- *Corresponding Author:
- L. J. Patel
Shri B. M. Shah College of Pharmacy, Modasa-383 315, India
E-mail: ljp353630@rediffmail.com
| Date of Submission | 14 October 2005 |
| Date of Revision | 01 March 2006 |
| Date of Acceptance | 19 December 2006 |
| Indian J Pharm Sci, 2006, 68 (6): 790-793 |
Abstract
Two simple, specific, accurate and precise methods, namely, reverse phase high performance liquid chromatography and high performance thin layer chromatography were developed for estimation of carvedilol in bulk drug and pharmaceutical formulations. For the high performance liquid chromatography method, Lichrospher 100 C-18, 5 µm column consisting of 200×4.6 mm i.d. in isocratic mode, with mobile phase containing 50 mM KH2PO4 buffer (pH 3.0±0.1): acetonitrile: methanol (60:50:10 v/v/v) was used. The flow rate was 1.0 ml/min and effluent was monitored at 242 nm. The retention time was 4.56±0.03 min. For the high performance thin layer chromatography method a Camag high performance thin layer chromatography system comprising of Linnomat V automatic sample applicator, Hamilton Syringe, Camag TLC Scanner-3, Camag Win CAT software with stationary phase precoated silica gel 60F254 and mobile phase consisting of ethyl acetate: toluene: methanol (1:4:3.5 v/v/v). The detection of spot was carried out at 242 nm. The Rf value was 0.65±0.02. The methods were validated in terms of linearity, accuracy and precision. The linearity curves were found to be linear over 1-35 µg/ml for high performance liquid chromatography and 50-300 ng/spot for high performance thin layer chromatography. The limit of detection and limit of quantification for high performance liquid chromatography were found to be 0.2 and 0.85 µg/ml, respectively, and for high performance thin layer chromatography, 10 and 35 ng/spot, respectively. The proposed methods were successfully used to determine the drug content of marketed formulations.
Carvedilol is chemically, 1-(9H-carbazol-4-yloxy)-3-[[2-(2-methoxyphenoxy)ethyl]amino]-2-propanol, which is a nonselective β-adrenergic blocker with α1-blocking activity [1]. It is used in the treatment of severe heart failure, bradycardia and hypertension [2]. The literature survey revealed that a few high performance liquid chromatography (HPLC) methods reported are applicable for analysis of carvedilol in body fluids [3-9] and in cardiac tissue [10]. Capillary electrophoresis method has been developed for enantiomers in serum [11]. Difference spectrophotometric [12] and UV spectrophotometric [13] methods have been advanced for determination of carvedilol in pharmaceuticals. There are no reports on the HPLC and HPTLC determination of carvedilol in pharmaceutical formulations. The present investigation describes precise, accurate and specific RP-HPLC and HPTLC methods for determination of carvedilol in bulk drug and in formulations.
All the reagents used were of HPLC and analytical grade. Reference standard of carvedilol was obtained from Intas Pharmaceuticals Limited, Ahmedabad. Carvedilol tablets of three different brands were purchased from local pharmacy. A standard stock solution of carvedilol (1 mg/ml) was prepared by dissolving 25 mg of the drug in 25 ml of methanol in a calibrated flask. For HPLC method, working standard solution (100 μg/ml) was obtained from stock solution by dilution with the mobile phase. For HPTLC, working standard solution (100 μg/ml) was obtained from stock solution by dilution with methanol.
HPLC, including a Hitachi pump L-7110 equipped with universal injector 77251 (Rheodyne) with injection volume 20 μl, Hitachi L-7420 UV/Vis detector, Merck-Hitachi HSM software, Lichrospher 100 C-18, 5 μm column having 200 mm length and 4.6 mm internal diameter, was used. Mobile phase was prepared by mixing 50 mM KH2PO4 (pH was adjusted to 3.0±0.1 with 10% v/v o-phosphoric acid), acetonitrile and methanol in proportion of 60:50:10 v/v/v, respectively. The mobile phase was filtered through 0.45 micron membrane filter paper and degassed by ultrasonication for 15 min. Linearity of the method was investigated by serially diluting the stock solution to give a concentration range of 1 to 35 μg/ml and injecting 20 μl with universal injector 77251 (Rheodyne). Calibration curve was constructed by plotting peak area against concentration.
