- Corresponding Author:
- Jadwiga Stachowicz
University of Life Sciences in Lublin, Department of Chemistry, 15 Akademicka Street, 20-950 Lublin
E-mail: jadwiga.stachowicz@up.lublin.pl
| Date of Submission | 10 June 2013 |
| Date of Revision | 25 April 2014 |
| Date of Acceptance | 03 May 2014 |
| Indian J Pharm Sci 2014; 76(4): 287-298 |
Abstract
Due to the increasing demand for new pharmaceuticals showing biological activity against pathogenic microorganisms, there is increasing search for new compounds with predicted biological activity. Variously substituted thioamide derivatives with 1.3 and 1.2 ring of thiazole and 1,3,4-thiadiazole, as well as pyrazole were assessed for their activity against Candida albicans. Activity of majority of tested thioamides was larger as compared with that of the reference drugs. The electron parameters of obtained N-heterocyclic thioamides were determined and dependencies on their biological activity against Candida albicans were studied. The best electron compliance of produced bindings with the activity against Candida albicans was observed for the derivatives containing 1,3,4-thiadiazole ring.
Keywords
Thioamides, antifungal activity, Candida albicans, electron parameters
Occurrence of pathogenic fungi infections has increased rapidly [1-3]. This is directly related to the increasing population of immune-compromised people in connection with the use of antibiotics [4-7]. Furthermore, fungal diseases are very difficult to treat and are associated with a number of relapses. Candida spp. seems to be the predominant etiologic agent of mycoses. With the reduced immunity of an organism, these pathogens are dangerous parasites, which cause systemic candidiasis. In recent years, an increased resistance to antifungal agents has been recorded and this problem is a major challenge for medicine and pharmacy, as well as for those who are involved in designing the compounds with potential antifungal activity. To solve the problem of drug resistance, diverse mechanisms of action against Candida should be taken into account during designing new biologically active compounds.
Heterocyclic bindings containing nitrogen and sulfur atoms are an important class of compounds in medicinal chemistry [8]; among others, thiazole ring is an integral part of many biologically active compounds such as the antifungal drug abafungin [8]. Similar effects have been reported for ring compounds composed of three heteroatoms such as thiadiazoles and triazoles that exhibit some pharmacological activity [9]. In recent years, various thiadiazole derivatives have been intensively studied, which revealed their multilateral biological activity such as antibacterial, antiinflammatory, antitubercular, and antifungal properties [10]. Imidazole, pyrazole, and thiazole derivatives also attracted the interest of those who are involved in the synthesis of biologically active compounds because of these properties [11-13].
In general, chemical descriptors associated with different properties of the molecules can be used in the QSAR approach commonly applied in the search for the relationship between chemical structure and biological activity of a molecule. These include electron (HOMO and LUMO), thermodynamic (molar refraction), and structural energies (surface area, volume of the compound). HOMO and LUMO orbitals are very important parameters used in quantum chemistry. Based on their characteristics, it can be specified how a molecule would interact with other molecules. The HOMO orbitals can be considered as an electron donor group, while the LUMO orbitals as free sites able to accept them. Due to the interaction between these orbitals, p-p* transition, with respect to the molecular orbital theory, is observed [14-16]. Energy of the HOMO orbitals can be directly linked to the ionization potential, whereas the LUMO orbital energy can be associated with the electron affinity. The difference between the orbital energies of HOMO and LUMO is referred to as energy gap, which is an important parameter that can determine the reactivity or stability of molecules [16].
The aim of present calculations was to determine the electron parameters of N-heterocyclic thioamides containing the modified heterocyclic systems as well as to search for any relationship with their biological activity against Candida albicans.
Materials and Methods
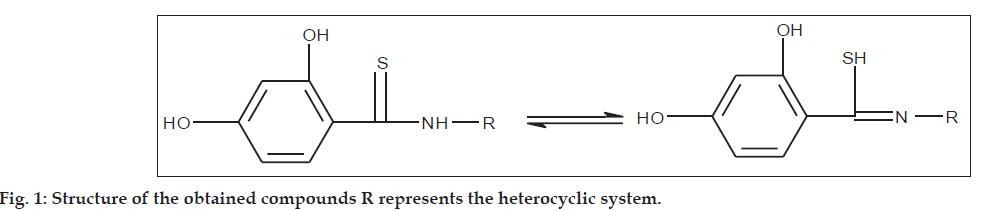
The study included 22 synthesized thioamide derivatives with 1.3 and 1.2 ring of thiazole, as well as 1,3,4 ring of thiadiazole and pyrazole (Table 1). The compounds were obtained using the corresponding amines and the patented thioarylillating reagent [17]. All derivatives of the general equilibrium formuloes shown in (fig.1) are provided with a 2,4-dihydroxyphenyl substituent determining the chemical properties and biological efficacy.
