- *Corresponding Author:
- I. Singhvi
Department of Pharmaceutical Sciences, M. L. Sukhadia University, Udaipur - 313 001, India
E-mail: indrajeet_s@yahoo.com
| Date of Submission | 24 June 2006 |
| Date of Revision | 21 March 2007 |
| Date of Acceptance | 17 August 2007 |
| Indian J Pharm Sci, 2007, 69 (4): 601-604 |
Abstract
Three simple, accurate, economical and reproducible UV spectrophotometric methods for simultaneous estimation of two component drug mixture of ranitidine hydrochloride and ondansetron hydrochloride from combined tablet dosage form have been developed. First developed method involves formation and solving of simultaneous equations at 267.2 nm and 314.4 nm. Second method was developed making use of first order derivative spectroscopy using 340.8 nm and 276.0 nm as zero crossing points for estimation of ranitidine hydrochloride and ondansetron hydrochloride respectively. Third method is based on two wavelength calculation, wavelengths selected for estimation of ranitidine hydrochloride were 266.1 nm and 301.8 nm and for ondansetron hydrochloride 305.7 nm and 319.2 nm. The results of analysis have been validated statistically and by recovery studies.
Ranitidine hydrochloride, chemically 1,1- ethenediamine-N- [2- [ [ [5- [(dimethylamino)methyl]- 2-furanyl]-methyl]thio]ethyl]-N’-methyl-2-nitro hydrochloride is an H2-receptor antagonist indicated for the duodenal ulcer [1]. Literature survey reveals that for ranitidine hydrochloride HPLC [3,4], spectrophotometric [5] and capillary electrophoresis [6,7] methods have been reported for its determination from human plasma and commercial formulation. Ondansetron hydrochloride, chemically 4H-carbazol-4-one-1,2,3,9-tetrahydro- 9-methyl-3- [(2-methyl-1H-imidazole-1-yl) methyl] hydrochloride is a selective 5-HT3 receptor antagonist indicated for the prevention of nausea and vomiting [2]. Three HPLC [8-10] and one LC [11] methods have been reported in literature for estimation of ondansetron hydrochloride from human plasma and commercial formulation. However no spectrophotometric method is yet reported for simultaneous analysis of two drugs from combined pharmaceutical dosage form.
A systronics UV/Vis spectrophotometer (model 2101) with 1 cm matched quartz cells was used for spectrophotometric analysis. Spectra were recorded using specific program of instrument, having specifications as, spectral band width 2 nm, wavelength accuracy ±0.5 nm, wavelength readability 0.1 nm increment. Double distilled water was used for the preparation of 0.1N hydrochloric acid. The tablet samples of combined dosage form of ranitidine hydrochloride and ondansetron hydrochloride [Ranidom-O (Mankind Laboratories, New Delhi), Doran-O (Bestochem Formulation Ltd, Delhi), Rani- O (Prime Life Pharmaceuticals, New Delhi)] were procured from the local market.
