- Corresponding Author:
- S. Nagarsenker
Department of Pharmaceutics, Bombay College of Pharmacy, Santacruz (E), Mumbai-400 098, India
E-mail: mangal_nag511@yahoo.co.in
| Date of Submission | 07 August 2013 |
| Date of Revision | 19 July 2013 |
| Date of Acceptance | 25 July 2013 |
| Indian J Pharm Sci 2013;75(5):591-598 |
Abstract
Solid lipid nanoparticles have been increasingly utilised for improving oral bioavailability of drugs. Simvastatin is biopharmaceutical class 2 drug with poor oral bioavailability of 5%. In the present study, simvastatin solid lipid nanoparticles were successfully prepared by hot melt emulsification process and optimised with respect to surfactant and lipid concentration, and drug loading. The nanoparticles were characterised for entrapment efficiency, particle size, morphology, crystallinity and thermal behavior. Optimised formulations prepared from solid lipids glyceryl behenate and glyceryl palmitostearate containing Tween 80 as surfactant exhibited satisfactory entrapment efficiencies above 96% and mean particle size below 200 nm. The electron micrographs indicated that lipid nanocarriers were almost spherical in appearance. X-ray diffraction and differential calorimetric studies proved that the drug was amorphised in the lipid matrix and did not crystallise out. To improve the physical as well as chemical stability of formulations, dry adsorbed nanoparticles were prepared by evaporative drying method using a carrier. The adsorbed nanoparticles demonstrated good flow properties and satisfactory reconstitution properties. Pharmacodynamic studies of simvastatin solid lipid nanoparticles revealed improved reduction in total cholesterol values as compared to pure drug powder indicating improved bioavailability.
Keywords
Bioavailability, hyperlipidemia, solid lipid nanoparticles, simvastatin.
Lipid−based drug delivery systems have been increasingly utilised to improve the bioavailability of BCS class 2 drugs. Market survey reveals oral lipid−based formulations account for about 4% of commercially available products in the US, UK and Japan market[1], which range from simple lipid solutions filled in capsules to self−emulsifying systems. The utilisation of disperse lipid based systems such as microemulsions, nanoemulsions, self−emulsifying (SEDDS)/self−microemulsifying (SMEDDS)/ self−nanoemulsifying (SNEDDS) drug delivery systems, and solid lipid nanoparticles for improving oral bioavailability of drugs has been substantially successful. The increase in bioavailability is mainly attributed to increased dissolution of drug, increase in residence time, and lymphatic uptake[2]. Solid lipid nanoparticles (SLNs) developed in early nineties, are sub−micron colloidal carriers (50−1000 nm) which are composed of physiological lipid dispersed in water or in an aqueous surfactant solution. SLNs are advantageous over other colloidal systems with regards to biocompatibility and scale up[3].
Simvastatin (SIM) is a potent inhibitor of 3−hydroy−3−methyl−glutaryl−coenzyme A (HMG−CoA) reductase which converts HMG CoA into mevalonate, a precursor in cholesterol synthesis. The biopharmaceutical classification system (BCS) classifies SIM as class 2 drug having low aqueous solubility and variable absorption. SIM also undergoes extensive first pass metabolism in intestinal gut and liver. Hence the oral bioavailability of SIM in its intact form is only 5%[4]. The poor bioavailability necessitates development of efficient delivery system which can improve the oral bioavailability of SIM. SLNs have been utilised for increasing the bioavailability of a number of drugs[5−7]. SIM SLNs have also been reported earlier. The efficacy of the SLN formulations was established by analyzing the pharmacokinetic parameters either indirectly by radiolabeling[8], or by analysis of SIM and its metabolite in plasma[9]. However, none of the researchers have attempted in analyzing the pharmacodynamic activity of SIM when given in form of SLNs. In the case of statins, the pharmacokinetic parameters are not sufficient descriptors of the pharmacodynamic activity[10]. The aim of the present study was to investigate the potential use of SLNs in improving the oral bioavailability of SIM. SLN formulations containing SIM were optimised and pharmacodynamic activity was studied for SIM SLNs.