Analytical investigations
Melting point measurements on a Boetius apparatus are given uncorrected. The EI-MS spectra were recorded with an AMD-604 mass spectrometer (electron ionization at 70 eV). The parameters of the basic band and characteristic fragmentation ions corresponding to the products of the primary fragmentations and to the structure relatively close to that of the tested compound are given. The 1H NMR spectra were recorded with an FT-NMR Tesla BS 567 A spectrometer (100 MHz) in relation to TMS. The spectra of compounds were registered mainly to confirm the structure and if possible to determine the chemical shift of thioamide proton. The oscillation spectra were recorded with a Perkin-Elmer apparatus (in KBr). In Table 1 the frequencies of stretching vibrations in the equilibrium states of the amidothione system typical of frequencies are also listed.
Antifungal activity
Material for the study on the Sabouraud medium consisted of yeast-like fungi isolated from different onthocoenoses. In order to define the antifungal activity of the compounds were tested against Candida albicans reference strains 10231 ATCC and 200 fresh clinical isolates of C. albicans. Identification of strains was made using the CandiSelect medium by Sanofi Diagnostics Pasteur. Their resistance was determined in relation to the reference formulations (fluconazole and itraconazole). Fluconazole (Pfizer Inc,) and itraconazole (Janssen Pharmaceutica) were obtained as reagent-grade powders from their respective manufacturers.
The assessment of compounds sensitivity was made by means of the plate dilution method. Minimum inhibitory concentration (MIC mg/ml) was determined on the classic Sabouraud medium (Bio-Rad). For yeasts MICs were determined by the agar dilution procedure according to the National Committee for Clinical Laboratory Standards (NCCLS) reference document M27 National Committee for Clinical Laboratory Standards. The reference method was applied for broth dilution antifungal susceptibility testing of yeasts. Starting inocula were adjusted by the spectrophotometric method densitometric (bioMerieux) to 1×105 CFU/ ml. A solvent control was included in each set of assays; the DMSO solution at the maximum final concentration of the 1% had no effect on fungal growth.
All compounds were dissolved in 1% DMSO solution and further dilutions were made applying 0.9% NaCl. The Sabouraud medium in the amount of 15 ml was poured into Petri dishes of 9 cm diameter. Once solidified, the substrate in the following dishes contained the tested compounds of the concentrations from 200 to 6.25 mg/l.
The test suspensions were obtained from 24 h cultures of pure fungi strains. Density of the suspension was determined using a densitometer (BioMerieux) and a set at approximately 105 cells per 1 ml of saline. The prepared suspensions were plated on the Petri dishes with the Sabouraud medium (pH 7.2) containing increasing concentrations of the compound. The first control system cultures were composed of tested strains inoculated to the medium not containing the compound, while the second substrate was a solution containing 1% DMSO. All results were read after 24 and 48 h incubation at 37°. Biological assays were performed at the Medical University of Bialystok. Table 2 summarizes the results of biological tests.
Computing
Spartan Pro 1.08 software (Wavefunction, Inc.) was used for calculations. It was helpful for determining the HOMO and LUMO energies of the obtained compounds using the Hartree-Fock ab initio method and the self-adjusted database 6-31**. The HOMO and LUMO energies of compounds were used to determine the size of the HOMO-LUMO gap. In addition, the descriptors characterizing: The electronegativity (x = -0,5 (HOMO?LUMO)), hardness index (η=0,5 (HOMO-LUMO)), softness index (Sm=1/η), and electrophilicity (?=x2/2η), taking into account both above measures, were determined (Table 3).
The values of the electron density, as well as the HOMO energy value were used to calculate the electron orbital density limit R(I), which represents the HOMO electron density at the sulfur atom within thioamide binding, that probably plays a decisive role in the possible interactions with the molecular target. The values of R(I) are expressed in the Eqn.; R(I)=(fr(i)/-EHOMO)×102 (1) where: fr(i) is the HOMO electron orbital density limit at an i-th atom, while EHOMO is the HOMO energy expressed in electronvolts (eV) and measured from the level 0 [18].