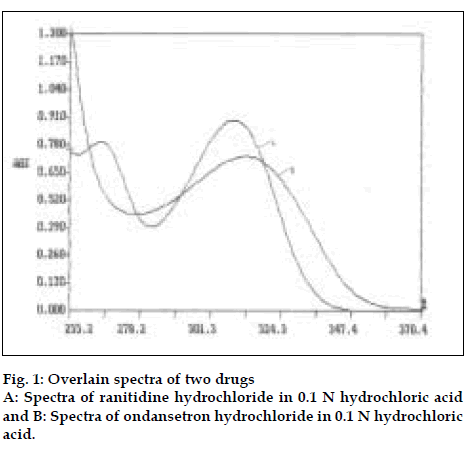
For method I pure drug sample of ranitidine hydrochloride and ondansetron hydrochloride were dissolved separately in 0.1 N hydrochloric acid so as to give six dilutions of standard in concentration range of 50-500 µg/ml of ranitidine hydrochloride and 2-30 µg/ml of ondansetron hydrochloride. All solutions were scanned in wavelength range of 220 nm and 380 nm. Fig. 1 represents the overlain spectra of ranitidine hydrochloride and ondansetron hydrochloride in 0.1N hydrochloric acid. Two wavelengths selected for formation and solving of simultaneous equations were 267.2 nm and 314.4 nm. Absorptivity coefficients of both the drugs were determined at selected wavelengths. Absorptivity coefficient for ranitidine hydrochloride at 267.2 nm and 314.4 nm were 25.32 and 35.78 cm-1g-1 l, while respective values for ondansetron hydrochloride were 461.0 and 481.66 cm-1 g-1 l. Set of two simultaneous equations thus formed are A1= 461.0C1± 25.32C2 and A2= 481.66C1±35.78C2, where A1 and A2 are absorbance of sample solution at 267.2 nm and 314.4 nm, respectively. C1 and C2 are concentration of ranitidine hydrochloride and ondansetron hydrochloride respectively in sample solution in g/l. Validity of above formed equations was checked by preparingfive mixed standards using pure drug sample of two drugs, results of which are reported in Table 1.
| Sample No. | Conc present (mcg/ml) | % Conc. Found | ||||||
|---|---|---|---|---|---|---|---|---|
| Method I | Method II | Method III | ||||||
| RDN | OST | RDN | OST | RDN | OST | RDN | OST | |
| 01 | 500 | 04 | 99.96 | 98.75 | 101.36 | 98.44 | 99.82 | 99.12 |
| 02 | 400 | 08 | 101.60 | 102.12 | 101.25 | 98.34 | 98.29 | 98.48 |
| 03 | 300 | 12 | 100.38 | 100.30 | 98.16 | 96.90 | 98.96 | 98.72 |
| 04 | 200 | 16 | 101.72 | 100.29 | 100.71 | 98.34 | 100.20 | 100.45 |
| 05 | 100 | 20 | 99.60 | 98.94 | 99.78 | 98.34 | 99.02 | 98.75 |
RDN denotes ranitidine hydrochloride and OST is ondansetron hydrochloride
Table 1: Results of validation studies for method I, II & III using mixed standards
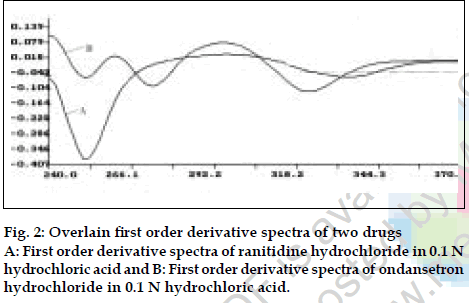
Twenty tablets were accurately weighed and average weight per tablet was determined. Tablets were ground to fine powder and tablet powder equivalent to 150 mg of ranitidine hydrochloride was accurately weighed and extracted four times with 20 ml portions of 0.1 N hydrochloric acid andfiltered through Whatmanfilter paper no. 41 in to a 100 ml volumetric flask, filter paper was washed with 0.1 N hydrochloric acid adding washings to thefilterate and volume was made up to the mark with the same. From the above filterate 1 ml was further diluted to 10 ml with 0.1N hydrochloric acid. Absorbance of this final diluted solution was measured at 267.2 nm and 314.4 nm respectively and concentration of two drugs in the sample were calculated using the simultaneous equations. Results of analysis of tablet formulation are reported in Table 2. The second developed method isfirst order derivative spectroscopy. From first order derivative spectra of ranitidine hydrochloride and ondansetron hydrochloride in 0.1 N hydrochloric acid (fig. 2) zero crossing points 340.8 nm and 276.0 nm were selected for simultaneous estimation of two drugs. Accurately weighed pure drug sample of ranitidine hydrochloride and ondansetron hydrochloride were dissolved in 0.1 N hydrochloric acid so as to give six dilutions in concentration range of 50-500 µg/ml for ranitidine hydrochloride and 2-30 µg/ml for ondansetron hydrochloride. The absorbance of these solutions was recorded in first derivative mode at 340.8 nm for estimation of ranitidine hydrochloride and 276.0 nm for estimation of ondansetron hydrochloride and respective calibration curves were prepared. Validity of proposed method was checked by preparingfive mixed standards using pure drug sample of two drugs and absorbance was measured at respective selected zero crossing points and determined concentration of two drugs using respective calibration curve. Results of validation studies are reported in Table 1.
