- *Corresponding Author:
- S. Saraf
Institute of Pharmacy, Pt. Ravishankar Shukla University, Raipur - 492 010, India
E-mail: rsofiop_gg@rediffmail.com
| Date of Submission | 10 March 2006 |
| Date of Revision | 16 April 2007 |
| Date of Acceptance | 13 October 2007 |
| Indian J Pharm Sci 2007, 69 (5): 692-694 |
Abstract
The combination of aceclofenac, paracetamol and chlorzoxazone is emerging as one of the widely prescribed combination in single dosage form. Aceclofenac is a typical Cox-2 inhibitor in combination with muscle relaxant chlorzoxazone and a traditional antipyretic drug paracetamol. Literature revealed that there is no single method for the simultaneous estimation of all these drugs in tablet dosage forms, which prompted us to develop a simple, rapid, accurate, economical and sensitive spectrophotometric method. The simultaneous estimation method is based on the additivity of absorbances, for the determination of aceclofenac, paracetamol and chlorzoxazone in tablet formulation. The absorption maxima of the drugs found to be at 276 nm, 282 nm and 248 nm respectively for aceclofenac, chlorzoxazone and paracetamol in methanol. All three drugs obeyed the Beer Lambert's law in the concentration range of 2-20 µg /ml. The accuracy and reproducibility of the proposed method was statistically validated by recovery studies.
Keywords
Simultaneous ace para and chloro
Aceclofenac is 2[(2,6-dichlorophenyl)amino]benzoic acid carboxymethyl ester is an analgesic and nonsteroidal antiinflammatory drug; paracetamol (4- hydroxy acetanilide) is used as an analgesic and anti pyretic drug and chlorzoxazone is 5-chloro- 2-benzoxazolol is a commonly prescribed muscle relaxant. Aceclofenac is official in BP [1], paracetamol in BP and IP [2,3] and chlorzoxazone in USP [4]. BP suggests a potentiometric assay method for aceclofenac in bulk drugs. The IP and BP both suggest titrimetric and UV spectrophotometric assay method for paracetamol in bulk and tablet formulations. Literature survey revealed that HPLC [5], densitometric [6], spectroß uorimetric [7] and colorimetric [8] methods have been reported for the estimation of aceclofenac in pharmaceutical dosage forms. With the advancement in the field of analytical chemistry and software technology different methods have been developed for simultaneous estimation of combination dosage forms. Though the combination is widely prescribed, no simultaneous method is reported for the estimation of the drugs in combined dosage forms. This prompted us to develop simple, rapid, accurate, economical and sensitive spectrophotometric simultaneous method.
The Shimadzu Pharmaspec 1700 UV/Vis spectrophotometer with 10 mm matched quartz cells was used for experiments. The chemicals used were of analytical grade. The commercially available tablets of aceclofenac, paracetamol and chlorzoxazone in combination were procured from local market. Aceclofenac, received as gift sample from Aristo Pharma Ltd., paracetamol (BDH) and chlorzoxazone from Mankind Pharma were used as such without further purification.
Standard stock solution of aceclofenac, paracetamol and chlorzoxazone were prepared separately by dissolving 100 mg each (accurately weighed) of standard aceclofenac, paracetamol and chlorzoxazone in methanol and made up the volume up to 100 ml with same solvent. Working standard solutions (10 μg/ml) (A), (B) and (C) were further prepared by taking 1 ml of stock solution of each drug solution in 100 ml volumetric ß asks separately and made up the volume up to the mark with methanol.
Overlain spectra of standard solutions of aceclofenac, paracetamol and chlorzoxazone were scanned (fig. 1). Aceclofenac shows absorption maxima at 276 nm, paracetamol shows at 248 nm and chlorzoxazone at 282 nm. The calibration curves for each were prepared in the concentration range of 2-20 μg/ml at each wavelength i.e. 276 nm, 248 nm and 282 nm. The absorptivity coefficients were determined for all the drugs at all the wavelengths and following equations were made. A1= 306.64 Cx+163.16 Cy+251.4 Cz..(1), A2= 109.52 Cx+908.22 Cy..(2) and A3= 293.77Cx+135.58Cy+325.52 Cz.. (3), where A1, A2 and A3 are absorbances at 276 nm, 248 nm and 282 nm, respectively and Cx Cy and Cz are concentrations of aceclofenac, paracetamol and chlorzoxazone respectively.
Tablet estimation was done on of two brands, Dolokind-MR (Mankind Pharma, Delhi) and Morcet- MR (Moraceae Lab, Luknow). Twenty tablets were weighed and crushed to a fine powder. Powder equivalent to 65 mg of paracetamol, 20 mg of aceclofenac and 50 mg chlorzoxazone (tablet contains 325 mg paracetamol, 100 mg aceclofenac and 250 mg chlorzoxazone) was extracted quantitatively with (4×20) ml of methanol and volume was made up to 100 ml. Insoluble excipients were separated by filtration. The filtrate was further diluted to get final concentration of both the drugs in the linearity range.