Results and Discussion
In majority cases, activity of tested thioamides against Candida albicans was larger compared to that of the reference drugs used (Table 2). The following compounds were particularly effective: 1e, 3, 5c, 5e, 5g, 5h. When seeking the QSAR dependence, the attempts to link the activity of compounds with the descriptors that quantify the electronic effects, were undertaken. The size of the HOMO-LUMO energy gap was calculated assuming that sulfur atoms of the equilibrium thioamide/imidothiole binding may be one of the most effective chemical moieties due to the largest electron density derived from the HOMO orbital (Table 3).
| Compound | MIC (µg/ml) | ||||
|---|---|---|---|---|---|
| Candida albicans | Candida albicans isolates | ||||
| 10231 ATCC | No 200 | ||||
| MIC | Min | Max | |||
| 1a | 100 | 180±80 | 50 | 200 | |
| 1b | 120 | 100±50 | 50 | 150 | |
| 1c | 200 | 160±70 | 50 | 200 | |
| 1d | 200 | 200± | 200 | 200 | |
| 1e | 25 | 47.5±30.5 | 25 | 100 | |
| 1f | 100 | 100±35 | 50 | 200 | |
| 1g | 100 | 170±55 | 50 | 200 | |
| 2 | 100 | 120±40 | 50 | 200 | |
| 3 | 25 | 20±6 | 10 | 50 | |
| 4a | 100 | 167.5±88.5 | 50 | 200 | |
| 4b | 100 | 100±40 | 50 | 150 | |
| 4c | 200 | 200±100 | 50 | 200 | |
| 4d | 150 | 167±80 | 50 | 200 | |
| 4e | 100 | 130±55 | 50 | 200 | |
| 5d | 100 | 180±70 | 50 | 100 | |
| 5a | 200 | 125±45 | 50 | 200 | |
| 5b | 200 | 160±80 | 100 | 200 | |
| 5c | 200 | 50±30 | 25 | 200 | |
| 5e | 50 | 18.3±12 | 6.25 | 50 | |
| 5f | 200 | 100±50 | 50 | 200 | |
| 5g | 50 | 28.3±15 | 25 | 50 | |
| 5h | 200 | 70±40 | 50 | 200 | |
| Itraconazole | 200 | 182.5±65 | 120 | 200 | |
| Fluconazole | 200 | 200±200 | 100 | 200 | |
Activity of thioamide derivatives from n-(1,3 and 1,2 thiazoles, 1,3,4-thiadiazoles, pyrazoles) groups expressed in MIC (µl/ml) in relation to candida (sabouraud medium, reading after 24 h of incubation)
Table 2: Mic Values of Thioamide Derivatives
| Compound | E HOMO (eV) | E LUMO (eV) | R (I) for sulfur atom | HOMO-LUMO gap | ? | ? | Sm | ? |
|---|---|---|---|---|---|---|---|---|
| 1a | -8.36 | 1.78 | 0.55 | 10.14 | 5.07 | -3.29 | -0.30 | -3.91 |
| 1a’ | -8.14 | 2.03 | 0.42 | 10.17 | 5.09 | -3.06 | -0.33 | -4.23 |
| 1b | -8.24 | 1.80 | 0.55 | 10.05 | 5.02 | -3.22 | -0.31 | -3.92 |
| 1b’ | -8.07 | 2.29 | 0.47 | 10.36 | 5.18 | -2.89 | -0.35 | -4.64 |
| 1c | -8.37 | 1.72 | 0.04 | 10.09 | 5.05 | -3.33 | -0.30 | -3.83 |
| 1c’ | -8.16 | 2.30 | 0.48 | 10.46 | 5.23 | -2.93 | -0.34 | -4.66 |
| 1d | -8.64 | 1.53 | 0.51 | 10.18 | 5.09 | -3.56 | -0.28 | -3.64 |
| 1d’ | -8.34 | 1.87 | 0.38 | 10.20 | 5.10 | -3.23 | -0.31 | -4.03 |
| 1e | -8.92 | 0.49 | 0.45 | 9.41 | 4.71 | -4.22 | -0.24 | -2.63 |
| 1e’ | -8.94 | 0.96 | 0.33 | 9.90 | 4.95 | -3.99 | -0.25 | -3.07 |
| 1f | -7.97 | 1.44 | 0.54 | 9.41 | 4.70 | -3.26 | -0.31 | -3.39 |
| 1f’ | -8.07 | 1.86 | -0.02 | 9.93 | 4.97 | -3.11 | -0.32 | -3.97 |
| 1g | -8.35 | 1.68 | -0.54 | 10.03 | 5.01 | -3.33 | -0.30 | -3.77 |
