| Date of Submission | 28 July 2011 |
| Date of Revision | 10 August 2012 |
| Date of Acceptance | 19 August 2012 |
| Indian J Pharm Sci, 2012, 74 (4): 365-367 |
Abstract
The present manuscript describes a simple, sensitive, accurate, precise and economical visible spectrophotometric method for the estimation of azithromycin from tablet formulation. The method is based on the reduction of potassium permanganate in alkaline medium with azithromycin. The measurement of decrease in absorbance of potassium permanganate at 547 nm was done, as it decolourises upon reduction by azithromycin. The method was used to determine between 2 and 20 μg/ml of azithromycin in the final measured solution. There is no interference from the ingredients commonly found in azithromycin tablets with this method. The results for the determination of azithromycin in tablets were in good agreement with the labelled quantities and related analytical parameters are calculated.
Keywords
Azithromycin, potassium permanganate, spectrophotometry, tablets
Azithromycin, chemically, (2R,3S,4R,5R,8R, 10R,11R,12S,13S,14R)-2-ethyl-3,4,10-trihydroxy- 3,5,6,8,10,12,14-heptamethyl-15-oxo-11-{ [3,4,6- trideoxy-3-(dimethylamino)-β-D-xylo-]oxy}-1- oxa-6-azacyclopentadec-13-yl 2,6-dideoxy-3-Cmethyl- 3-O-methyl-α-L-ribo-hexopyranoside is a semisynthetic macrolide antibiotic widely used in the treatment of respiratory tract infections, such as pharyngitis, pneumonia, chronic bronchitis and bronchopneumonia. The most commonly used techniques for the determination of azithromycin in pharmaceutical dosage forms are high performance liquid chromatography [1,2], liquid chromatography-mass spectrometry [3], microbiological [4], differential pulse voltammetric [5-7], amperometric [8] diffuse reflectance near infrared spectroscopy [9] and spectrophotometric methods [10-14].
However, chromatographic techniques require long experimental procedures for sample clean up and demand expensive equipment. In differential pulse voltammetric method, the adsorption of the drug on the electrode surface has not been sufficiently strong and hence it has not been analytically useful. Lakshmi [10] have reported a visible spectrophotometric method for the determination azithromycin in tablets is not specific. Most of the reported methods are highly sophisticated, costly and time-consuming.
The objective of the present work was to develop a simple spectrophotometric method for the determination of azithromycin in pharmaceutical formulations. The present procedure neither requires any extraction nor heating nor any elaborate equipment and the method is less time-consuming.
Azithromycin was kindly supplied by Max India’s Pharmaceutical Division, Nanjanagud, Mysore. Azee (azithromycin; 500 mg/tablet and 50 mg/tablet) of Cipla Limited, Goa, India. Azid kid (Azithromycin; 100 mg/tablet) of Indi Pharma Pvt. Ltd., Mumbai, India and Aziwin (azithromycin; 100 mg/tablet) of Bal Pharma Ltd., Rudrapura, India were purchased from local market. Potassium permanganate and anhydrous potassium carbonate were procured from Glaxo Ltd., Mumbai, India and were of analytical grade. UV/Vis spectrophotometer with 10-mm matched quartz cells were used for absorbance measurement.
Standard stock solution of azithromycin (10 μg/ml) was prepared by accurately weighing and dissolving 10 mg of azithromycin in distilled water and diluted to 100 ml. Potassium permanganate (0.0012 mol/l) was prepared by dissolving 0.02 g of potassium permanganate in distilled water and diluted to 100 ml. Potassium carbonate (0.1 mol/l) was prepared by dissolving 1.3831 g of potassium permanganate in distilled water and diluted to 100 ml.
Aliquots of the standard azithromycin containing 2-20 μg/ml were transferred to a series of 10 ml volumetric flasks. To each one of these flasks, 1.0 ml potassium permanganate (0.01 M) followed by 1.0 ml of potassium carbonate were added. The volume was made up to 10 ml with water and mixed thoroughly. Absorbance of these solutions was measured at 547 nm after 30 min making zero absorbance with distilled water.
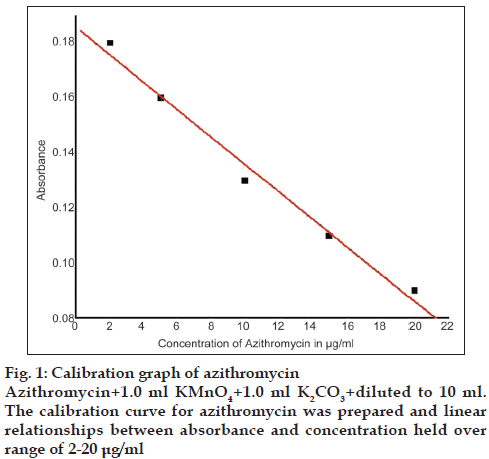
The calibration curve for azithromycin was prepared by the recommended procedure as shown in fig. 1. Linear relationships between absorbance and concentration held over a range of 2-20 μg/ml and other parameters are given in Table 1.
