- Corresponding Author:
- Madhuri D. Game
Vidyabharati College of Pharmacy, C. K. Naidu Road Camp, Amravati - 444 602
E-mail: game_madhuri@yahoo.co.in
| Date of Submission | 24 December 2009 |
| Date of Revision | 22 September 2010 |
| Date of Acceptance | 13 January 2011 |
| Indian J Pharm Sci, 2011, 73 (1): 70-74 |
Abstract
Two simple, accurate and precise spectrophotometric methods have been developed for simultaneous determination of nitazoxanide and ofloxacin in tablets. Method I is Q-absorbance ratio method which involves Q-absorbance at isobestic point (306.25 nm) and max (347.5 nm) of nitazoxanide, while method II is two wavelength method, where 244.6 nm and 273.0 nm were selected as 1 and 2 for determination of nitazoxanide and 294.3 nm and 388.1 nm were selected as 3 and 4 for determination of ofloxacin. Both drugs obeyed the Beer’s law in the concentration range 2-30 µg/ml,correlation coefficient (r2<1). Both methods were validated statistically and recovery studies were carried out to confirm the accuracy. Commercial tablet formulation was successfully analyzed using the developed methods.
Keywords
Nitazoxanide, ofloxacin, Q-absorbance ratio method, spectrophotometry, two wavelength method
Nitazoxanide (NIT) [1-5], chemically N-(5-nitro- 2-thiazoyal) salicylamide acetate), is used as an antiprotazoal, antihelmenthic, giardiasis [2] and cryptosporidiosis [2] in immune-compromised patient, including those with AIDS or HIV infection. It has been used in helmentic infection [3-7]. It is not official in any pharmacopoeia and extensive literature survey revealed its estimation by UV spectrophotometric methods in bulk drugs. Ofloxacin (OFL) [8,9] belongs to flouroquinolone group of antimicrobial agent. Chemically, it is (±)-9-fluoro-2,3-dihydro–3–methyll- 10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido- [1,2,3-de]-1,4-benzoxazine-6-carboxylic acid. It is mainly used as antibacterial for the treatment of urinary tract infection and sexually transmitted diseases. OFL is official in BP [8], USP [9] and EP [10]. Literature reported a number of analytical methods for quantitative determination of ofloxacin alone or in combination with other drugs and some of these methods include potentiometry and conductometry [10], spectrophotometry [11-16], HPLC [17-23], electrophoresis [24,25] and LC/MS/MS [26,27] methods.
The aim of the present work was to develop simple, precise, selective and economical instrumental spectrophotometric methods for the simultaneous estimation of NIT and OFL in tablets. Pure drugs of NIT and OFL were obtained as gift samples from Lupin Pharmaceuticals, Aurangabad, India. A.R. grade methanol from Qualigens, Mumbai was used as solvent for preparing solutions. Commercially available (Nizonide-O, Lupin Pharmaceuticals) containing 500 mg of NIT and 200 mg of OFL per tablet, was randomly selected for the study and were procured from the local market. The solution of 0.1N HCl was prepared in double distilled water as per IP 1996 procedure. A Shimadzu UV/Vis 1601 double beam spectrophotometer with a fixed slit width (2 nm) and 1 cm matched quartz cells was used for all the spectral measurements.