| 1g’ | -8.24 | 2.27 | 0.37 | 10.51 | 5.25 | -2.99 | -0.33 | -4.62 |
| 2 | -8.66 | 1.42 | 0.53 | 10.08 | 5.04 | -3.62 | -0.28 | -3.51 |
| 2’ | -8.77 | 2.14 | 0.37 | 10.90 | 5.45 | -3.31 | -0.30 | -4.48 |
| 3 | -8.44 | 1.78 | 0.51 | 10.21 | 5.11 | -3.33 | -0.30 | -3.92 |
| 3’ | -8.88 | 2.25 | 0.33 | 11.14 | 5.57 | -3.32 | -0.30 | -4.67 |
| 4a | -8.55 | 1.66 | 0.50 | 10.22 | 5.11 | -3.45 | -0.29 | -3.79 |
| 4a’ | -8.53 | 2.18 | 0.41 | 10.71 | 5.36 | -3.17 | -0.32 | -4.52 |
| 4b | -8.48 | 1.63 | 0.49 | 10.11 | 5.06 | -3.42 | -0.29 | -3.73 |
| 4b’ | -8.47 | 2.23 | 0.42 | 10.71 | 5.35 | -3.12 | -0.32 | -4.59 |
| 4c | -8.54 | 1.68 | 0.36 | 10.22 | 5.11 | -3.43 | -0.29 | -3.81 |
| 4c’ | -8.57 | 2.31 | 0.37 | 10.88 | 5.44 | -3.13 | -0.32 | -4.73 |
| 4d | -8.39 | 1.55 | 0.51 | 9.94 | 4.97 | -3.42 | -0.29 | -3.61 |
| 4d’ | -8.67 | 2.27 | 0.40 | 10.94 | 5.47 | -3.20 | -0.31 | -4.67 |
| 4e | -8.64 | 1.48 | 0.34 | 10.12 | 5.06 | -3.58 | -0.28 | -3.57 |
| 4e’ | -8.54 | 1.77 | 0.35 | 10.31 | 5.16 | -3.39 | -0.30 | -3.93 |
| 5a | -7.95 | 2.55 | 0.71 | 10.49 | 5.25 | -2.70 | -0.37 | -5.10 |
| 5a’ | -8.28 | 3.30 | 0.46 | 11.58 | 5.79 | -2.49 | -0.40 | -6.73 |
| 5b | -8.34 | 2.12 | 0.62 | 10.46 | 5.23 | -3.11 | -0.32 | -4.40 |
| 5b’ | -8.10 | 2.62 | 0.45 | 10.72 | 5.36 | -2.74 | -0.36 | -5.24 |
| 5c | -7.85 | 2.06 | 0.67 | 9.91 | 4.95 | -2.90 | -0.35 | -4.23 |
| 5c’ | -7.80 | 2.56 | 0.47 | 10.36 | 5.18 | -2.62 | -0.38 | -5.12 |
| 5d | -7.30 | 1.92 | 0.82 | 9.22 | 4.61 | -2.69 | -0.37 | -3.95 |
| 5d’ | -7.33 | 2.73 | 0.59 | 10.06 | 5.03 | -2.30 | -0.43 | -5.50 |
| 5e | -7.85 | 1.57 | 0.66 | 9.42 | 4.71 | -3.14 | -0.32 | -3.53 |
| 5e’ | -7.91 | 2.21 | 0.45 | 10.12 | 5.06 | -2.85 | -0.35 | -4.50 |
| 5f | -8.62 | 1.62 | 0.55 | 10.24 | 5.12 | -3.50 | -0.29 | -3.75 |
| 5f’ | -8.68 | 2.21 | 0.38 | 10.89 | 5.45 | -3.24 | -0.31 | -4.58 |
| 5g | -8.20 | 2.68 | 0.61 | 10.88 | 5.44 | -2.76 | -0.36 | -5.37 |
| 5g’ | -8.62 | 3.12 | -0.14 | 11.73 | 5.87 | -2.75 | -0.36 | -6.26 |
| 5h | -8.72 | 1.65 | 0.50 | 10.37 | 5.18 | -3.53 | -0.28 | -3.80 |
| 5h’ | -8.98 | 2.39 | 0.38 | 11.37 | 5.69 | -3.30 | -0.30 | -4.90 |
Descriptors quantifying the electron effects of thioamides from n-(1,3 and 1,2 thiazoles, 1,3,4-thiadiazoles, and pyrazoles) groups. Denotations X, X? are assigned to the thioamide and imidothiole forms, respectively
Table 3: Descriptors Quantifying the Electron Effects of Thioamides
When considering the structure of the obtained compounds in computing relationships, it can be found that due to the energy of molecular orbitals, they belong to hard nucleophiles (relatively low HOMO energy at LUMO energy remaining on the average level). If the conclusions about the relationship between HOMOLUMO gap size and the reactivity of molecules are taken into account, quite high capacity for cellular interactions can be predicted for most of the obtained compounds [19].
