- Corresponding Author:
- I. Rojas-oviedo
Departamento de Sistemas Biológicos, Universidad Autónoma Metropolitana-Xochimilco, Calzada del Hueso 1100, Col. Villa Quietud. C.P. 04960 México, D.F
E-mail: irma19@correo.xoc.uam.mx
| Date of Submission | 5 October 2011 |
| Date of Revision | 15 November 2012 |
| Date of Acceptance | 17 November 2012 |
| Indian J Pharm Sci., 2012, 74 (6): 505-511 |
Abstract
Mechanochemical activation is a practical cogrinding operation used to obtain a solid dispersion of a poorly water soluble drug through changes in the solid state molecular aggregation of drug-carrier mixtures and the formation of noncovalent interactions (hydrogen bonds) between two crystalline solids such as a soluble carrier, lactose, and a poorly soluble drug, indomethacin, in order to improve its solubility and dissolution rate. Samples of indomethacin and a physical mixture with a weight ratio of 1:1 of indomethacin and lactose were ground using a high speed vibrating ball mill. Particle size was determined by electron microscopy, the reduction of crystallinity was determined by calorimetry and transmission electron microscopy, infrared spectroscopy was used to find evidence of any interactions between the drug and the carrier and the determination of apparent solubility allowed for the corroboration of changes in solubility. Before grinding, scanning electron microscopy showed the drug and lactose to have an average particle size of around 50 and 30 μm, respectively. After high speed grinding, indomethacin and the mixture had a reduced average particle size of around 5 and 2 μm, respectively, showing a morphological change. The ground mixture produced a solid dispersion that had a loss of crystallinity that reached 81% after 30 min of grinding while the drug solubility of indomethacin within the solid dispersion increased by 2.76 fold as compared to the pure drug. Drug activation due to hydrogen bonds between the carboxylic group of the drug and the hydroxyl group of lactose as well as the decrease in crystallinity of the solid dispersion and the reduction of the particle size led to a better water solubility of indomethacin.
Keywords
Co-grinding, indomethacin, mechanochemical activation, noncovalent interactions, solid dispersion
A major obstacle that has prevented the commercialisation of many new promising, but poorly water soluble drugs is dissolution rate-limited oral bioavailability. The problem of poorly water soluble and low dissolution rate drugs has received extensive academic and industrial attention and numerous methods have been undertaken to enhance their dissolution and drug bioavailability. Examples of these methods include grinding or milling techniques [1], pH adjustment [2], cosolvency [3], inclusion-complexation [4] and micellisation combined with one of the methods [2,3,5], such as freeze-drying and cryogenic engineering [5,6], supercritical fluid processing [7], precipitation techniques [8] and solid dispersion formulations [9].
Currently, solid dispersions are one of the most promising strategies to improve the oral bioavailability of poorly water soluble drugs and they can be prepared by different methods and used to design amorphous or crystalline multicomponent systems [9,10]. With solid dispersions it is possible to choose a suitable carrier to modify the molecular mobility, relaxation times and intermolecular interaction [11]. Some of the first substances employed in solid dispersions were crystalline carriers such as urea and sugars [9]. These kinds of carriers produce crystalline or partially crystalline solid molecular dispersions, which are more thermodynamically stable than amorphous ones. For these reasons, more studies are necessary in order to obtain solid dispersions systems where drug and carrier particle size are reduced to a minimum and new noncovalent molecular interactions are formed. Hence, it is possible to improve drug wettability and optimal stability.
Actually, concepts of supramolecular chemistry and crystal engineering broaden the knowledge about specific intermolecular noncovalent interactions between molecules, such as hydrogen bonds, ionic, van der Waals and p?p interactions. These are responsible for molecular assemblies in multicomponent systems, comprised of a drug substance and a complementary neutral or charged molecule carrier [12]. These kinds of intermolecular interactions can be used in the design of amorphous or crystalline multicomponent systems and in the explanation of their structures. Etter set up a series of rules and guidelines for hydrogen bonding in crystals that apply to the design of molecular assemblies. The simplest of these rules states that all available proton donor and acceptor groups will be used in the hydrogen bond, noncovalent intermolecular patterns of most organic molecules in the crystalline state [13]. For instance, the carboxylic acid group of indomethacin forms a strong hydrogen bond with the more basic amide carbonyl group of polyvinylpyrrolidone (PVP) [14,15], and stabilizing effects are explained through the hydrogen bonding patterns of the PVP carrier on amorphous organic substances such as indomethacin in molecular dispersions [11].