| Parameters | Azithromycin |
|---|---|
| λmax, nm | 547 |
| Beer’s limit (μg/ml) | 2‑20 |
| Molar absorptivity (l/mol/cm) | 2.1994×104 |
| Sandell’s sensitivity (μg/cm2/0.001 A) | 0.03405 |
| Correlation coefficient | -0.99343 |
| Regression equation | |
| Slope (b) | -0.00496 |
| Intercept (a) | 0.1856 |
| Coefficient of variance (10 μg/ml of Azithromycin) | 0.64 (N=5) |
| Relative standard deviation (%) | 0.612 |
Optical characteristics for azithromycin such as Beer’s law, molar absorptivity and sandell’s sensitivity are shown
Table 1: Optical Characteristics Of The Proposed Procedure
No interference was observed from the presence of starch, lactose and most commonly used tablet excipients. For analysis of the formulation, 10 tablets of Azithromycin were weighed accurately and average weight per tablets was determined. The tablets were powdered and powder equivalent to 10 mg was weighed accurately and dissolved in water. The residue was filtered through Whatman Filter Paper No. 41 into a 100 ml volumetric flask. The residue was washed with distilled water and washings were added to the filtrate. Final volume of filtrate was made up to the mark with distilled water and was analysed according to the recommended procedure. Results were in good agreement with the label claim of the drug. The amount and relative standard deviation (RSD) (%) for the drugs azee of 500 mg and 250 mg, Azid Kid 100 mg and aziwin 100 mg and 250 mg were found to be 500.05, 0.15 and 250.25, 0.30, 100.6, 0.54, and 100.0, 0.70 and 250.12, 0.34, respectively.
The method is based on the reduction of potassium permanganate in alkaline medium with azithromycin. Permanganate decolourises as it is reduced quantitatively to manganese dioxide in alkaline solution. The colour is stable for about 1 h. The result of the analysis of tablet formulation by the proposed method is reproducible, reliable and in good agreement with the label claim of the drug. Method was validated and found to be simple, sensitive, accurate and precise. The excipients present in the tablet dosage form did not interfere with determination of azithromycin. Hence, this method can be used successfully for the routine analysis of azithromycin in tablet formulation.
References
- Shaikh KA, Patil SD, Devkhile AB. Development and validation of a reversed-phase HPLC method for simultaneous estimation of ambroxol hydrochloride and azithromycin in tablet dosage form. J Pharm Biomed Anal 2008;48:1481-4.
- Yang ZY, Wang L, Tang X. Determination of azithromycin by ion-pair HPLC with UV detection. J Pharm Biomed Anal 2009;49:811-5.
- Debremaeker D, Visky D, Chepkwony HK, Van Schepdael A, Roets E, Hoogmartens J. Analysis of unknown compounds in azithromycin bulk samples with liquid chromatography coupled to ion trap mass spectrometry. Rapid Commun Mass Spectrom 2003;17:342-50.
- Breier AR, Garcia CV, Oppe TP, Steppe M, Schapoval EE. Microbiological assay for azithromycin in pharmaceutical formulations. J Pharm Biomed Anal 2002;29:957-61.
- Nigović B. Adsorptive stripping voltammetric determination of azithromycin at a glassy carbon electrode modified by electrochemical oxidation. Anal Sci 2004;20:639-43.
- Farghaly OA, Mohamed NA. Voltammetric determination of azithromycin at the carbon paste electrode. Talanta 2004;62:531-8.
- Nigović B, Simunić B. Voltammetric assay of azithromycin in pharmaceutical dosage forms. J Pharm Biomed Anal 2003;32:197-202.
- Palomeque ME, Ortíz PI. New automatized method with amperometric detection for the determination of azithromycin. Talanta 2007;72:101-5.
- Ji XD, Wen BZ, Yan CF, Dan QS, Chang QH. Quantitative calibration models for the determination of azithromycin and decladinosylazithromycin in azithromycin injection powders using diffuse reflectance near infrared spectroscopy. J Near Infrared Spectrosc2011;19:265-75.
- Lakshmi S, Arul MM, Jayashankar L, Ramu P, Raja TK. Visible spectrophotometric methods for the determination of azithromycin in tablets. Indian J Pharm Sci 2004;66:249-51.
- Rachidia M, Elhartia J, Diguab K, Cherraha Y, Bouklouzea A. New spectrophotometric method for azithromycin determination. Anal Lett 2006;39:1917-26.
- Carlos ER, Vanessa GK, Ricardo CJ. Spectrophotometric method for the determination of azithromycin in pharmaceutical formulations basedon its charge transfer reaction with quinalizarin. J BrazChemSoc 2010;21:1664-71.
- Sultana N, Arayne MS, Hussain F, Fatima A. Degradation studies of azithromycin and its spectrophotometric determination in pharmaceutical dosage forms. Pak J Pharm Sci 2006;19:98-103.
- Rufino JL, Pezza HR, Pezza L. Flow-injection spectrophotometric determination of azithromycin in pharmaceutical formulations using p-chloranil in the presence of hydrogen peroxide. Anal Sci 2008;24:871-6.