SIM is a labile molecule and is prone to oxidative and hydrolytic degradation[11]. Hence its stability in SLN dispersion would undoubtedly be of concern. SLN dispersions by themselves have few issues regarding physical stability since these systems are prone to gellation with loss in colloidal stability. Dilute SLN dispersions containing appropriate stabilisers are stable for three years when stored at 25º[12]. Another option is to convert SLN dispersions into dry forms. Lyophilisation and spray drying are established techniques for removal of water from SLNs. Lyophilisation is a costly time consuming process, whereas spray drying can cause melting of the lipids during the process. Hence in the present study dry SLNs were obtained by adsorbing on suitable carriers. The process was optimised such that SLNs would remain in nanometer range after reconstitution of the dry form.
Materials and Methods
Simvastatin (SIM) was obtained as gift sample from Sun Pharmaceutical Industries, Limited, Mumbai. Solid lipids such as Compritol ATO888 (glyceryl behenate), Precirol ATO5 (glyceryl palmitostearate), Geleol (glyceryl monostearate), Gelucire 50/13 (PEG glyceride) were obtained as gift sample from Gattefosse India Pvt. Ltd. Dynasan 114 (trimyristin), Dynasan 116 (tripalmitin) were obtained as gift samples from Sasol GmbH, Germany. Pluronic F68 (poloxamer) was obtained as gift sample from BASF, India. Polysorbate 80 was purchased from S. D. Fine- Chem., Mumbai. All solvents and reagents used were of analytical grade.
Screening of lipids
The solubility of SIM in different solid lipids was determined by a semi quantitative method. Two hundred milligrams of lipid was taken in test tube and heated at a temperature of 5º above its melting point. Ten milligrams of the drug was added to the melted lipid and vortexed using cyclomixer (Remi, Mumbai). The drug was added in increments of 10 mg. The quantity after which there is no further solubilisation of drug was taken as the end point of drug solubility.
Formulation and optimisation of SIM SLNs
SIM SLNs were prepared hot melt emulsification method. The lipid was melted at a temperature 5º above its melting point on a water bath (Superfit, India). SIM was added to the hot lipid melt and dissolved with the aid of cyclomixer. Aqueous phase containing surfactant maintained at same temperature was added to the lipidic phase and vortexed to give a coarse emulsion. The globule size of the emulsion was reduced by sonication for 4 min using ultrasonifier (Branson 250, USA) at a power of 40 W. The nanoemulsions formed after size reduction process, were cooled gradually to room temperature to give SLNs. Blank SLNs were prepared in similar manner by omitting the step of addition of drug. The formulation was optimised with respect to lipid concentration, type and concentration of surfactant and drug loading.
Determination of drug content
SLN dispersion (0.1 ml) was diluted to 10 ml with methanol in volumetric flask. This solution was sonicated for 2 min and filtered through 0.45 μm syringe filter. This solution was diluted suitably and analyzed by validated HPLC method. Drug content=(amount of drug obtained/amount of drug added)×100.
Percent entrapment efficiency (%EE) of SIM
SLN dispersion was aggregated with the help of sodium sulfate. The aggregate was then centrifuged at 10,000 rpm using Minispin centrifuge (Eppendorf, Germany) to get pellet. The supernatant was discarded and the pellet was air dried. The semidried pellet was dispersed in methanol and sonicated for drug extraction. The methanol solution was filtered through 0.45 μm syringe filter and analyzed by UV spectrophotometry at wavelength of 240 nm. Blank SLN dispersion was treated in similar manner and UV absorbance of blank SLNs was subtracted from drug−loaded SLNs and this reading was used to determine the entrapment efficiency. The percent entrapment efficiency was found using the formula, Percent entrapment efficiency=(amount of drug in SLNs/amount of drug added) × (100/Drug content)×100.
Particle size and zeta potential
The particle size was measured on N5 Beckman Coulter Sub−micron Particle Size analyzer, (Beckman Coulter, USA) which works on the principle of photon correlation spectroscopy (PCS). All experiments were carried out at 25ο using disposable polystyrene cells, after appropriate dilution with double distilled water. The zeta potential was measured on Brooke Haven instrument at 25ο using zeta cells after appropriate dilution with double distilled water.