A Camag HPTLC system comprising of Linnomat V automatic sample applicator, Hamilton Syringe, Camag TLC Scanner-3, Camag Win CAT software, Camag twin trough chamber and stationary phase precoated silica gel 60F254 were used. Ethyl acetate:toluene:methanol (1:4:3.5 v/ v/v) was used as mobile phase. The detection of spot was carried out at 242 nm. TLC plates were prewashed with methanol. Activation of plates was done in an oven at 50° for five min. The chromatographic conditions maintained were precoated silica gel 60F254 aluminum sheets as stationary phase, ethyl acetate: toluene: methanol (1:4:3.5 v/v/v) as mobile phase, chamber and plate saturation time of 30 min, migration distance allowed was 80 mm, wavelength scanning was done at 242 nm keeping the slit dimension at 6×0.45 mm. A deuterium lamp provided the source of radiation. Aliquots of standard solution (100 μg/ ml) of carvedilol (0.5, 1, 1.5, 2, 2.5 and 3 μl) were applied on the TLC plate. The TLC plate was dried, developed and analyzed as described earlier.
Assay of three different marketed products with brand names, Cardivas 3.125 mg (Sun Pharmaceuticals Ltd., Mumbai), Carca 3.125 mg (Intas Pharmaceuticals Ltd., Ahmedabad) and Carloc 12.5 mg (Cipla Ltd., Mumbai) were performed. Twenty tablets were separately weighed and powdered. An amount of powder equivalent to 25 mg of carvedilol was dissolved in methanol to obtain 1 mg/ml concentration, ultrasonicated and filtered through 0.45 μm cellulose nitrate filter. The solution was subjected to analysis by HPLC and HPTLC methods as described earlier after suitable dilution. From the peak area of carvedilol the amount of drug in the sample was computed using regression equation.
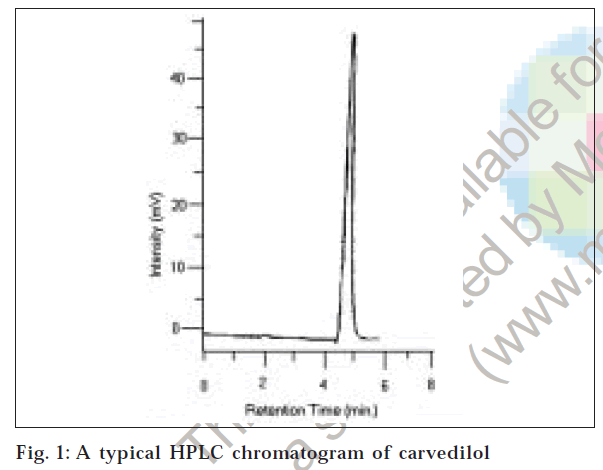
To optimize the HPLC parameters, several mobile phase compositions were tried. Satisfactory peak symmetry was obtained with mobile phase consisting of 50 mM KH2PO4: methanol (60:50:10 v/v/v). Quantification was achieved with UV detection at 242 nm based on peak area. A representative chromatogram is shown in fig. 1. Parameters of chromatogram are shown in Table 1.