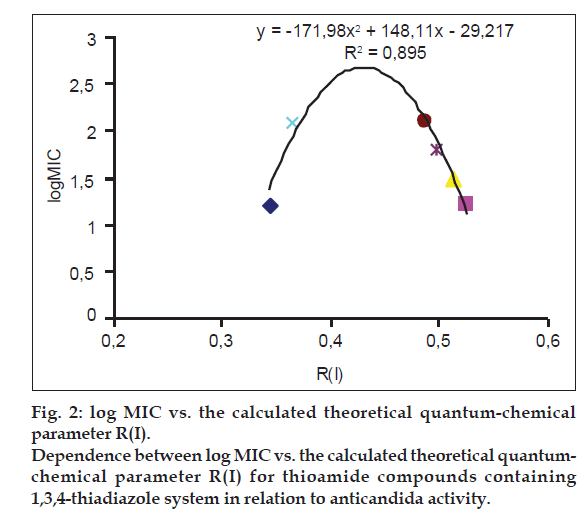
Dependencies observed between the listed log MIC values and the theoretical parameter R(I) are the confirmation of the adopted assumption defining the role of sulfur atoms within thioamide/imidothiole binding during inhibitory processes. The directional tautomerization resulting from the influence of heterocyclic systems has some impact on the electron states of sulfur atom, as indicated by the correlation parameter R(I) with the activity of the compounds. High correlation of the descriptor describing the thioamide form with activity towards Candida albicans was reported in the group of derivatives with thiadiazole ring (Table 4, fig. 2). In the group of pyrazole derivatives, the compounds with thiazole and thiazoline systems, there were obtained correlations were average (Table 4).
| Parameter | Equation | R2 |
|---|---|---|
| Thiadiazole | ||
| R (I) | y1 = -171.98×2+148.11x-29.217 | 0.895 |
| ? | y1=36.255×2+236.26x+386.35 | 0.9325 |
| S | y1=3924×2+2412.7x+372.35 | 0.9309 |
| Pyrazole | ||
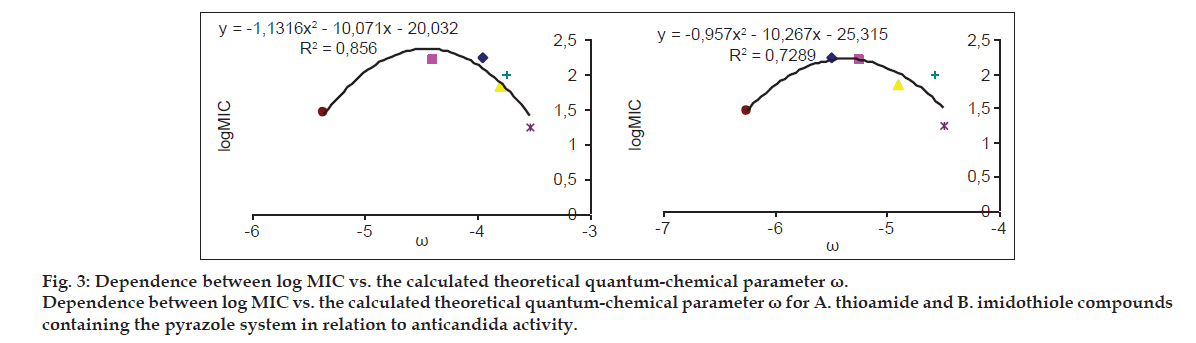
| ? | yT= -1.1316×2-10.071x-20.032 | 0.856 |
| y1= -0.957×2-10.267x-25.315 | 0.7289 |
Correlation equations between the thioamide activity against candida albicans vs. the descriptors quantifying the electron effects. The denotations T, I are assigned to the thioamide and imidothiole forms, respectively
Table 4: Correlation Equations between Thioamide Activity against Candida Albicans vs. Descriptors Quantifying the Electron Effects
The relationships of these compounds and other descriptors quantifying the electron effects were also considered, but the level of correlation for particular descriptors depended on a tautomeric form. In general, better equations were obtained in the analysis of imidothiole tautomers and describing parameters η and Sm (hardness and softness indices, respectively). Within the thiazole group, weak or average correlations between the activity and descriptors quantifying the electron effects (x, ?, Sm and ?) were recorded. A similar level of correlation of these parameters and activity was observed in a number of pyrazoles, and only for the electrophilicity descriptor ? determined for the thioamide forms, a large similarity of this parameter to the activity was reported (Table 4, fig. 3).
In the mycological literature there are numerous studies on the resistance of yeast-like fungi to currently used antifungal agents [20-22]. A correlation has been demonstrated between the amount of phospholipase produced and virulence in C. albicans strains and other yeast species. Some fungi such as: Mucor, Rhizopus, Aspergillus, Penicillium and Candida, have the ability of releasing hydrolytic enzymes into the environment, which break down the multimolecular compounds? polysaccharides, proteins, lipids, hydrocarbons [23].