Morphology of SLNs
The morphology of SLNs was determined by Philips CM 200 transmission electron microscope (Philips, Netherland). A drop of appropriately diluted SLNs was placed on a formvar−coated 300 mesh size copper grid and 5 min was allowed for some of the nanocarriers to stick to the grid. The drop of SLNs was drawn off by touching the edge of the grid with a piece of tissue paper held at 90° to the plane of grid. The SLN film was dried and observed under electron microscope. The electron micrographs were obtained in the range of 30000 to 85000× magnification.
Crystallinity studies
X−ray powder diffraction (pXRD) patterns were recorded on a Philips PW 17291 powder X−ray diffractometer (Philips, the Netherland) using Ni−filtered, Cu−K radiation, a voltage of 40 kV, and a 25 mA current. The scanning rate used was 1° min−1 over the 10-40° 2θ range. pXRD diffraction patterns were recorded for plain drug, bulk lipids and drug−loaded SLNs. The SLNs were dried under vacuum at room temperature for removing water before pXRD studies.
Thermal behavior
Differential scanning calorimetric (DSC) analysis was done to confirm the drug−lipid association in nanoparticulate formulations and crystallinity of drug with the help of Mettler TA STARe system equipped with DSC 822e cell (Mettler Toledo, US). Samples containing 40 mg of SLNs were weighted accurately in aluminum pans. DSC scans were recorded at a heating and cooling rate of 5º/min. The DSC analysis was done for plain drug, blank SLNs and drug−loaded SLNs. The SLNs were dried under vacuum at room temperature for removing water before DSC studies.
Preparation of dry adsorbed SLNs
The drug loaded SLNs were converted into dry granules (DANs) by evaporative drying using on suitable carriers. Five millilitres of SLN dispersion was taken in mortar. About 2/3 parts of adsorbent was dissolved in SLN dispersion and the aqueous phase was concentrated by evaporating in vacuum desiccator at room temperature. To the pasty mass obtained after drying process, the remainder 1/3 part of adsorbent material was added and finally dried in vacuum desiccator at room temperature. This was then sieved through mesh no #22 along to obtain free flowing granules. The concentration of carrier was kept at 20% and the lipid load in the dispersion was 5%.
Characterisation of DANs
The DANs were characterised for release of SLNs after reconstitution (redispersibility), flow properties and water content. For redisperisbility, DANs corresponding to one dose (5 mg SIM) were dispersed in dissolution flasks containing 500 ml media, maintained at 37º and stirred using paddle at 50 rpm. The media selected was distilled water and 0.5% Tween 80 in distilled water. Aliquot was withdrawn after 6 h and mean particle size and polydispersity index (PI) of the released SLNs was noted using N5 submicron particle size analyzer (Beckman Coulter, USA). For insoluble carriers, the aliquot was filtered using Whatman filter no. 42 and the filtrate was analyzed for particle size. Also, the residue remained on the filter was dispersed in methanol, vortexed, and filtered. The filtrate analyzed for drug content using UV spectrophotometer (V530, Jasco, Japan).
The flow properties of DANs such as flow rate, angle of repose and bulk density were determined by standard pharmacopoeial methods. Water content in DANs was checked by Karl-Fischer method.
Pharmacodynamic study of SIM SLNs
The anticholesterolemic effects of different SLN formulations were compared by poloxamer induced hyperlipidemia model. The study protocol was approved by the Institutional Animal Ethics Committee of Bombay College of Pharmacy, Mumbai. Female Sprague Dawley (SD) rats of weight range 200−250 g were obtained from Glenmark Research Centre, Mahape, Mumbai and housed into groups of six, maintained on a standard diet with free access to water. The area was maintained at 25±2º with a 12 h dark/light cycle throughout the study. The animals were divided into 4 groups of six animals, each group receiving different formulations. Group I received plain water which served as control group, group II received SIM drug suspension, group III received formulation F1 and group IV received formulation F2. The drug suspension was prepared by suspending 10 mg of SIM in 10 ml of distilled water containing 5% w/v sodium carboxymethyl cellulose.