| Method | Brand | Label claim (mg/tab) | % of label claim estimated* | Standard deviation | % Recovery** | ||||
|---|---|---|---|---|---|---|---|---|---|
| RDN | OST | RDN | OST | RDN | OST | RDN | OST | ||
| Method I | A | 150 | 04 | 99.77 | 99.58 | 0.464 | 0.542 | 99.65 | 99.02 |
| B | 150 | 04 | 98.93 | 100.23 | 0.542 | 0.621 | 98.98 | 99.23 | |
| C | 150 | 04 | 99.22 | 101.04 | 0.422 | 0.508 | 99.29 | 99.11 | |
| Method II | A | 150 | 04 | 99.48 | 99.12 | 0.489 | 0.512 | 100.11 | 99.58 |
| B | 150 | 04 | 100.58 | 98.82 | 0.528 | 0.488 | 99.66 | 101.13 | |
| C | 150 | 04 | 100.62 | 98.97 | 0.569 | 0.501 | 101.21 | 100.72 | |
| Method III | A | 150 | 04 | 99.83 | 99.22 | 0.569 | 0.603 | 98.62 | 100.19 |
| B | 150 | 04 | 99.25 | 99.29 | 0.572 | 0.452 | 99.28 | 98.92 | |
| C | 150 | 04 | 100.01 | 98.94 | 0.621 | 0.585 | 98.98 | 99.44 | |
RDN denotes ranitidine hydrochloride and OST is ondansetron hydrochloride. *Average of three determinations and **Average of determination at three different concentration levels. Brand A is Ranidom-O, Brand B is Doran-O and Brand C is Rani-O
Table 2: Results of analysis of commercial formulation
Tablet sample solution was prepared in similar manner as for method I, absorbance offinal sample solution was recorded at 340.8 nm and 276.0 nm from first derivative spectra of sample and amount of two drugs were calculated using respective calibration curve. Results of analysis are reported in Table 2.
The third developed method is two wavelength calculation method. From absorption spectra of ranitidine hydrochloride and ondansetron hydrochloride (fig.1), set of two wavelengths λ1 (266.1 nm) and λ2 (301.8 nm) for estimation of ranitidine hydrochloride and λ3 (305.7 nm) and λ4 (319.2 nm) for estimation of ondansetron hydrochloride were selected on basis of principle that absorbance difference between two points on a mixture spectra is directly proportional to concentration of component of interest and independent of interfering component [12]. Five mixed standards containing different concentration of pure drug sample of two drugs were prepared in 0.1 N hydrochloric acid. All standards were scanned at respective set of selected wavelengths. Absorbance difference was measured and respective calibration curve was plotted.
Tablet sample solution was prepared in similar manner as for method I, final sample solution was analyzed by scanning at respective set of wavelength and absorbance difference values were noted, the concentration of ranitidine hydrochloride and ondansetron hydrochloride was calculated from the respective calibration curve. Result of analysis is reported in Table 2.
To study the accuracy, reproducibility and precision of the above developed methods recovery studies were carried out by addition 0.5, 1.0 and 1.5 ml of standard drug stock solution (100 µg/ml) to pre-analyzed tablet sample solutions. Results of recovery studies were found to be satisfactory and are reported in Table 2.