Our results are in accordance with the previous reports [24-26]. Bujadakova et al. [24], assessed the anticandida activity of 6-amino-2-n-pentylthiobenzothiazole, benzylester of (6-amino-2-benzothiazolylthio) acetic acid and of 3-butylthio-(1,2,4-triazolo)-2,3-benzothiazole
The compounds were active against Candida strains. The first compound exhibited inhibitory activity on the germ-tube formation and mycelial growth in the C. albicans strains, while the others were not active in these tests. All the compounds tested were highly active on a nystatin-resistant C. albicans strains [24]. Similar findings were also obtained by Kucukbay [25].
In the earlier paper we evaluated antifungal activity of new 2,5-disubstituted amino-oxomety-loso-arylothiadiazole (AOAT) derivatives against Candida albicans, non-Candida albicans. The mean MIC of the Candida albicans strains was 141.6 (37.5-200) mg/l on the Sabouraud?s medium. The mean MIC of AOATs against the non-Candida albicans strains was 153.3 (50-200) mg/l.
High activity of compounds 1e, 3, 5c, 5e, 5g and 5h suggests the need for further research on the synthesis of new combinations containing variously modified heterocyclic systems. Much data indicates that the biological activity of various compounds can be related to the energy difference between the molecular orbitals HOMO and LUMO [27,28], which is the largest at the values about 9 eV. It is quite commonly accepted that the ability of a compound to react with the molecular target is independent of the mechanism of its action. These relationships were examined ?among others? in research upon the activity of sulfonourea herbicides [28] and imidazoline derivatives [29]. The symmetry of HOMO and LUMO orbitals distribution attracted some interest, which led to the conclusion that when they are localized at the same sites of the molecule, their activity decreases [28]. The team headed by Vasanthanathan linked the high fungicidal activity of derivatives containing the furan ring also with the electron parameters and energy of HOMO and LUMO orbitals [30,31].
These findings seem to confirm the results that the active compounds are characterized by the difference of HOMO-LUMO orbitals energy by about 9-10.5 eV (Table 3). Although these are slightly higher values than those quoted by the literature data, the most active compound (1e) in from the thiole group is characterized by relatively the lowest gap (9.41 eV). The LUMO energy value calculated for this compound (0.49 eV) is also the lowest in that group of bindings, which can suggest its specific abilities to form covalent bonds with nucleophiles. Symmetrical distribution of HOMO and LUMO orbitals in the imidothiole forms of 1c, 2, 4a, 4b, 4c, 5b compounds (Tables 5 and 6) is reflected in their relatively lower biological activity, whereas variable distributions of HOMO orbitals in the heterocyclic systems and particular atoms do not allow for determining their univocal correlations with the activity.
The obtained N-pyrazolthioamides group is characterized by the HOMO-LUMO gap value of about 9-11 eV, yet for the most active compounds (5e, 5g, and 5d), the values are similar to very active azoles[29,31-35]. The good correlation between the activity and R (I) indicator for thioamides with the thiadiazole system suggests that sulfur atom of the thioamide binding, due to the largest electron density of HOMO orbitals, would be the most sensitive towards the reactions associated with the electron transfer or binding to other atoms as well as responsible for the reactions with biological systems. The large activity of these compounds can be also associated with the impact of sulfur atom within thioamide binding, showing the ability to form hydrogen bonds with the enzyme thioles associations as well as close and long-range hydrophobicity regulators. Similar results were obtained by Karimian et al.[36-39] when studying three groups of fungicidal 1,2,4-thiadiazoles. These authors also indicated the possibility of reaction of these compounds with cysteine-dependent enzymes. They also demonstrated that the probability of N-S bond cleavage within the ring and the ability to form hydrogen associates is proportional to the charge accumulation on the heteroatoms of the ring.
This allows for generalization that the calculated values of R(I) for the sulfur atom in thioamide/ imidothiole binding as well as the size of HOMOLUMO gap are important parameters that describe general QSAR relationships. Also Xu et al.[40] tested the compounds containing 1H-pyrazole ring and demonstrated that the HOMO-LUMO gap of about 4-5 eV is well correlated with their fungicidal activity
The best correspondence between the electron parameters of the obtained compounds and their activity against Candida albicans is observed for the derivatives with 1,3,4-thiadiazole ring. In this group of compounds, the best correlation equations were obtained for the imidothiole forms with the parameters defining the softness (SM) and hardness indices (?). Considering the whole group, there was a good correlation between the activity vs. the electrophilicity descriptor (?) that describes the thioamide forms (fig. 3). When analyzing the most active compounds from the N-pyrazole group (5c, 5e, 5g), it was found that their activity increases with the increasing value of Sm, which may be related to the results of Pearson?s, who pointed out to the fact that soft particles also quickly reach a molecular target[41].