The rats were fasted overnight prior to study with free access to water. Hyperlipidemia was induced by intraperitoneal injection of 1.0 g/kg of poloxamer F−127 solution (20% w/v). The rats were dosed orally with 0.1 ml of plain drug and formulations once after 12 h of injection of poloxamer. This procedure was repeated for 4 days. The blood was withdrawn after 24, 48 h, and at the end of 4 days of induction of hyperlipidemia. The serum was separated and analysed for total cholesterol (TC) and high density lipoproteins (HDL) by in vitro diagnostic kit (Beacon Diagnostic Pvt. Ltd., Mumbai, India).
Statistical analysis
The statistical analysis for the determination of the difference in the lipid levels of control and treatment groups was treated by unpaired t test and Mann−Whitney test and results with P<0.05 were considered significant.
Results and Discussion
The melting points and solubility of SIM in different lipids is given in Table 1. SIM showed high solubility in geleol, followed by compritol and precirol. Geleol is a monoglyceride whereas the other two lipids contain mixture of fatty acid esters. These lipids have fewer tendencies to crystallise into different polymorphic forms and thus can incorporate more amount of drug. As against this, dynasan 114, 116, 118 are triglycerides of myristic, palmitic, and stearic acid. These lipids are highly crystalline in nature and incorporate less amount of drug[13]. Hence the solubility of SIM in these lipids was found to be low. High solubility is a prerequisite for obtaining good drug loading and also ensures good association of drug with the lipid system in vivo.
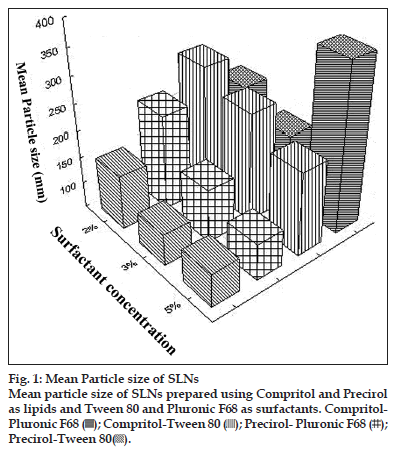
Melt emulsification process has been the method of choice for drugs having good solubility in molten lipids. Based on the solubility studies compritol, precirol were selected for preparation of SLNs. Geleol, being a monoglyceride, is prone to oxidative degradation and hence was not used for preparation of SLNs even though it demonstrated good affinity for SIM. Two surfactants, Tween 80 and pluronic F68 were utilised for stabilisation of SLNs formulations. These were used in concentration of 2, 3 and 5% and the effect on mean particle size and polydispersity index (PI) was noted. The increase in surfactant concentration from 2 to 3% caused a decrease in particle size and PI for all SLNs prepared using either of the surfactants. Further increase to 5% was not of substantial help and increase in PI was noted. Hence, all formulations were prepared with surfactant concentration of 3%. The results are summarised in fig. 1.
| Solid lipid | Melting point | Solubility % w/w |
|---|---|---|
| Compritol 888 | 65−77° | 13 |
| Precirol ATO5 | 52−55° | 13 |
| Geleol | 55−60° | 15 |
| Dynasan 114 | 56−57° | 5 |
| Dynasan 116 | 66° | 5 |
| Dynasan 118 | 68−70° | 5 |
Table 1: Solubility Of Sim In Different Lipids
The lipid concentration is also responsible for particle size distribution of the formulation. The lipid concentration in SLN dispersion was varied and formulations were prepared containing SLNs at lipid concentration of 2, 5 and 10%. Results indicated that there was increase in mean particle size with the increase in lipid load and at 10% lipid load all formulations comprised visible particles and gelled on storage. The mean particle size of SLNs remained below 200 nm for lipid load of 5%.
The effect of drug loading on particle size was observed by preparing SLNs containing 2 and 6% drug load. The increased drug load did not affect the particle size and PI of precirol SLNs. However in the case of compritol SLNs there was almost 4−fold increase in particle size. The increase in particle size can be attributed to the viscosity of the lipid matrix. The incorporation of drug could have caused significant increase in viscosity and hence there was an increase in particle size. For this purpose, compritol SLNs were prepared having lipid load of 2%. In all formulations no drug crystals were noticed at 6% drug loading when observed with the help of phase contrast microscope. The composition of final formulations selected for the study is as given in Table 2.