Absorbance was noted at the selected wavelengths and concentrations were determined by using the Eqns. 1, 2 and 3.
The method was found to be accurate, simple and rapid, for routine simultaneous analysis of the formulations without prior separation. The content of the aceclofenac, paracetamol and chlorzoxazone was directly found from the Eqns. 1, 2 and 3 using matrices (Cramer’s rule).
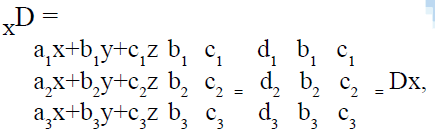
similarly using the same approach the other determinant Dy and Dz can also be found out.
The reproducibility, repeatability and accuracy of the proposed method were found to be satisfactory which is evidenced by low values of standard deviation, percent relative standard deviation and standard error (Table 1). The percent range of error (within 95% confidence limits) showed precision of the method. The accuracy and reproducibility of the proposed method was confirmed by recovery experiments, performed by adding known amount of the drugs to the pre analyzed formulations and reanalyzing the mixture by proposed method (Table 2). The percent recovery obtained indicates non-interference from the excipients used in the formulations. Thus the method developed in the present investigation found to be simple, sensitive, accurate and precise and can be successfully applied for the simultaneous estimation of aceclofenac, paracetamol and chlorzoxazone in tablets.
| Tablet brand | Tablet component | Label claim* (mg/tab) | Amount found* (mg/tab) | SD* | %RSD* | SE* | ‘t’ Calc.* |
|---|---|---|---|---|---|---|---|
| A | Aceclofenac | 100 | 99.47 | 0.2218 | 0.0796 | 0.6174 | 0.3514 |
| Paracetamol | 325 | 323.87 | 0.0049 | 0.0489 | 0.2315 | 0.8251 | |
| Chlorzoxazone | 250 | 248.14 | 0.2876 | 0.0214 | 0.1479 | 0.3954 | |
| B | Aceclofenac | 100 | 99.14 | 0.0868 | 0.0014 | 0.0157 | 0.8471 |
| Paracetamol | 325 | 324.78 | 0.0789 | 0.0127 | 0.1896 | 0.2354 | |
| Chlorzoxazone | 250 | 249.04 | 0.2156 | 0.0046 | 0.2310 | 0.9623 |
Table 1: Compilation Of Results Of Statistical Analysis Of Commercial Formulations
| Tablet brand | Recovery level (Added amount) | Percent recovery + SD* | ||
|---|---|---|---|---|
| Aceclofenac | Paracetamol | Chlorzoxazone | ||
| A | 50% | 99.47+0.0234 | 99.85+0.0150 | 99.34+0.1054 |
| B | 99.14+0.0158 | 99.90+0.0070 | 99.04+0.2163 | |
| A | 100% | 98.94+0.1023 | 99.96+0.0134 | 99.09+0.1897 |
| B | 99.12+0.0189 | 99.59+0.0698 | 98.98+0.1247 | |
| A | 150% | 99.87+0.0146 | 99.21+0.0197 | 99.01+0.1235 |
| B | 99.29+0.0698 | 99.35+0.0524 | 99.27+0.1754 | |
Table 2: Compilation of Results of Drug Recovery Study
Acknowledgements
The authors wish to thank Director, Institute of Pharmacy, Pt. Ravishankar Shukla University Raipur (CG) for providing necessary facilities, also thanks to Aristo Pharma Ltd., Mandideep and Mankind Pharma Delhi for providing the authentic sample of drugs.
References
- British Pharmacopoeia, Vol. I, London: Her Majesty ' s Stationary office; 1998; p. 33.
- British Pharmacopoeia, Vol. II, London: Her Majesty ' s Stationary office; 1998; p. 1854.
- Indian Pharmacopoeia, Vol. II, New Delhi: the controller of publications, Govt. of India; 1996, p. 554.
- United State Pharmacopoeia XXVII22, Rockville, MD: United States Pharmacopoeial Convention INC; 2004, p. 441.
- Hinz B, Auge D, Rau T, Rietbrock S, Brune K and Werner U. Simultaneous Determination of Aceclofenac and Three of its Metabolites in Human Plasma by High-Performance Liquid Chromatography. Biomed Chromatog 2003; 17: 268.
- El-Saharty YS, Refaat M and el-Khateeb SZ. Stability Indicating Spectrophotometric and Densitometric Methods for Determination of Aceclofenac. Drug Develop Int Pharm 2002; 28: 571.
- El-Kousy NM. Spectrophotometric and Spectrofluorimetric Determination of Etodolac and Aceclofenac. J Pharm Biomed Anal 1999; 20: 185.
- Zawilla NH, Mohammad MA, el-Kousy NM and el-Moghazy AS. Determination of Aceclofenac in Bulk and Pharmaceutical Formulations. J Pharm Biomed Anal 2002; 27: 243.
