± 100 | +++ | ||
| Gel IV | 0 | 100 ± 0.00 | 15,850 ± 100 | +++ | |
| 3 | 97.91 ± 1.06 | 15,950 ± 75 | +++ |
Table 4: Results of stability studies
References
- Barman, J.M., Pecoraro, V. and Astore, I., J. Gerontol., 1969, 24, 163
- Novak, E., Franz, T.J., Headington, J.T. and Wester, R.C., Int. J. Dermatol., 1985, 2, 82
- Sintov, A., Serafimovich, S. and Gilhar, A., Int. J. Pharm., 2000, 194, 125
- Paraskevas, D., Rekkas, D., Choulis, N., Hatzis, J. and Stratigos, J., Arch Dermatol., 1987, 11, 1433
- Bazzano, G.S., Terezakis, N. and Galen, W., J. Amer. Acad. Dermatol., 1986, 15, 880
- Comacho, F., Medical treatment of androgenetic alopecia. In: Comacho, F., Montagna, W., Eds. Trichology: Diseases of the Pilosebaceous Follicle. Madrid (Spain), Avla Medica Group, SA, 1997, 357-386 pp.
- Sintov, A., Serafimovich, S. and Gilhar, A., Int. J. Pharm., 2000, 194, 125
- Tsai, J.C., Gordon, L., Flynn, G.L., Weiner, N. and Ferry, J.J., Int. J. Pharm., 1993, 96, 111
- Chiang, C.M., Flynn, G.L., Weiner, N.D. and Szpunar, G.J., Int. J. Pharm., 1989, 50, 21.
- Vlasses, P.H., Ribeiro, L.G.T., Rotmensch, H.H., Bondi, J.V., Loper, A.E., Hitchens, M., Dunlay, M.C. and Ferguson, R.K.,.J. Cardiovasc. Pharmacol., 1985, 7, 245
- Kibbe, H.A., In: Hand Book of Pharmaceutical Excipients, 3rd Edn., Pharmaceutical Press, London, 2000, 41.
- Tsai, J.C., Cappel, M.J., Flynn, G.L., Weiner, N.D., Kreuter, J. and Ferry, J.J., J. Pharm. Sci., 1992, 81, 736
- Ghosh, B. and Reddy, L.H., Indian J. Exp. Biol., 2001, 7, 710