- *Corresponding Author:
- L. J. Patel
Shri B. M. Shah College of Pharmacy, Modasa-383 315,India
E-mail: ljp353630@rediffmail.com
| Date of Submission | 19 September 2005 |
| Date of Revision | 1 March 2006 |
| Date of Acceptance | 18 September 2006 |
| Indian J Pharm Sci, 2006, 68 (5): 635-638 |
Abstract
A simple, specific, accurate and precise reverse phase high performance liquid chromatographic method was developed for the simultaneous estimation of bisoprolol fumarate and hydrochlorothiazide in tablet dosage form. A lichrospher 100 C-18, 5 µm column 20 cm × 4.6 mm in isocratic mode, with mobile phase containing water, acetonitrile and tetrahydrofuran in proportion of 80:20:5 v/v/v were used. The flow rate was 1 ml/min, and effluent was monitored at 225 nm. The retention time of bisoprolol fumarate and hydrochlorothiazide were 1.48 ± 0.02 and 4.72 ± 0.03 min respectively, and the resolution factor was 9.0. Linearity values for bisoprolol fumarate and hydrochlorothiazide were in the range of 10-150 µg/ml and 1-90 µg/ml respectively. The limit of detection and limit of quantification for bisoprolol fumarate was found to be 3.5 and 8.5 µg/ml respectively; and for hydrochlorothiazide, 0.4 and 0.9 µg/ml respectively. The proposed method is accurate, precise, specific and rapid for simultaneous estimation of bisoprolol fumarate and hydrochlorothiazide in tablet dosage form.
Bisoprolol fumarate (BSF) is a cardioselective ß1- adrenergic blocker. Chemically, BSF is (±)-1-[4-[[2-(1- methylethoxy)ethoxy]methyl]phenoxy]-3-[(1 1methylethyl)amino]-2-propanol(E)-2-butenedioate (2:1) [1]. Various analytical methods such as HPLC [2-8], LC-MS [9] and capillary isotechophoresis [10] are reported in the literature for the estimation of bisoprolol fumarate alone in biological fluids/pharmaceutical formulations. Hydrochlorothiazide (HCTZ) is thiazide diuretic and administered orally in the treatment of hypertension and oedema. Chemically, HCTZ is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7- sulphonamide-1,1-dioxide [11]. It is official in IP, BP and USP. The literature describes HPLC [12-19], non-aqueous titration [20,21], differential pulse anodic voltameter [22], LC-MS [23] and fluoromeric [24] methods for the estimation of hydrochlorothiazide alone in biological fluids/ pharmaceutical formulations. Fixed dose combination containing BSF and HCTZ is available in tablet dosage form in the market. Analytical methods for estimation of BSF and HCTZ in combination are not found reported in literature. The present paper describes a precise, accurate, specific and sensitive RP-HPLC method for simultaneous estimation of BSF and HCTZ in tablet dosage forms.
Materials and Methods
High performance liquid chromatography including a Hitachi pump L-7110 equipped with universal injector 77251 (Rheodyne), Hitachi L-7420 UV/Vis detector, Merck-Hitachi HSM software, Lichrospher 100 C-18, 5 μm column having dimensions 20 cm × 4.6 mm i.d. was used. Reference standard of bisoprolol fumarate was obtained from Intas Pharmaceuticals Ltd., Ahmedabad, and hydrochlorothiazide was obtained from Torrent Pharmaceuticals Ltd., Ahmedabad. Tablets of two different brands having combination of BSF and HCTZ were purchased from a local pharmacy. Acetonitrile, water and tetrahydrofuran used were of HPLC grade.
Preparation of mobile phase and standard stock solution
Mobile phase was prepared by mixing acetonitrile, water and tetrahydrofuran in proportion of 80:20:5 v/v/v. The mobile phase was filtered through 0.45 micron membrane filter and degassed by ultrasonicated for 15 min. The standard stock solutions (1 mg/ml) of BSF and HCTZ were prepared by dissolving 25 mg of drug in 25 ml of methanol and ultrasonicated for 15 min. The standard stock solutions were further diluted with mobile phase to obtain concentration range of 10-150 μg/ml and 1-90 μg/ ml for BSF and HCTZ respectively.