The drug content of all formulations was around 98%. HPLC data confirmed that there was no drug degradation during preparation of SLNs. The entrapment efficiencies of all formulations were 96-97%. This confirmed that the drug dissolved in lipid matrix remained associated with the matrix and there was no drug diffusion.
The particle size of blank and drug−loaded SLNs were measured by photon correlation spectroscopy (PCS). The particle size obtained for formulations F1 to F4 and their blank BF1 to BF4 are as mentioned in Table 3. The particle size of drug−loaded formulations was greater than the blank ones. All formulations had particle size in nanometer range and acceptable polydispersity index of around 0.4 to 0.5 indicating unimodal distribution.
| Formulation | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Lipids (in mg) | ||||
| Compritol | 200 | 200 | ||
| Precirol | 500 | 500 | ||
| Surfactant (in mg) | ||||
| Tween 80 | 200 | 300 | ||
| Pluronic F68 | 200 | 300 | ||
| SIM (in mg) | 12 | 30 | 12 | 30 |
| Water (ml) | 10 | 10 | 10 | 10 |
Table 2: Final Sln Formulations For The Study.
| Formulation | Zeta potential (mV) |
Particle size (in nm) |
Polydispersity index |
|---|---|---|---|
| BF1 | −23.50 | 149.4 | 0.356 |
| BF2 | −24.82 | 129.9 | 0.158 |
| BF3 | −8.97 | 151.6 | 0.244 |
| BF4 | −4.77 | 73.4 | 0.334 |
| F1 | −19.59 | 397.2 | 0.499 |
| F2 | −15.64 | 314.4 | 0.479 |
| F3 | −1.15 | 236.7 | 0.340 |
| F4 | −6.51 | 136.5 | 0.281 |
Table 3: Particle Size And Zeta Potential Of Sln Formulations.
The zeta potential values obtained for formulations F1 to F4 and their blank BF1 to BF4 are as mentioned in Table 3. The formulations showed negative zeta potential since lipid nanoparticles have negative charge on their surface[14]. Incorporation of pluronic F68 (formulations F3 and F4) decreased the absolute value of zeta potential. The results are in accordance with findings from other research workers where similar decreased the absolute value of zeta potential was observed for SLNs stabilised with pluronics[15].
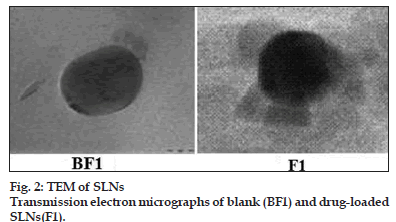
Formulations BF1 and F1 were characterised for their overall shape using transmission electron microscopy. The electron micrographs of blank and drug−loaded lipid nanocarriers are shown in fig. 2. The morphological studies indicated that lipid nanocarriers were almost spherical in appearance with average diameter of around 100 nm. Platelet−like structures were also observed. It is reported that chemically heterogeneous lipids in combination with heterogeneous surfactants lead to the formation of almost spherical particles. Homogeneous lipids tend to form platelet−like structures due to lipid crystallisation. Since the lipids used in this study are of heterogeneous nature, we were able to observe spherical particles. Also, there was no indication of drug crystallisation in the formulation.
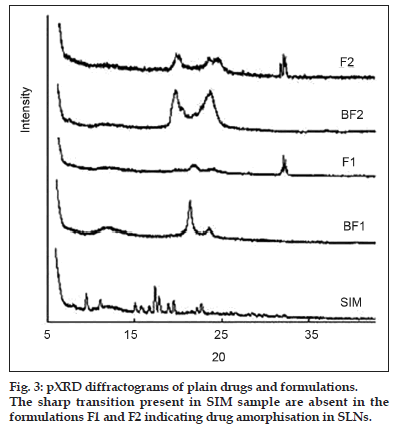
Fig. 3 shows the X−ray diffraction patterns of SIM, blank formulations and drug−loaded formulations. In the X−ray diffraction pattern of SIM, the sharp peaks at a diffraction angle (2θ) of 10.98°, 15.70°, 16.62°, 17.32°, 17.82°, 18.88°, 19.50°, 22.12°, and 22.66° are present, and it reveals that drug is present in crystalline form. X ray diffraction patterns of formulations F1 and F2 were characterised by diffuse spectra and no characteristic peaks of drug. These results confirm that there was no crystallisation of drug inside the lipid nanocarrier matrix.