Indomethacin was selected as a model drug because it is poorly soluble in water, a crystalline non-steroidal, antiinflammatory, analgesic and antipyretic drug. Indomethacin has also been frequently shown to have low bioavailability when administered orally. In the last few years, additional uses have been found for indomethacin as an effective agent for decreasing the risk of several types of cancer [16].
Meanwhile, lactose (L) is a widely used excipient in the manufacture of solid dosage forms because of its suitable properties, including hydrophilicity, low hygroscopicity, good compression and ease of purchase at a high quality [17]. Therefore, lactose was selected in order to design a dispersion with indomethacin, using a mechanochemical activation to obtain a minimum particle size and to increase the number of hydrogen bonds.
The term ?mechanochemical activation? is defined as the accumulation of defects (amorphisation or crystallinity loss) in solids, and occurs using different ground media [18]. In this process the mechanical energy is transferred to solid material surfaces, resulting in sufficient intensity to produce a local energy accumulation in submicroscopic zones. This creates a metastable structure that must release part of the accumulated energy to reach a more stable thermodynamic stage resulting in processes such as the propagation and interaction of dislocations, phase transformations and mechanochemical reactions such as the rupturing of chemical bonds. The main part of the supplied energy is converted into heat but the concentration of the strain at particular crystal sites produces crystal crushing and the formation of new surfaces. Furthermore, the energy supplied yields an accumulation of defects into the crystal and finally leads to complete amorphisation. The experimental verification of mechanochemical activation falls under the broad field of solid state characterization and there are many techniques that are useful for this purpose (X-ray, infrared, Raman spectroscopy, differential scanning calorimetry (DSC) and electron microscopy) [19]. The purpose of the present study was to obtain a multicomponent solid dispersion using a high speed vibrating ball mill over a crystalline bicomponent system of indomethacin and lactose (IL) whose main effect is to enhance drug solubility in water and thus, drug bioavailability. The changes were examined through calorimetric studies [20], electron microscopy, determination of the apparent solubility [21] and by observing changes in the infrared spectra [22].
Materials and Methods
Indomethacin 99% (Auzohu-Konch Pharmaceutica, Mexico) with an average particle size of 50 µm, lactose USP (Lactochem, Mexico), a Millipore membrane 0.45 µm, a Vibrating ball mill (Pen Walt S.S. White), a Ceramic ball mill (Erweka), Carver Press, a Microbalance Autobalance AD-4 (Perkin-Elmer), a DSC-7 (Perkin-Elmer), and UV-201 Spectrophotometers (Perkin-Elmer) and FTIR Spectrophotometers,Tensor 27 (Bruker) were used.
Preparation of the physical mixtures
Physical mixtures of IL were prepared by mixing equal weights of the drug and carrier and tumbling in a plastic receptacle for 10 min to assure a homogeneous mixture.
Preparation of the ground samples
Samples of indomethacin alone and the physical mixtures (IL) were ground in a porcelain ball mill (60 rpm) for about 4 h in order to compare with those which were ground in a vibrating ball mill at different times at 1700 rpm for 20 and 30 min (pure indomethacin, I20 and I30, and the physical mixtures IL20 and IL30, respectively). The properties assessed were particle size distribution, degree of crystallinity loss, changes in the infrared spectra and apparent solubility.
Characterization
The morphology and the size distribution of the particles were obtained through images of secondary electrons using the JEOL JSM-5600 scanning electron microscopy (SEM). The samples were placed on the surface of a bronze sample holder using double sided adhesive tape. The images were digitized and the size distribution was calculated from these images using the Imago Web for Windows and Digital Micrograph Processor as well as the Statistical Package for the Social Sciences (SPSS) software, version 12.0.