Based on the study, it can be assumed that the electron parameters, regardless of their level of correlation, make the character of interactions between particular groups within compounds closer and hence they can be used in a rational design of bindings for the synthesis after setting general dependencies defining their kinetic properties.
References
- Garber G. An Overview of Fungal Infections Drugs. Drugs 2001;61 Suppl 1:1-12.
- Weinberg ED. In: Burger?s medicinal chemistry and drug discovery. Vol. 2. New York: Wiley; 1996. p. 637-47.
- Singh UP, Gahtori P, Singh RK. In vitro Antifungal Activity of some 1,3,5-triazine Derivatives Nature Proceedings: hdl:10101/ npre.2011.5751.1: Posted 1 Mar 2011. Available from: http:// precedings.nature.com/documents/5751/version/1/files/npre20115751-1. pdf?origin=publication_detail [Last accessed on 2014 Jan 08].
- Da Costa KR, Ferreira JC, Komesu MC, Candido RC. Candida albicans and candida tropicalis in oral candidosis: Quantitative analysis, exoenzyme activity, and antifungal drug sensitivity. Mycopathologia 2009;167:73-9.
- Kuriyama T, Williams DW, Bagg J, Coulter WA, Ready D, Lewis MA. In vitro susceptibility of oral Candida to seven antifungal isolates. Oral Microbiol Immun 2005;20:349-53.
- Blignaut E, Messer S, Hollis RJ, Pfaller MA. Antifungal susceptibility of South African oral yeast isolates from HIV/ AIDS patients and healthy individuals. Diagn Microbiol Infect Dis 2002;44:169-74.
- Munoz P, Fernandez-Turegano CP, Alcala L, Rodriguez- Creixems M, Pelaez T, Bouza E. Frequency and clinical significance of bloodstream infections caused by C. albicans strains with reduced susceptibility to fluconazole. Diagn Microbiol Infect Dis 2002;44:163-7.
- Kumar A, Kumar R. A review on synthesis of Schiff?s bases of 2-amino 4-phenyl thiazole. Int Res J Pharm 2011;2:11-2.
- Siddiqui N, Ahuja P, Asan W, Pandeya SN, Alam MS. Thiadiazoles: Progress Report on Biological Activities, J Chem Pharm Res 2009;1:19-30.
- Singh AK, Bose S, Singh UP, Jana S, Shukla R, Singh V, et al. Synthesis and biological activity of some new thiadiazole derivative. Trends Pharm Res 2009;2:133-7.
- Singaravel M, Sarkkarai A, Kambikudi RM. Synthesis, Characterization and Biological activity of Some Novel Sulphur Bridged Pyrazoles. Int J Pharm Sci Res 2010;1:391-8.
- Abunada NM, Hassaneen HM, Kandile NG, Miqdad OA. Synthesis and Antimicrobial Activity of Some New Pyrazole, Fused Pyrazolo[3,4- d]-pyrimidine and Pyrazolo[4,3-e][1,2,4]- triazolo[1,5-c]pyrimidine Derivatives. Molecules 2008;13:1501-17.
- Ezawa M, Garvey DS, Janero DR, Khanapure SP, Letts LG, Martino A, et al. Design of a Heteroaryl Modified, 1,5-Disubstituted Pyrazole Cyclooxygenase-2 (COX-2) Selective Inhibitor. Lett Drug Des Discov 2005;2:40-3.
- Fukui K. Theory of orientation and stereoselection. Berlin: Springer- Verlag; 1975.
- Fukui K. Role of frontier orbitals in chemical reactions. Science 1982;218:747-54.
- Buyukuslu H, Akdogan M, Yildirim G, Parlak C. Ab initio Hartree- Fock and density functional theory study on characterization of 3-(5-methylthiazol-2-yldiazenyl)-2-phenyl-1H-indole. Spectrochim Acta A 2010;75:1362-9.
- Niewiadomy A, Matysiak J, Macik-Niewiadomy G. New thioamides, the intermediate product for preparing New thioamides. Poland Patent. 2000. P. 330263.
- Nakayama A, Hagiwara K, Hashimoto S, Shimoda S. QSAR of fungicidal D3-1,2,4-thiadiazolines. Reactivity-activity correlation of SH-Inhibitors. Quant Struct Act Relat 1993;12:251-5.
- Galeazzi R, Marucchini C, Orena M, Zadra C. Stereoelectronic properties and activity of some imidazolinone herbicides: A computational approach. J Mol Struc-Theochem 2003;640:191-200.