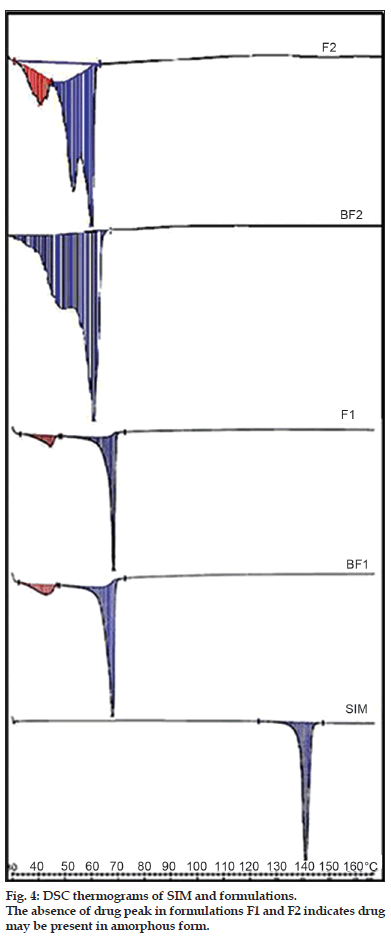
The DSC thermograms of SIM, BF1, BF2, F1 and F2 are as shown in fig. 4. SIM shows a sharp melting endotherm at 140º which corresponds to its melting point. This melting endotherm was absent in DSC of formulations. The probable reason could be that SIM has completely solubilised in the lipid matrix. Also, changes in onset of melting points as well as enthalpies of lipids compritol and precirol were noted, indicating interaction between lipids and SIM.
The DANs represent a simple technique for preparation of dry powders from SLNs which is amenable to scale up. In the present study 4 different carriers were tried for preparation of DANs. The water−soluble adsorbents were selected due to ease of characterisation of such systems, since it was assumed that the carriers would dissolve with the release of SLNs when dispersed in water. The water−insoluble ones such as aerosil, neusilin were selected because they have large surface area for adsorption and hence would be required in lesser quantities. No attempt was made to remove surfactant from the system since surfactants are helpful to prevent coalescence of particles during the process of drying of SLNs and also to aid in redispersion of SLNs from the dry form. Also, surfactants help in reducing the crystallinity of lipids and thus can be helpful in preventing the drug expulsion during storage[15].
The mean particle size and PI of precirol SLNs adsorbed on different carriers is as given in Table 4. The redispersibility of DANs in water was not satisfactory. There was increase in particle size which could be attributed to particle aggregation. Better results were obtained when the DANs were dispersed in 0.5% Tween 80 solution.
Cavalli et al. have reported evaporative drying wherein a specially designed minidessicator was used to evaporate the aqueous phase of SLNs at lower temperature[16]. DANs5 was dried in similar manner without the use of any adsorbent at room temperature. The process was lengthy and took 72 h for complete removal of water. The obtained dry mass was a sticky lipidic material which was scrapped from the evaporating dish. It was observed that the SLNs remained aggregated and the particle size after redispersion in 0.5% Tween solution was found to be 865 nm with PI of 1.2. The carriers were helpful in minimising the aggregation of SLNs. The water insoluble carriers were satisfactory in redispersing the SLNs and were required in less quantity due to large surface area. However, on analyzing the adsorbent material for the presence of in residue left after redispersion, it was observed that only about 50% of SLNs were redispersed from the carriers in 6 h. As against this water−soluble carriers were found suitable in releasing the SLNs as well as keeping the particle size in nanometer range. These systems also exhibited good flow with angle of repose was less than 25°. The water content was found to be 6.2%.