Calibration curve
Linearity of the method was investigated by serially diluting the stock solutions to give a concentration range of 10-150 μg/ml and 1-90 μg/ml for BSF and HCTZ respectively. An aliquot (20 μl) was injected using mixture of water, acetonitrile and tetrahydrofuran in proportion of 80:20:5 v/v/v as mobile phase. Calibration curves were constructed by plotting peak area against concentration. The flow rate was maintained at 1.0 ml/ min. Temperature of the column was kept at ambient, and the effluent was monitored at 225 nm. The retention times were 1.48±0.02 and 4.72±0.03 min, respectively with resolution factor of 9.0.
Analysis of marketed formulations
Assays of tablets of two different brands – Lodoz tablet of Merck Limited having combination of BSF 2.5 mg and HCTZ 6.25 mg (Brand 1); and Carbis-H tablet of Unichem Laboratories Ltd. having combination of BSF 5.0 mg and HCTZ 6.25 mg (Brand 2) – were performed. Twenty tablets each of Brand 1 and Brand 2 were separately weighed and powdered. The powder equivalent to 25 mg of BSF and 62.5 mg of HCTZ for Brand 1; and 25 mg of BSF and 31.25 mg of HCTZ for Brand 2 was dissolved in 25 ml of methanol, ultrasonicated for 15 min and filtered through 0.45 micron membrane filter. Standard solutions of tablets were further diluted with mobile phase to obtain concentration of 25 μg/ml of BSF and 62.5 μg/ml of HCTZ for Brand 1; and 25 μg/ml of BSF and 31.25 μg/ml of HCTZ for Brand 2; and were subjected to HPLC analysis as described earlier. From the peak area of BSF and HCTZ, the amount of drugs in samples was computed.
Results and Discussion
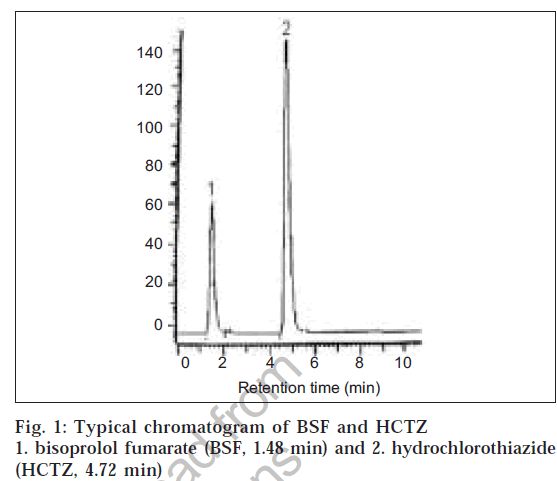
Several mobile phase compositions were tried to resolve the peaks of BSF and HCTZ. The optimum mobile phase containing water : acetonitrile : tetrahydrofuran (80:20:5 v/ v/v) was selected because it was found ideal to resolve the peaks of BSF (RT = 1.48 ± 0.02) and HCTZ (RT = 4.72 ± 0.03) with resolution factor of 9.0. Quantification was achieved with UV detection at 225 nm based on peak area. A representative chromatogram is shown in fig. 1. Parameters of chromatogram are shown in Table 1. As per USP XXIII, system suitability tests were carried out on freshly prepared standard stock solutions of bisoprolol fumarate and hydrochlorothiazide, and parameters obtained with 20 μl injection volume are summarized in Table 1.
| Parameters | BSF | HCTZ |
|---|---|---|
| Retention time (min) | 1.48±0.02 | 4.72±0.03 |
| Linearity range (µg/ml) | 10 –150 | 1 – 90 |
| Correlation coefficient (r2) | 0.9988 | 0.9996 |
| Regression equation (y=mx+c)* Slope (m) | 12172 | 104432 |
| Intercept (c) | -3653 | 102682 |
| Tailing factor | 1.14 | 1.81 |
| Theoretical plates | 539 | 2799 |
| Resolution factor | - | 9.0 |
| % RSD (n = 5) | 0.512 | 0.620 |
| Limit of detection (µg/ml) Limit of quantification(µg/ml) | 3.5 8.5 |
0.40 0.90 |
BSF = Bisoprolol fumarate; HCTZ = Hydrochlorothiazide; y = Peak area; x = concentration in μg/ml, RSD = Relative Standard Deviation
Table 1: Validation And System Suitability Parametersn
Linear regression data showed a good linear relationship over a concentration range of 10-150 μg/ml for BSF and 1-90 μg/ml for HCTZ. The correlation coefficients (r2) were 0.9988 and 0.9996 for BSF and HCTZ respectively. The limit of detection and limit of quantification were found to be 3.5 and 8.5 μg/ml respectively for BSF and 0.40 and 0.90 μg/ml respectively for HCTZ. The values indicate that the method is sensitive. The intra-day and inter-day precisions were determined by analyzing standard solutions in the concentration range of 20-90 μg/ ml and 5-40 μg/ml for BSF and HCTZ respectively (Table 2). The lower values of % RSD indicate that the method is precise. Repeatability was performed by injecting 50 μg/ml solution of BSF and 20 μg/ml solution of HCTZ, five times, and peak areas were measured. The % RSD was found to be 0.512 and 0.620 for BSF and HCTZ respectively.