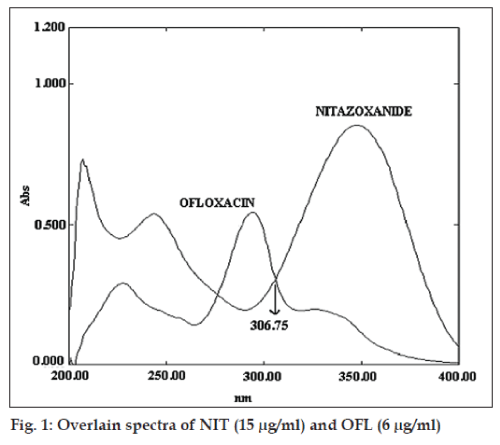
Standard stock solutions (100 μg/ml) of NIT and OFL were prepared separately by dissolving 10 mg of NIT and OFL, respectively in 100 ml methanol. Suitable aliquot of standard stock solutions were diluted with 0.1 N HCl to obtain solutions of NIT (15 μg/ml) and OFL (6 μg/ml). The resulting solutions were scanned in the range of 200-400 nm in 1 cm cells against solvent as blank. The UV absorption overlain zero order spectrum of NIT and OFL is depicted in fig. 1. From the overlain spectra the wavelengths 306.25 nm (iso-absorptive point) and 347.5 nm λmax of NIT were selected for Q-absorbance ratio method and for twowavelength method set of two wavelengths selected were λ1 (244.6 nm) and λ2 (273.0 nm) for estimation of NIT and λ3 (294.3 nm) and λ4 (388.1 nm) for the estimation of OFL. Standard stock solutions of NIT and OFL were diluted with 0.1N HCl to obtain concentration range of 2-30 μg/ml and absorbances were measured at selected wavelengths. The concentration of drug against absorbance was plotted to obtain calibration curves and the curves were found to be linear in the concentration range under study. Q-absorbance ratio method (method I) uses the ratio of absorbances at two selected wavelengths one is the isobestic point and other being the λmax of one of two compounds The absorptivity values (A 1%, 1cm) of each drug at selected wavelengths were determined. The absorptivity values of NIT at 306.25 nm and 347.5 nm were 185.4 and 512.7 while respective values for OFL were 460.1 and 195.82. Amount of each drug was estimated by substituting the absorbance and absorptivity values in the following equations. CNIT= (Qm–Qy)/(Qx –Qy)×A1/ax1 and COFL= (Qm–Qx)/(Qy–Qx)×A1/ay1, where A1 and A2 are the absorbances of mixtures at 306.25 nm and 347.5 nm. ax1 is absorptivity value of NIT at 306.25 nm, ax2 is absorptivity value of NIT at 347.5 nm, ay1 is absorptivity value of OFL at 306.25 nm and ay2 is absorptivity value of OFL at 347.5 nm and Qm=A2/ A1, Qy= ay2/ay1 and Qx= ax2/ax1.
The prior criteria for two-wavelength method (method II) is the existence of two such wavelengths where interfering component shows same absorbance where as component of interest shows significant difference in absorbance. On the basis of this principle that absorbance difference between two points on mixture spectra is directly proportional to concentration of component of interest and independent of interfering component, five mixed standards containing pure drug samples of NIT and OFL in the ratio (5:2) were prepared. All mixed standards were scanned at respective set of selected wavelengths. The difference in absorbances at 244.6 nm and 273.0 nm was plotted against the concentration of NIT and that at 294.3 nm and 388.1 nm was plotted against the concentration of OFL to construct two separate calibration curves for NIT and OFL. Calibration curves for mixed standards ensure that varying concentrations are not affecting the absorbances of analyzing components. Curves are results of mixed standards and Statistical data of calibration curves for NIT and OFL is summarized in (Tables 1 and 2).
| Standard laboratorymixture concentration(µg/ml) | Difference in absorbance for NIT at 244.6 and 273.0 nm | Difference in absorbancefor OFL at 294.3 and 388.1 nm | |
|---|---|---|---|
| NIT | OFL | ||
| 5 | 2 | 0.091 | 0.171 |
| 10 | 4 | 0.184 | 0.337 |
| 15 | 6 | 0.268 | 0.535 |
| 20 | 8 | 0.368 | 0.7 |
| 25 | 10 | 0.455 | 0.886 |
| 30 | 12 | 0.54 | 1.021 |
Table 1: Data Of Calibration Curves For Method Ii
| Parameters | For NIT* | For OFL** |
|---|---|---|
| Wavelength (nm) | 224.6-273.0 nm | 294.3-388.1 nm |
| Beer’s Law limit (µg/ml) | 2-30 | 2-30 |
| Correlation coefficient (r2<1) | 0.9997 | 0.9987 |
| Regression equation | Y=0.0181x +0.001 | Y=0.0867x +0.0011 |
*Difference in absorbance at 224.6 nm and 273.0 nm. **Difference in absorbance at 294.3 nm 389.1 nm.
Table 2: Statistical Data Of Calibration Curves For Nit And Ofl
For analysis of both NIT and OFL in tablets, twenty tablets were accurately weighed and average weight was calculated. Tablets were finely powdered and mixed thoroughly. Quantity of tablet powder equivalent to 5 mg of NIT was weighed accurately, dissolved in 20 ml methanol and sonicated for 20 min. The solution was filtered through Whatman filter paper (No. 41) and transferred to 50 ml volumetric flask and volume was made up to mark with methanol. The aliquot portion of filtrate was further diluted with 0.1 N HCl to get final concentration of about 15 μg/ml of NIT and 6 μg/ml of OFL.
For method I, absorbances of tablet sample solutions were recorded at 306.25 nm and 347.5 nm and the concentration of each drug was obtained by using mentioned formulae. For method II, tablet sample solutions prepared above were analyzed by scanning at respective set of wavelengths and absorbance difference values were noted and concentration of each drug was calculated from respective calibration curve.