Many researchers have developed lipid−based delivery systems for SIM. Kang et al.[17], have developed self−microemulsifying drug delivery system (SMEDDS) for SIM. The authors have conducted bioavailability studies in beagle dogs and were successful in achieving 1.5 fold increase in oral bioavailability. Similar studies are also recently reported by Zhang et al.[9] where a 2 fold increase in oral bioavailability was observed for SIM SLNs. These results are encouraging. However, in case of statins the pharmacokinetic data is less predictive of pharmacodynamic efficacy and conclusive results can be estimated only by a pharmacodynamic study. Hence, in the present study the efficacy of formulations was studied by comparing the anticholesterolemic effect of formulations and drug suspension using poloxamer induced hyperlipidemia model. Since the DANs released SLNs after reconstitution, SLN formulations were used in the pharmacodynamic study instead of DANs. Formulations F1 and F2 were chosen for the study to observe the effect of different lipids on the efficacy of the formulations. Formulations F3 and F4 differed only with respect to the stabiliser used and it was assumed that this will not dictate the efficacy of the formulations. Hence these were not used for determining the efficacy of SLN formulations.
| Formulation | Adsorbent | Particle size in nanometer | |
|---|---|---|---|
| Water* | 0.5% tween 80* | ||
| DANs1 | Lactose | 1230.2±624.14 (1.433) | 525.4±15.67 (0.685) |
| DANs2 | Mannitol | 764.5±146.1 (1.220) | 573.5±42.18 (0.921) |
| DANs3 | Aerosil 200 |
614.8±42.54 (1.179) | 178.8±2.61 (1.51) |
| DANs4 | Neusilin | 425.4±43.34 (1.172) | 446.7±212.5 (1.365) |
| DANs5 | - | 4873.8±308.8 (1.707) | 865.5±100.82 (1.204) |
Table 4: Mean Particle Size Of Slns After Redispersion.
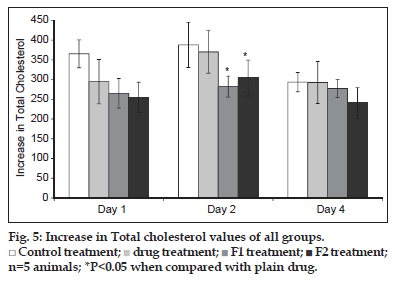
The individual values of total cholesterol (TC) are given in fig. 5. After 24 h of poloxamer injection, there was 4 to 4.8 fold increase in the TC level of the control. Similar rise was observed in case of treatment groups, which showed around 3.5 to 4 fold increase in the TC levels. The SLN formulations as well as plain drug were able to inhibit the increase in TC levels. Since considerable variation was observed in TC levels, the parameter selected for comparison of results was increase in total cholesterol (ITC) rather than the total cholesterol. This term takes into consideration the difference in basal cholesterol values of individual animal and can eliminate the false negative results obtained due to difference in basal cholesterol levels. The parameter was calculated as, (Increase in total cholesterol)nthday=(Total cholesterol)nthday–(Total cholesterol)0day.
The ITC values after 24 h of treatment was significantly higher in control group as compared to the treated groups (P<0.05). Although the TC levels were low in case of groups treated with formulations as compared to plain drug a substantial variability in results was noted which is depicted by high standard deviation values. There was a further increase in ITC values of control and drug treated group on day 2, whereas in case of formulations, the ITC was considerably lowered. Thus, the formulations depicted a superior cholesterol lowering activity which can be attributed to increase bioavailability of SIM.
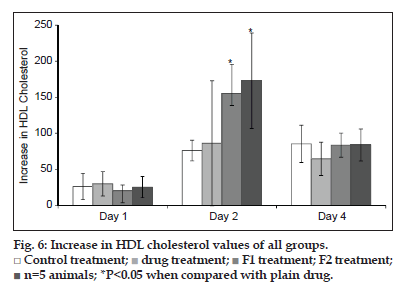
Statins are also known to increase the HDL cholesterol. The HDL cholesterol was determined for all groups on all four days. The HDL values of all groups are as given in fig. 6. The increase in HDL was calculated for all groups as mentioned for total cholesterol. It was observed that the HDL cholesterol values doubled at the end of day 1 for all groups. On day 2, a statistically significant increase in HDL cholesterol was observed in formulation groups when compared with the control group which again can be attributed to increased bioavailability of SIM.