The degree of crystallinity loss was determined through a DSC process, where sample weights were kept within a range of 5-10 mg and the temperature from 50° to 250° at a constant rate of 10°/min in the presence of a nitrogen draft. This was further confirmed by the use of a high resolution transmission electron microscopy (TEM) using HF-2000FEG at 200 keV (Hitachi, Tokyo, Japan). The dry samples were collected on a standard lacey formvar coated electron microscope copper grid and images and diffracting patterns digitized. Fourier transform-infrared spectra (FTIR) were obtained on a system using the KBr disk method.
Solubility was evaluated from the dissolution of 10 mg of each sample in 100 ml of water at 37±0.5°, stirring at 60 rpm for a 2 h period, then filtering and measuring absorbance at a wavelength of 254 nm. Measurements were carried out in triplicate. By monitoring the dissolution for a period of 6 h, it was noticed that after the first 2 h, the value of the absorbance became constant, therefore, all other tests were carried out in 2 h periods.
Results
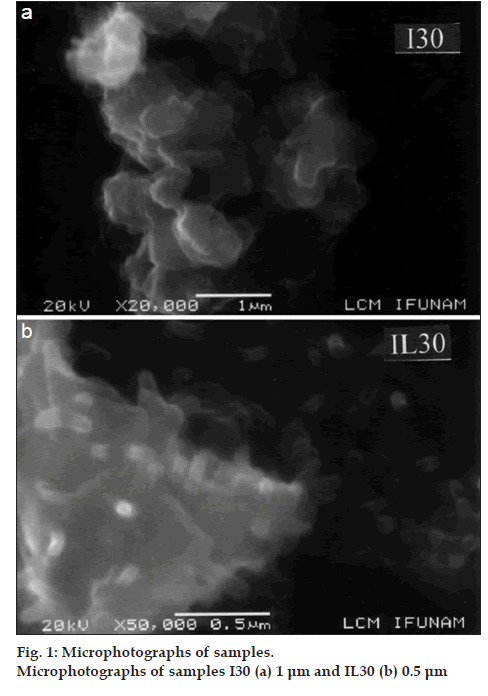
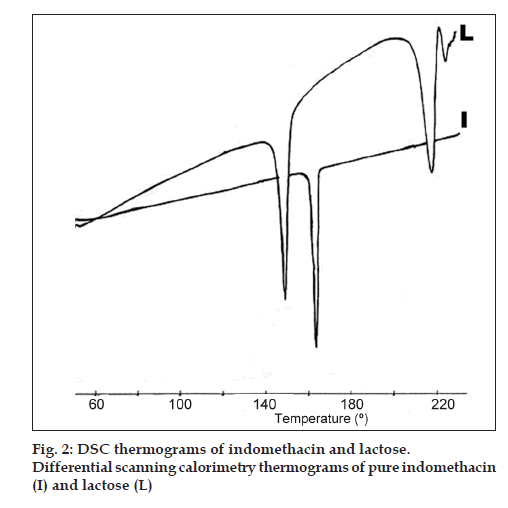
Fig. 1 shows the microphotographs of I30 and IL30, where the size of the aggregates are around 5 and 2 µm, respectively and morphology of both is different. In the first sample, the appearance is a flake like but the second one resembles a coral structure. Fig. 2 shows the thermograms of I and L, whose melting points were 162° and 219°, respectively. Another signal for the a form of lactose monohydrate appears at 151.3°, and it is associated to the loss of water.
The experiments were carried out using a common porcelain ball mill and there were no changes to the endotherm at 162° after 4 h, which are results obtained by Etman and Nada [23].
The loss of crystallinity for samples I20 and I30 was observed through the decrease in the endotherms around 162° by 30.6% and about 56%, respectively, compared to the endotherm of unground indomethacin.
Taylor and Zografi [24] worked only with indomethacin with similar equipment and reported a 36.5% loss of crystallinity at 30 min whereas with our equipment, the efficiency was 1.5 times higher in the same amount of time.