Both the methods were validated statistically as per ICH/USP16 guidelines for all the parameters like accuracy, linearity, precision, ruggedness and specificity. To study the accuracy of the proposed methods, recovery studies were carried out by standard addition method at three different levels (80, 100 and 120% of the test concentration). A known amount of drug was added to pre analyzed tablet powder and percentage recoveries were calculated. The results of recovery studies were satisfactory and are presented in (Table 3). Linearity was constructed in the range of 2-30 μg/ml (r2<1). NIT and OFL in tablets were found to be linear in the range ± 20% of test concentration. Precision was studied by analyzing three replicates of sample solutions and concentrations were calculated. Ruggedness was established by carrying out experiment at different conditions like intra-day, interday and by different analyst. Specificity of the method was ascertained by analysing standard drug and sample. There was no interference of the excipients present in the formulation. By observing validation parameter (Table 4) the methods described were found to be specific, accurate, precise and economical and can be successfully applied to analyze commercially available tablets containing NIT and OFL The results obtained are in good agreement with the labeled content, summarized in Table 5.
| Level of standard addition (%) | % Recovery±SD* | ||||
|---|---|---|---|---|---|
| Method I | Method II | ||||
| NIT | OFL | NIT | OFL | ||
| 80 | 99.78±0.375 | 100.47±0.617 | 99.58±0.480 | 100.25±0.991 | |
| 100 | 100.29±0.991 | 99.49±0.732 | 98.69±0.461 | 99.56±1.10 | |
| 120 | 100.00±1.50 | 100.43±1.281 | 100.50±1.025 | 99.21±0.99 | |
Method I is Q- absorbance ratio method and method II is two-wavelength method. *mean of three estimations, SD is standard deviation
Table 3: Recovery Status Data
| Parameters | Method I | Method II | |||
|---|---|---|---|---|---|
| NIT | OFL | NIT | OFL | ||
| Linearity range | ±20 % of test conc. | ±20 % of test conc. | ±20 % of test conc. | ±20 % of test conc. | |
| Beer’s Law limit (µg/ml) | 2 to 50 | 2 to 50 | 2 to 50 | 2 to 50 | |
| Precision, % Drug found±SD* (n=3) | 100.22±0.2792 | 99.98 ±0.3912 | 100.38±0.5012 | 100.57±0.4129 | |
| Ruggedness, % Label Claim (n=3) | |||||
| Intraday | 100.79 | 100.32 | 100.81 | 100.35 | |
| Interday | 100.45 | 99.66 | 101.24 | 100.29 | |
| Different analyst | 100.41 | 99.73 | 100.79 | 100.39 | |
Method I is Q- absorbance ratio method and method II is two-wavelength method, (n=3) results are mean of three determinations, SD is standard deviation
Table 4: Summary Of Validation Parameters
| Method | Drug | % Label Claim* | ±SD* |
|---|---|---|---|
| I | NIT | 99.58 | 0.4641 |
| OFL | 100.09 | 0.5799 | |
| II | NIT | 99.68 | 0.2097 |
| OFL | 100.18 | 0.6286 | |
Method I is Q- absorbance ratio method and method II is two- wavelength, *results are mean of six sample solutions, SD is standard deviation
Table 5: Results Of Tablet Formulation Analysis.
Due to high sensitivity and simple sample preparation, the methods described can be used for undergraduate studies. Moreover simple spectrophotometric methods have obvious advantages over sophisticated instrumental analysis such as HPLC. Hence, simple and economical instrumental methods always have a role in pharmaceutical analysis.
Acknowledgements
The authors are grateful to Lupin Laboratories, SIDCO industrial Complex, Jammu for providing NIT and OFL pure drugs as gift samples. Authors are also thankful to Vidyabharati College of Pharmacy, Amaravati for providing necessary facilities for the research work.
References
- Sweetman SC, editors. Martindale: The Complete Drug Reference, 33rd ed. London: Pharmaceutical Press; 2002. p. 598-9.
- O’Neil MJ, editors. In; The Merck Index, An Encyclopedia of Chemicals, Drugs and Biologicals, 13th.ed. Whitehouse Station, NJ: Merck and Co. Inc.; 2001. p. 1177.
- Cavier R. Nitazoxanide in treatment of anthelminthiasis. Eur J Med ChemChimTher 1978;13:539-49.
- Dubreuil L, Houcke I, Mouton Y, Rossignol JF. In vitro evaluation of activities of nitazoxanide and tizoxanide against anaerobes and aerobic organisms. Antimicrob Agents Chemother 1996;40:2266.