| Parameters | RP-HPLC Method | HPTLC Method |
|---|---|---|
| Retention time (min) | ~5 | |
| Rf value | - | 0.65 ± 0.02 |
| Linearity range | 1-35 mg/ml | 50-300ng/spot |
| Correlation coefficient (r2) | 0.9997 | 0.9942 |
| Regression equation (y=mx+c) | ||
| Slope (m) | 119096 | 11.321 |
| Intercept (c) | -15051 | -176.25 |
| Tailing factor | 1.75 | |
| Theoretical plates | 3191 | |
| Limit of detection (LOD) | 0.2 mg /ml | 10 ng/spot |
| Limit of quantification (LOQ) | 0.85 mg /ml | 35 ng/spot |
Table 1: Validation And System Suitability
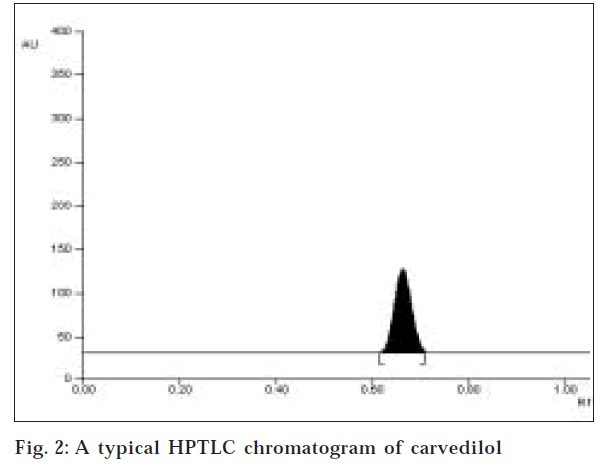
In HPTLC method, several mobile solvent system were tried to accomplish a good chromatogram. Using the solvent system ethyl acetate: toluene: methanol (1:4:3.5 v/ v/v) and precoated silica gel 60F254 aluminum plate a good chromatogram was obtained where Rf value was found to be 0.65±0.02 (fig. 2). The quantification of the drug was carried out at 242 nm.
The regression data showed a good linear relationship over a concentration range of 1 to 35 μg/ml for HPLC and 50 to 300 ng/spot for HPTLC. The limit of detection and limit of quantification for HPLC were found to be 0.2 and 0.85 μg/ml, respectively, and for HPTLC 10 and 35 ng/spot, respectively. As per the USP XXIII [14], system suitability tests for HPLC were carried out on freshly prepared standard stock solution of carvedilol and parameter obtained with 20 μl injection volume are summarized in Table 1. The intra-day and inter-day precision were determined by analyzing standard solutions in the concentration range of 5 to 25 μg/ml for HPLC and 150 to 270 ng/spot for HPTLC. The intra-day and inter-day precision results are given in Table 2. The results of the analysis of marketed formulations are well agreed with the label claim (Table 3). To study accuracy of the developed methods, recovery studies were carried out using standard addition method at four different levels for all the three brands and the % recovery was calculated (Table 4). The results revealed no interference from the excipients.
| Concentration | Intra?day (%RSD) | Inter?day (%RSD) | |||
|---|---|---|---|---|---|
| HPLC µg/ml |
HPTLC ng/spot |
HPLC | HPTLC | HPLC | HPTLC |
| 5 | 150 | 1.927 | 1.931 | 1.486 | 0.533 |
| 10 | 180 | 1.364 | 1.983 | 1.691 | 0.847 |
| 15 | 210 | 0.472 | 0.595 | 1.083 | 0.262 |
| 20 | 240 | 0.673 | 1.079 | 1.411 | 1.237 |
| 25 | 270 | 1.240 | 1.823 | 1.632 | 0.334 |
RSD = Relative standard deviation
Table 2: Intra-Day And Inter-Day Precision Study (N = 3)
| Formulation | Method | Amount found* mg/tablet ± SD | % of label claim ± SD |
|---|---|---|---|
| Cardivas | HPLC | 3.109 ± 0.03 | 99.5 ± 0.03 |
| 3.125 mg | HPTLC | 3.185 ± 0.02 | 101.9 ± 0.59 |
| Carca | HPLC | 3.067 ± 0.02 | 98.15 ± 0.50 |
| 3.125 mg | HPTLC | 3.158 ± 0.01 | 101.1 ± 0.29 |
| Carloc | HPLC | 12.33 ± 0.10 | 98.84 ± 0.51 |
| 12.5 mg | HPTLC | 12.28 ± 0.06 | 98.25 ± 0.51 |