Three spectrophotometric methods have been developed for simultaneous estimation of ranitidine hydrochloride and ondansetron hydrochloride from combined tablet dosage form. The first developed method involving formation and solving of simultaneous equations is very simple and requires only the accurately determined absorptivity of the two drugs at two selected wavelengths. The method just requires recording of absorbance and few calculations that can be manually done, thus method can be used with any model of spectrophotometer. Once the equations are framed the method is very fast. Framed equations were validated using laboratory prepared mixed standards of two drugs which gave satisfactory results.
Second developed method for simultaneous analysis of ranitidine hydrochloride and ondansetron hydrochloride from combined dosage form make use of first derivative ultraviolet spectrophotometry based on principle that at zero crossing point of one component the other component have substantial absorbance.
Third developed method for simultaneous analysis of ranitidine hydrochloride and ondansetron hydrochloride make use of two wavelength calculation so as to remove interference between two components. Proper selection of two wavelengths for estimation of a component is critical.
The results of analysis of two drugs from tablet formulation using all the three developed methods were found close to 100% for both ranitidine hydrochloride and ondansetron hydrochloride, standard deviation was satisfactorily low indicating accuracy and reproducibility of the methods. Recovery studies were satisfactory which shows that there is no interference of excipients. The developed methods were found to be simple, rapid, accurate and can be used for routine analysis of two drugs from tablet formulations.
References
- Keith GT, In; Gennaro, AR. eds., Remington: The Science and Practice of Pharmacy, 20thEdn., Vol. II, Maryland, USA: Lippincott Williams and Wilkins; 2000, p. 1225.
- Keith GT, In; Gennaro, AR. Eds., Remington: The Science and Practice of Pharmacy, 20th Edn., Vol. II, Maryland, USA: Lippincott Williams and Wilkins; 2000, p.1236.
- Ho C, Huang HM, Hsu SY, Shaw CY, Chang BL. Simultaneous HPLC analysis for famotidine ranitidine hydrochloride, cimetidine nizatidine in commercial products. Drug Develop Ind Pharm 1999;25:379-85.
- Kanumula GV, Bhanu R. Simultaneous determination of ranitidine hydrochloride and domperidone in pharmaceutical dosage by RP-HPLC. Indian Drugs 2000;37:375-78.
- Walash M, Sharaf-El DM, Metwalli ME, RedaShabana M. Spectrophotometric determination of nizatidine and ranitidine through charge transfer complex formation. Arch Pharm Res 2004;27:720-6.
- Wu SM, Ho YH, Wu HL, Chen SH, Ko HS. Simultaneous determination of cimetidine famotidine nizatidine and ranitidine in tablets by capillary zone electrophoresis. Electrophoresis. 2001;22:2758-62.
- Wu SM, Ho YH, Wu HL, Chen SH, Ko HS. Head column field- amplified sample stacking in capillary electrophoresis for the determination of cimetidine famotidine nizatidine and ranitidine hydrochloride in plasma. Electrophoresis. 2001;22:2717-22.
- Liu J, Stewart J T. HPLC analysis of ondansetron enantiomers in human serum using a reversed-phase cellulose-based chiral stationary phase and solid-phase extraction. J Chromatogr B Biomed Sci Appl. 1997;694:179-84.
- Kelly JW, He L, Stewart JT. HPLC separation of ondansetron enantiomers in serum using cellulose derivatized stationary phase and solid phase extraction. J Chromatogr. 1993;622:291-5.
- Bauer S, Stormer E, Kaiser R, Tremblay PB, Brockmoller J, Roots I. Simultaneous determination of ondansetron and tropisetron in human plasma using HPLC with UV detection. Biomed Chromatogr 2002; 16:187-90.
- Depot M, Leroux S, Caille G. High resolution liquid chromatographic method using ultraviolet detection for determination of ondansetron in human plasma. J Chromatogr B Biomed SciAppl 1997;693:399-06.
- Singhvi I. Spectrophotometric and HPLC methods for simultaneous estimation of nimesulide and paracetamol from tablets. Philippine Journal of Science 2002;131:59-64.