| Bisoprolol fumarate | Hydrochlorothiazide | ||||
|---|---|---|---|---|---|
| Conc. (μg/ml) | % RSD | Conc. (μg/ml) | % RSD | ||
| Intra-day | Intra-day | Intra-day | Inter-day | ||
| 20 | 1.846 | 0.914 | 5 | 1.197 | 1.785 |
| 35 | 1.168 | 0.191 | 10 | 0.834 | 0.736 |
| 50 | 1.418 | 0.844 | 20 | 0.643 | 0.364 |
| 70 | 1.459 | 0.115 | 30 | 1.137 | 1.161 |
| 90 | 1.060 | 0.155 | 40 | 0.831 | 1.205 |
RSD = Relative Standard Deviation (n = 3)
Table 2: Intra-Day And Inter-Day Precision Studyn
Analysis was carried out using optimized mobile phase for tablets of Brand 1 and Brand 2 containing combination of bisoprolol fumarate and hydrochlorothiazide. The average contents of BSF and HCTZ were 2.489±0.007 and 6.249±0.090 respectively for Brand 1 and 4.959±0.034 and 6.179±0.058 respectively for Brand 2, which are in good agreement with the label claim (Table 3).
| Formulations | Bisoprolol fumarate | Hydrochlorothiazide | ||||
|---|---|---|---|---|---|---|
| Label claim mg/tab | Amount found* mg/tab ± SD | % assay ± SD | Label claim mg/tab | Amount found* mg/tab ± SD | % assay ± SD | |
| Brand I | 2.5 | 2.489±0.007 | 99.51±0.29 | 6.25 | 6.249±0.090 | 99.99±1.47 |
| Brand II | 5.0 | 4.959±0.034 | 99.18±0.69 | 6.25 | 6.179±0.058 | 99.86±0.93 |
*Average of three determinations; SD = Standard deviation
Table 3: Assay Results of Combined Tablet Dosage Forms
To study accuracy of the developed method, recovery study was carried out using standard addition method at four different levels for both the brands, and the % recoveries were calculated. The average % recoveries were 100.34±0.67 and 101.75±0.41 for BSF and HCTZ respectively in Brand 1; and 100.89±0.27 and 101.73±0.53 for BSF and HCTZ respectively in Brand 2 (Table 4).The results revealed that there was no interference of excipients. The proposed RP-HPLC method is accurate, precise, sensitive, selective and rapid for simultaneous estimation of BSF and HCTZ in combined tablet dosage form.
| Formulations | Bisoprololfumarate | Hydrochlorothiazide | ||||
|---|---|---|---|---|---|---|
| Amount added (µg/ml) | Amount recovered* (µg/ml) | % recovery ±SD | Amount added (µg/ml) | Amount recovered* (µg/ml) | % recovery ±SD | |
| Brand I | 5 | 4.96 | 99.33±0.42 | 5 | 4.97 | 99.33±0.47 |
| 10 | 10.19 | 101.47±1.13 | 10 | 10.21 | 102.32±0.39 | |
| 15 | 14.86 | 98.88±0.59 | 15 | 14.99 | 99.89±0.13 | |
| 20 | 20.30 | 101.69±0.52 | 20 | 20.29 | 101.46±0.65 | |
| Average recovery | Average recovery | |||||
| 100.34±0.67 | 100.75±0.41 | |||||
| Brand II | 5 | 5.10 | 102.09±0.26 | 5 | 4.99 | 100.45±1.43 |
| 10 | 10.12 | 101.17±0.22 | 10 | 10.25 | 102.50±0.02 | |
| 15 | 14.95 | 99.68±0.39 | 15 | 15.42 | 102.78±0.03 | |
| 20 | 20.13 | 100.63±0.22 | 20 | 20.28 | 101.20±0.63 | |
| Average recovery | Average recovery | |||||
| 100.34±0.67 | 101.73±0.53 | |||||
*Average of three determinations
Table 4: % Recovery of Bisoprolol Fumarate And Hydrochlorothiazide In Combined Dosage Form
Acknowledgements
The authors are grateful for the gift samples received from Intas Pharmaceuticals Ltd., Ahmedabad (bisoprolol fumarate); and Torrent Pharmaceuticals Ltd., Ahmedabad (hydrochlorothiazide). Authors also thank Shri B. M. Shah College of Pharmaceutical Education and Research, Modasa, for providing facilities to carry out this work.