- Murphy JR, Friedmann JC. Pre-clinical toxicology of nitazoxanide-a new antiparasitic compound. J ApplToxicol 1985;5:49-52.
- Stockis A, Deroubaix X, Lins R, Jeanbaptiste B, Calderon P, Rossignol JF. Pharmacokinetics of nitazoxanide after single oral dose administration in six healthy volunteers. Int J ClinPharmacolTher 1996;34:349.
- Rossignol JF, Maisonneuve H. Nitazoxanide in the treatment of TaeniasaginataandHymenolepis nana.Am J Trop Med Hyg 1984;33:511-2.
- British Pharmacopoeia, Licensing division, Norwich: HMSO; 2003. p. 357.
- United States Pharmacopoeia, United States Phamacopoeial Convention Inc,: Rockville, MD; 2004. p. 1355.
- European Pharmacopoeia. 5th ed. Strasbourg, France: EDQM, Council of Europe; 2005. p. 2131.
- Mathur SC, Kumar Y, Murugesan N, Rathore YK, Sethi PD. Spectrophotometric determination of Ofloxacin in Pharmaceutical formulations. Indian Drugs 1992;29:376.
- Immanuel C, Kumar AK. Simple and rapid high performance liquid chromatography method for the determination of Ofloxacin concentrations in plasma and urine. J Chromatogr B Biomed Sci Appl 2001;760:91-5.
- Panzade PD, Mahadik KR. Simultaneous estimation of Ofloxacin and Tinidazole in tablet dosage form. Indian Drugs 2001;38:368-70.
- Kasture VS, Bhagat AD, Puro NC. Spectrophotometric method for simultaneous estimation of ofloxacin and ornidazole in tablet dosage form. Indian Drugs 2004;41:51-3.
- Halkar UP, Ankalkope PB. Reverse phase high performance liquid chromatographic determination of ofloxacin and tinidazole in tablets. Indian Drugs 2000;37:585-8.
- Kamble NS, Venkatachalam A. High performance liquid chromatographic determination of ornidazole and ofloxacin in solid dosage form. Indian Drugs 2005;42:723-5.
- Patel MB, Patel KM, Patel GS, Suhagia BN, Prajapati AM. Development and validation of a stability-indicating HPTLC densitometric method for Satranidazole. J LiqChromatogrRelTechnol 2007;30:2459-71.
- Natarajan S, Raman B. HPLC determination of satranidazole in bulk and pharmaceutical dosage forms. Asian J Chem 2008;20:1833-40.
- Mruthyunjayaswamy BH, Patil SM, Raju SA. Spectrophotometric determination of satranidazole in bulk drug and formulations. J Indian ChemSoc 2003;80:863-5.
- Wankhede SB, Nanda RK, Prakash A, Chitlange SS. Simultaneous Spectrophotometric estimation of Ofloxacin and Satranidazole in tablet dosage form. J Pharm Res 2008;7:92-4.
- Sun HW, Hea P, Lva YK, Lianga SX. Effective separation and simultaneous determination of seven fluoroquinolones by capillary electrophoresis with diode array detector. J ChromatogrB 2007;852:145-51.
- A. Yin XB, Kang J, Fang L, Yang X, Wang E. Short-capillary electrophoresis with electrochemiluminescence detection using porous etched joint for fast analysis of lidocaine and Ofloxacin. J Chromatogr2004;1055:223-8.
- A. Lea HB, Pearta TE, Svobodab ML. Determination of ofloxacin, norfloxacin and ciprofloxacin in sewage by selective solid phase extraction, liquid chromatography with fluorescence detection and liquid chromatography–tandem mass spectrometry. J Chromatogr 2007;1139:45-52.
- Tuerk J, Reinders M, Dreyer D, Kiffmeyer TK, Schmidt KG, Kuss HM. Analysis of antibiotics in urine and wipe samples from environmental and biological monitoring–comparison of HPLC with UV, single MS and Tandem MS Detection. J Chromatogr B 2006;831:72-80.
- Sherje AP, Chokshi J, Satam CA, Chaudhary D, Vanshiv SD. Determination of nitazoxanide and ofloxacin in combined dosage form. J Pharm Res 2010;3:72-4.
- Senthilraja. Simultaneous UV spectrophotometric method for the estimation of nitazoxanide and ofloxacin in combined dosage form. Res J Pharm Tech 2008;1:469.
- Kalta RR, Sharma R, Chaturvedi SC. Simultaneous RP-HPLC determination of nitazoxanide and ofloxacin in combined dosage form. Indian J Pharm Sci 2008;70:491-4.