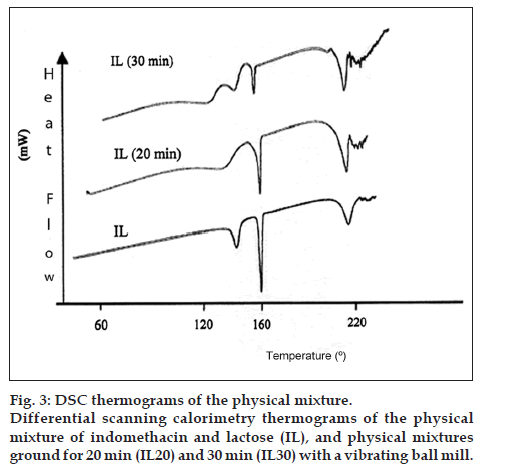
Fig. 3 shows the thermograms for the physical mixture (IL), as well as the samples ground for 20 and 30 min each (IL20 and IL30). The thermogram of sample IL20 shows a reduction of the peak around 162° resulting in a 43% loss of crystallinity for indomethacin while the endotherm at 151.3° belonging to lactose almost disappeared.
Sample IL30 presents a vitreous transition at 136°, two lactose endotherms at 151.7° and 209°, and a significant decrease of the area for the melting peak of indomethacin at 162°. This resulted in a loss of crystallinity of almost 81% compared to Etman and Nada [23] who reported a crystallinity loss of 63% using a ball mill for about 90 min.
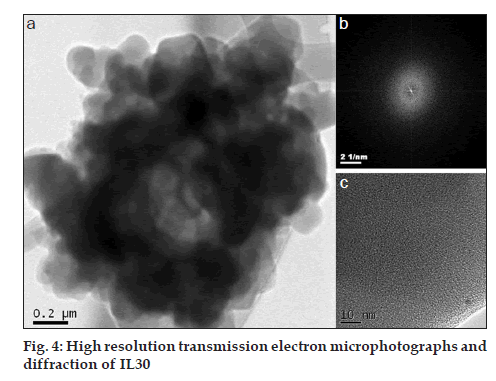
The IL30 has also been examined by transmission electron microscopy and electron diffraction. Both techniques demonstrate a high degree of crystallinity loss as proposed from the DSC analysis. Fig. 4a shows a bright field high resolution image of a grain of the IL30 sample. The 2 µm particles are made up of smaller nanoparticles of average 200 nm in average size, although there are smaller particles. Diffraction patterns of such particles were obtained, which clearly show the amorphous nature because broad rings were observed (fig. 4b), which was confirmed by image (fig. 4c).
The confirmation that we were dealing with IL30 was demonstrated by obtaining energy dispersive X-ray spectra, which clearly shows the presence of the Cl due to the indomethacin (fig. 5).
From the analysis of indomethacin FTIR spectrum (fig. 6, I), it can be observed that there are hydroxyl bands at 3371 cm-1 (free OH) and 2927_3350 cm-1 (OHassociated), two carbonyl bands at 1716 cm-1 (acid group) and 1692 cm-1 (amide group). Comparing the infrared spectrum of the physical mixture (fig. 6, IL) with that of lactose (fig. 6, L) it is clear that the bands of indomethacin are overlapped by those of lactose. In addition, no change in the carbonyl region was found. Therefore, there is no sign of an interaction in the physical mixture. On the contrary, with the ground indomethacin, a gradual diminution of the band is evident at 1716 cm-1 (fig. 6, I30), and practically disappears in the case of the mixture of indomethacinlactose that was ground for 30 min (IL30).
The data contained in Table 1 show that the apparent solubility of indomethacin improves in the physical mixture with lactose, almost doubling when compared to that of the pure drug sample indomethacin. Table 1 also shows a 2.22 fold increase when the indomethacin is ground for a 30 min period. Finally, a 2.76 fold increase is reached for the mixture of IL30 which was also ground for an equivalent period of 30 min.
| Sample | Absorbance | Solubility | Standard deviation |
|---|---|---|---|
| (254 nm) | (µg/ml) | ||
| I | 0.263 | 14.153 | 0.016 |
| IL | 0.518 | 29.062 | 0.005 |
| I30 | 0.559 | 31.461 | 0.007 |
| IL30 | 0.691 | 39.183 | 0.004 |
Table 1: Aparent Solubility of Samples in Water
Discussion
Analyses of the unground drug and lactose showed an average particle size of around 50 and 30 µm, respectively. After high speed grinding, indomethacin (I30) and the mixture IL30 had a reduced average particle size of around 5 and 2 µm, respectively, however, the latter showed cylindrical structures, which could increase the surface area and therefore, improve its wettability.