References
- Budavari, S., Eds., In; The Merck Index, 13th Edn., Merck & Co., Inc., Whitehouse Station, NJ, 2001, 1294.
- Suzuki, T., Horikiri, Y., Mizobe, M. and Noda, K., J. Chromatogr., 1993, 619, 267.
- Braza, A.J., Modamio, P., Lastra, C.F. and Marino, E.L., Biomed. Chromatogr., 2002, 16, 517.
- Clarice, M.B.R., Liberato, B., Marico, F., Marcelo, D.M., Lisiane, B. and Sergio, L.D., J. Liq. Chromatogr. Related Techno., 2005, 28, 477.
- Zhang, X., Ouyang, J., Baeyens, W.R.G., Zhai, S., Yang, Y. and Huang, G., J. Pharm. Biomed. Anal., 2003, 31, 1047.
- Eastwood, R.J., Jerman, J.C, Bhamra, R.K. and Holt, D.W., Biomed. Chromatogr., 1990, 4, 178.
- Buhring, K.U. and Garbe, A., J. Chromatogr., 1986, 382, 215.
- Caudron, E., Laurent, S., Billud, E.M. and Prognon, P., J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci., 2004, 801, 339.
- Pitarch, E., Hernandez, F., Ten, H.J., Meiring, H., Niesing, W., Dijkman, E., Stolker, L. and Hogendoorn, E., J. Chromatogr., 2004, 1031, .
- Hercegova, A., Sadecka, J. and Polonsky.J.,Acta. Pol. Pharm.,1998, 55, 167.
- Budavari, S., Eds., In; The Merck Index, 13th Edn., Merck & Co., Inc., Whitehouse Station, NJ, 2001, 4802.
- United State Pharmacopoeia, XXIV, NF XIX, US Pharmacopoeial Convention, Inc., Rockville MD, 2000, 820.
- Farthing, D., Fakhry, I., Ripley, E.B. and Sica, D., J. Pharm. Biomed. Anal., 1998, 17, 1455.
- Zendelovska, D., Stafilov. T. and Milosevski, P., Biomed. Chromatogr., 2004, 18, 71.
- Kuo, B.S., Mandaaqere, A., Osborne, D.R. and Hwang, K.K., Pharm. Res., 1990, 7, 1257.
- Tisdall, P.A., Mpyer, T.P. and Anhalt, J.P., Clin. Chem., 1980, 26, 702.
- Hsieh, J.Y., Lin, C., Matuszewski, B.K. and Dobrinska, M.R., J. Pharm. Biomed. Anal., 1994, 12, 1555.
- Soldin, S.J., Hach, E. and Pollard, A., Ther. Drug. Monit., 1979, 1, 399.
- Azumava, C.T., J. Chromatogr., 1990, 532, 168.
- Indian Pharmacopoeia, Vol. I, Government of India, Ministry of Health and Family Welfare, the Controller of Publication, New Delhi. 1996, 371.
- British Pharmacopoeia, Vol. I, Ministry of Health and Family Welfare, H.M. Stationary Office, London, 1993, 330.
- Razak, O.A., J. Pharm. Biomed. Anal., 2004, 34, 433.
- Stumph, M.J. and Noall, M.W., J. Anal. Toxicol., 1984, 8, 170.
- Schafer, M., Geissler, H.E. and Mutschler, E., J. Chromatogr., 1977, 143, 15.