SLNs have been one of the systems of choice for improving the oral bioavailability of drugs. In the present study SIM SLNs were successfully prepared by melt emulsification method with high entrapment efficiency, drug loading, satisfactory particle size distribution and favorable crystalline behavior. To increase the chemical stability of SIM as well as protect colloidal stability of SLNs, the dry forms were prepared by adsorbing on suitable carriers. The water−soluble carriers gave satisfactory results leading to free−flowing SLN granules which released SLNs in contact with aqueous system. Such granules can be further compressed into Tablets or filled in capsules for efficient patient friendly delivery system. The pharmacodynamic activity of SIM SLNs was better compared to plain drug which could be attributed to increase bioavailability. Researchers have demonstrated increase uptake of SIM SLNs using in situ intestinal absorption model[9]. The superior pharmacodynamic profile achieved in the present study correlated with the increased absorption depicted in the earlier study.
References
- Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res 2004;21:201-30.
- Aungst BJ. Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism. J Pharm Sci 1993;82:979-87.
- Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery: A review of the state of the art. Eur J Pharm Biopharm 2000;50:161-77.
- Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: Drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther 2006;112:71-105.
- Patel K, Padhye S, Nagarsenker M. Duloxetine HCl Lipid Nanoparticles: Preparation, Characterization, and Dosage Form Design. AAPS PharmSciTech 2012;13:125-33.
- Suresh G, Manjunath K, Venkateswarlu V, Satyanarayana V. Preparation, characterization, and in vitro and in vivo evaluation of lovastatin solid lipid nanoparticles. AAPS PharmSciTech 2007;8:162-70.
- Vivek K, Reddy H, Murthy RS. Investigations of the effect of the lipid matrix on drug entrapment, in vitro release, and physical stability of olanzapine-loaded solid lipid nanoparticles. AAPS PharmSciTech 2007;8:16-24.
- Tiwari R, Pathak K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: Comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharm 2011;415:232-43.
- Zhang Z, Bu H, Gao Z, Huang Y, Gao F, Li Y. The characteristics and mechanism of simvastatin loaded lipid nanoparticles to increase oral bioavailability in rats. Int J Pharm 2010;394:147-53.
- Nagarsenker MS, Date AA. Novel delivery systems of atorvastatin should be evaluated for pharmacodynamics instead of pharmacokinetics. J Pharm Pharmacol 2007;59:1583-4.
- Dixit RP, Barhate CR, Padhye SG, Viswanathan CL, Nagarsenker MS. Stability indicating RP-HPLC method for simultaneous determination of simvastatin and ezetimibe from Tablet dosage form. Indian J Pharm Sci 2010;72:204-10.
- Freitas C, Müller RH. Spray-drying of solid lipid nanoparticles (SLNTM). Eur J Pharm Biopharm 1998;46:145-51.
- Westesen K, Bunjes H, Koch MH. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. J Control Release 1997;48:223-36.
- Schwarz C. Solid lipid nanoparticles (SLN) for controlled drug delivery II. Drug incorporation and physicochemical characterization. J Microencapsul 1999;16:205-13.
- Han F, Li S, Yin R, Liu H, Xu L. Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: Nanostructured lipid carriers. Colloids Surf Physicochem Eng Aspects 2008;315:210-6.
- Cavalli R, Gasco MR, Barresi AA, Rovero G. Evaporative drying of aqueous dispersions of solid lipid nanoparticles. Drug Dev Ind Pharm 2001;27:919-24.
- Kang BK, Lee JS, Chon SK, Jeong SY, Yuk SH, Khang G et al. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int J Pharm 2004;274:65-73.
 Control treatment;
Control treatment;  drug treatment;
drug treatment;  F1 treatment;
F1 treatment;  F2 treatment;
n=5 animals; *P<0.05 when compared with plain drug.
F2 treatment;
n=5 animals; *P<0.05 when compared with plain drug.