- Tipton KF. Nomeclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). Enzyme nomenclature. Recommendations 1992. Supplement: Corrections and additions. Eur J Biochem 1994;223:1-5.
- Davey KG, Holmes AD, Johnson EM, Szekely A, Warnock DW. Comparative evaluation of FUNGITEST and broth microdilution methods for antifungal drug susceptibility testing of Candida species and Cryptococcus neoformnas. J Clin Microbiol 1998;36:926-30.
- Durupinar B, Günaydin M, Sanic A, Müjgan P, Leblebiciloglu H. In vitro susceptibility of yeast isolates from various clinical specimes to antifungal agents. Mikol Lek 1996;3:9-12.
- Beck-Sague C, Jarvis WR. Secular trends in the epidemiology of nosocomial fungal infections in the United States. 1980-1990: National Nosocomial Infections Surveillance System. J Infect Dis 1993;167:1247-51.
- Bujdakova H, Kuchta T, Sidoova E, Gvozdjakova A. Anti-Candida activity of four antifungal benzothiazoles. FEMS Microbiol Lett 1993;112:329-33.
- Kucukbay H, Durmaz B. Antifungal activity of organic and organometallic derivatives of benzimidazole and benzothiazole. Arzneim Forsch 1997;47:667-70.
- Cetinkaya E, Alici B, Durmaz Y, Gunal S. New derivatives of benzimidazole and their antimicrobial activity. J Chemother 1999;11:83-9.
- Maynard AT, Covell DG. Reactivity of Zinc Finger Cores: Analysis of Protein Packing and Electrostatic Screening. J Am Chem Soc 2001;123:1047-58.
- Galeazzi R, Marucchini C, Orena M, Zadra C. Molecular structure and stereoelectronic properties of herbicide sulphonylureas. Bioorgan Med Chem 2002;10:1019-24.
- Galeazzi R, Marucchini C, Orena M, Zadra C. Stereoelectronic properties and activity of some imidazolinone herbicides: A computational approach. J Mol Struc-Theochem 2003;640:191-200.
- Vasanthanathan P, Lakshmi M, Babu MA, Gupta AK, Kaskhedikar SG. QSAR study of 3-phenyl-5-acyloxymethyl-2H,5H-furan-2-ones as antifungal agents: The dominant role of electronic parameter. Chem Pharm Bull (Tokyo) 2006;54:583-7.
- Vasanthanathan P, Lakshmi M, Babu MA, Kaskhedikar SG. Influence of thermodynamic parameter in lanosterol 14 alpha-demethylase inhibitory activity as antifungal agents: A QSAR approach. Biol Pharm Bull 2006;29:1262-6.
- Tuppurainen K, Lötjönen S, Laatikainen R, Vartiainen T, Maran U, Strandberg M, et al. About the mutagenicity of chlorine-substituted furanones and halopropenals. A QSAR study using molecular orbitals indices. Mutat Res 1991;247:97-102.
- Pearson RG. Absolute electronegativity and hardness: applications to organic chemistry. J Org Chem 1989;54:1423-30.
- López-Romero B, Evrard G, Durant F, Sevrin M, George P. Molecular structure and stereoelectronic properties of sarmazenil - a weak inverse agonist at the omega modulatory sites (benzodiazepine receptors): Comparison with bretazenil and flumazenil. Bioorg Med Chem 1998;6:1745-57.
- Werbovetz KA, Bhattacharjee AK, Brendle JJ, Scovill JP. Analysis of stereoelectronic properties of camptothecin analogues in relation to biological activity. Bioorg Med Chem 2000;8:1741-7.
- Karimian K, Tam TF, Leung-Toung RCSH, Li W, Bryson SP, Wodzinska JM. Thiadiazole compounds useful as inhibitors of cysteine activity dependent enzymes. US Patent 6,468,977, B1, 2002.
- Karimian K, Tam TF, Desilets D, Lee S, Cappelletto T, Li W. Process for scavenging thiols. US Patent 6,114,537, A, 2000
- Karimian K, Tam TF, Desilets D, Lee S, Cappelletto T, Li W. Imidazo(1,2-d)-thiadiazole compounds; peptic ulcer treatment. US Patent 6,093,738, A, 2000.
- Karimian K, Tam TF, Leung-Toung R, Li W. Thiadiazole compounds useful as inhibitors of H+ /K+ ATPase. US Patent 6,060,472, A, 2000.
- Xu L, Huang Y, Yu G, Si G, Zhu QI. Experimental, theoretical and biological activity study on 1-(4,5-dihydro-3-arylpyrazol-1-yl)-2-(1H- 1,2,4-triazol-1-yl)-etha-none. Struct Chem 2006;17:235-9.
- Pearson RG. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Natl Acad Sci USA 1986;83:8440-1.