From the analysis of the thermograms, there is a gradual reduction of area of the endotherms at 162° (I20 and I30) observed in fig. 3. This could be interpreted as a change in the crystalline state of the particle because even though it is known that the grinding process reduces the particle size, this would increase the rate of dissolution, but not the solubility of a drug. As a matter of fact, the apparent solubility listed in Table 1, where solubility itself has increased, allowed us to corroborate that this is due to the destruction of the crystalline net of indomethacin instead of the presence of other polymorphic forms. This can be verified since those forms would appear at 134° and 154° and there are no signals in those regions [25].
In the case of the physical mixture, it is reasonable to expect that the improvement in the apparent solubility could be due only to the hydrophilic properties of lactose, because no change was found in the thermogram nor in the infrared spectrum of that sample (IL).
It can be seen from the thermogram of the mixture ground for 30 min (fig. 3) that the crystallinity loss was stronger than I30 (without lactose) and the vitreous transition as well as the endotherm at 209° could be an indication of interactions between indomethacin and lactose. Furthermore, our solid dispersions present a yellow colour which coincides with the observations of Cavallari et al. [21], who prepared tablets containing indomethacin, lactose and Polyethylene glycol with an ultrasound assisted compaction machine and the yellow colour of the tablets was due to the destruction of the crystal lattice.
Related to the interaction between the drug and the excipient which was strongly evidenced by the thermal analysis, it was important to determine its nature. In this case, the disappearance of the band in the infrared at 1716 cm-1 observed in fig. 6 (IL30) which was identified as an acid carbonyl stretch [22], supported the idea that hydrogen bonds formed between the acidic function of indomethacin and the OH groups of lactose.
This idea is in agreement with other studies, such as those conducted by Valizadeh et al. [10], who have observed similar changes in the FTIR spectra when they worked with indomethacin and dextrin. In addition, Taylor and Zografi [14] reported the hydrogen bond formation between PVP amide carbonyl and indomethacin carboxylic acid hydroxyl in the amorphous solid dispersion prepared by coprecipitation. Furthermore, Watanabe [26] proposed the interaction between indomethacin and the silanol groups in the ground mixture of the drug with SiO2.
It has been proven that the apparent solubility of the ground solid dispersion of indomethacin and lactose (IL30) increased almost 3 fold when compared to that of the pure indomethacin. This could be explain with the connection of two factors, first the crystallinity loss of indomethacin in presence of lactose, corroborated by the DSC thermograms and TEM. Thermal analysis also enabled the exclusion of polymorphism phenomena as a consequence of the mechanical treatment. Secondly, the number of interactions between the carboxyl group of indomethacin and the hydroxyl groups of lactose for the ground sample increased through the mechanoactivation using the vibrating ball mill.
Acknowledgements
We wish to thank José Reyes-Gasga, Eréndira García Ríos, Eileen Philips, Gabriela Salcedo, Antonio Ramírez and Lourdes Carrasco, for their technical assistance.
References
- Choi WS, Kim HI, Kwak SS, Chung HY, Yamamoto K, Oguchi T, et al. Amorphous ultrafine particle preparation for improvement of bioavailability of insoluble drugs: Grinding characteristics of fine grinding mills. Int J Miner Proc 2004;74:S165-72.
- Yalkowsky SH. Solubility and Solubilization in Aqueous Media. New York: Oxford University Press, USA; 1999.
- He Y, Tabibi SE, Yalkowsky SH. Solubilization of two structurally related anticancer drugs: XK-469 and PPA. J Pharm Sci 2006;95:97-107.
- Loftsson T, Magnúsdóttir A, Másson M, Sigurjónsdóttir JF. Self-association and cyclodextrin solubilization of drugs. J Pharm Sci 2002;91:2307-16.