*Average of three determination; SD = Standard deviation
Table 3: Assay Results Of Carvedilol In Pharmaceutical Formulations
| Formulation | RP-HPLC method | HPTLC method | ||||
|---|---|---|---|---|---|---|
| Conc. added µg/ml | Conc. recovered µg/ml* | % recovery ± SD | Conc. added ng/spot | Conc. recovered ng/spot* | % recovery ± SD | |
| Cardivas | 5 | 4.992 | 99.84 ± 0.86 | 30 | 29.61 | 98.72 ± 1.67 |
| 3.125 mg | 10 | 10.143 | 101.45 ± 0.35 | 60 | 60.55 | 100.31 ± 0.02 |
| 15 | 15.334 | 102.29 ± 0.41 | 90 | 91.80 | 102.0 ± 0.56 | |
| 20 | 20.007 | 100.04 ± 0.11 | 120 | 123.24 | 102.7 ± 0.34 | |
| Carca | 5 | 4.925 | 98.5 ± 1.27 | 30 | 30.7 | 102.33 ± 1.89 |
| 3.125 mg | 10 | 9.597 | 95.47 ± 0.15 | 60 | 59.42 | 99.03 ± 0.51 |
| 15 | 14.851 | 99.00 ± 0.01 | 90 | 88.78 | 98.64 ± 0.67 | |
| 20 | 20.084 | 100.42 ± 0.38 | 120 | 117.07 | 97.56 ± 0.34 | |
| Carloc | 5 | 5.103 | 102.05 ± 0.71 | 30 | 30.405 | 101.35 ± 2.76 |
| 12.5 mg | 10 | 10.262 | 102.62 ± 0.31 | 60 | 59.22 | 97.92 ± 1.09 |
| 15 | 15.244 | 101.63 ± 0.27 | 90 | 89.51 | 99.92 ± 0.65 | |
| 20 | 20.197 | 100.99 ± 0.45 | 120 | 121.34 | 100.05 ± 1.50 | |
*Average of three determination
Table 4: % Recovery Study
The developed methods are simple, precise and accurate. The statistical data proved that methods are reproducible and selective for the analysis of carvedilol in bulk drug and its marketed formulations.
Acknowledgements
The authors thank M/s Intas Pharmaceuticals Ltd., Ahmedabad for providing gift sample of carvedilol and, the Management, Shri B. M. Shah College of Pharmaceutical Education and Research, Modasa, for providing facilities to carry out this work.
References
- Paul, A.I, In; Gilman, A.G., Rall, T.W., Nies, A.S. and Taylor, P. Eds., Goodman and Gilman’s, The Pharmacological Basis of Therapeutics, 8th Edn., McGraw Hill, New York, 1996, 239.
- Heber, M.E., Bridgen, G.S., Caurana, M.P., .Lahiri, A. and Raftery, E.B., Amer. J. Cardiol., 1987, 59, 400.
- Cloths, L. and Mcerlane, K.M., J. Pharm. Biomed. Anal., 2003, 31, 407.
- Lamprecht, G., Gruber, L., Stochitzky, K. and Lindner, W., Chromatographia Suppl., 2002, 56, S-25
- Behn, F., Laer, S., Mir, T.S. and Scholz, H., Chromatographia, 2001, 53, 641.
- Huai, Y.W., Yan X. and Juan, H., Anal. Sci., 2005, 21, 537.
- Yang, E., Wang, S., Kratz, J. and Cyronak, M.J., J. Pharm. Biomed. Anal., 2004, 36, 609.
- Von, O.H. and Clarke, S., Eur. J. Clin. Pharmacol., 1997, 52, A 118.
- Lamprecht, G. and Stoschitzky, K., Chromatographia, 2004, 59, 551.
- Behn, F., Laer, S. and Scholz, H., J. Chromatogr. Sci., 2001, 39, 121.
- Cloths, L. and McErlane, K.M., J. Pharm. Biomed. Anal., 2001, 24, 545.
- Patel, P. M. and Mashru, R. C., Indian J. Pharm. Sci., 2005, 67, 389.
- Jain, P.S., Talele, G.S., Talele, S.G. and Surana, S.J., Indian J. Pharm. Sci., 2005, 67, 358.
- The United State Pharmacopoeia, XXIII, National Formulary, XVIII, US Pharmacopoeial Convention, Inc., Rockville M.D., 1995, 1776.