- Rogers TL, Nelsen AC, Hu J, Brown JN, Sarkari M, Young TJ, et al. A novel particle engineering technology to enhance dissolution of poorly water soluble drugs: Spray-freezing into liquid. Eur J Pharm Biopharm 2002;54:271-80.
- Hu J, Rogers TL, Brown J, Young T, Johnston KP, Williams RO 3rd. Improvement of dissolution rates of poorly water soluble APIs using novel spray freezing into liquid technology. Pharm Res 2002;19:1278-84.
- Gong K, Viboonkiat R, Rehman IU, Buckton G, Darr JA. Formation and characterization of porous indomethacin-PVP coprecipitates prepared using solvent-free supercritical fluid processing. J Pharm Sci 2005;94:2583-90.
- Zhang X, Xia Q, Gu N. Preparation of all-trans retinoic acid nanosuspensions using a modified precipitation method. Drug Dev Ind Pharm 2006;32:857-63.
- Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today 2007;12:1068-75.
- Valizadeh H, Nokhodchi A, Qarakhani N, Zakeri-Milani P, Azarmi S, Hassanzadeh D, et al. Physicochemical characterization of solid dispersions of indomethacin with PEG 6000, Myrj 52, lactose, sorbitol, dextrin, and Eudragit E100. Drug Dev Ind Pharm 2004;30:303-17.
- Shalaev E, Zografi G. The concept of ?structure? in amorphous solids from the perspective of the pharmaceutical sciences. In, Levine H. Amorphous Food and Pharmaceutical Systems. Cambridge: The Royal Society of Chemistry; 2002. p. 11-30.
- Blagden N, de Matas M, Gavan PT, York P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv Drug Deliv Rev 2007;59:617-30.
- Etter MC. Hydrogen-bonds as design elements in organic chemistry. J Phys Chem 1991;95:4601-10.
- Taylor LS, Zografi G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm Res 1997;14:1691-8.
- Matsumoto T, Zografi G. Physical properties of solid molecular dispersions of indomethacin with poly (vinylpyrrolidone) and poly (vinylpyrrolidone-co-vinyl-acetate) in relation to indomethacin crystallization. Pharm Res 1999;16:1722-8.
- Ackerstaff E, Gimi B, Artemov D, Bhujwalla ZM. Antiinflammatory agent indomethacin reduces invasion and alters metabolism in a human breast cancer cell line. Neoplasia 2007;9:222-35.
- Sipos E, Szekely P, Redai E. Lactose, as pharmaceutical excipient. II. Influence of lactose type on tablet properties. Farmacia 2007;55:88-92.
- Colombo I, Grassi G, Grassi M. Drug mechanochemical activation. J Pharm Sci 2009;98:3961-86.
- Bugay DE. Characterization of the solid-state: Spectroscopic techniques. Adv Drug Deliv Rev 2001;48:43-65.
- Legendre B, Feutelais Y. Polymorphic and thermodynamic study of indomethacin. J Therm Anal Calorim 2004;76:255-64.
- Cavallari C, Albertini B, Rodriguez L, Rabasco AM, Fini A. Release of indomethacin from ultrasound dry granules containing lactose-based excipients. J Control Release 2005;102:39-47.
- Lin SY, Cherng JY. Polymorphic transformation of indomethacin in precirol solid dispersion system. J Therm Anal 1995;45:1565-77.
- Etman MA, Nada AH. Evaluation of simple and ball mill ground mixtures of two NSAIDs with different hydrophilic carriers. Alex J Pharm Sci 1999;13:135-40.
- Taylor LS, Zografi G. The quantitative analysis of crystallinity using FT-Raman spectroscopy. Pharm Res 1998;15:755-61.
- Del Rio LA. Preformulation study of the development of polycrystalline indomethacin in tablets. Ars Pharm 2002;43:147-71.
- Watanabe T, Wakiyama N, Usui F, Ikeda M, Isobe T, Senna M. Stability of amorphous indomethacin compounded with silica. Int J Pharm 2001;226:81-91.