- Corresponding Author:
- Galvina Ferreira
Department of Pharmaceutical Science and Technology, Medicinal Natural Products Research Laboratory, Institute of Chemical Technology, Mumbai–400 019, India
E–mail: galvinaferr@gmail.com
| Date of Submission | 28 December 2012 |
| Date of Revision | 28 March 2013 |
| Date of Acceptance | 30 March 2013 |
| Indian J Pharm Sci 2013;75(2):246-250 |
Abstract
Embelin, a naturally occurring benzoquinone, is obtained from fruits of Embelia ribes, which plays a vital role in the Ayurvedic system of medicine. The benzoquinone has diverse pharmacological and therapeutic properties and now constitutes a part of modern medicine. The aim of our study was to observe the effect of various stress conditions on this potential drug candidate. Embelin was subjected to stress conditions of acid and alkaline hydrolysis, oxidation, photolysis and thermal degradation. A significant degradation was found to occur by acid hydrolysis, oxidation and to a lesser extent under thermal stress and alkaline hydrolysis; the compound was found to be stable to photolytic stress. Further, the degradation product resulting from the acid hydrolysis was isolated and its structure was elucidated using spectroscopic techniques. Stress degradation studies on embelin provide an insight for its stability and storage considering the formulation aspects of modern medicine.
https://www.mobafire.com/profile/mavituronline12-1123034?profilepage https://www.youmagine.com/mavituronline12/designs https://www.myminifactory.com/users/Mavituronline12 http://qooh.me/Mavituronline12 https://linktr.ee/mavituronline12 https://pubhtml5.com/homepage/jhvxj/ https://telegra.ph/Mavituronline12-09-26 https://www.diggerslist.com/65128797aea49/about https://allmyfaves.com/Mavituronline12 https://www.metal-archives.com/users/Mavituronline12 https://www.catchafire.org/profiles/2507511/ https://www.fimfiction.net/user/640194/Mavituronline12 https://www.hebergementweb.org/members/mavituronline12.558486/ https://www.sqlservercentral.com/forums/user/mavituronline12 https://www.twitch.tv/mavituronline12/about https://www.roleplaygateway.com/member/Mavituronline12/ https://www.provenexpert.com/mavituronline12/?mode=preview https://www.intensedebate.com/people/Mavituronline12 https://www.indiegogo.com/individuals/35343393 https://visual.ly/users/mavituronline/portfolio https://slides.com/mavituronline12 https://letterboxd.com/mavituronline/ https://fliphtml5.com/dashboard/public-profile/cfres https://community.windy.com/user/mavituronline12 https://speakerdeck.com/mavituronline12 https://trello.com/u/mavituronline https://myanimelist.net/profile/Mavituronline12 https://3dwarehouse.sketchup.com/user/8e8085e4-3ac1-4dc0-a599-cfe9385842dd/Mavituronline12-M https://www.wattpad.com/user/Mavituronline12 https://www.goodreads.com/user/show/170217678-mavituronline12 https://gravatar.com/mavituronline https://tr.pinterest.com/mavituronline/ https://www.mapleprimes.com/users/Mavituronline12 https://medium.com/@mavituronline https://www.ted.com/profiles/45143312 https://www.infragistics.com/community/members/c4e29067eaf88a9857b8317d72e3aa1a43d33c4f?_ga=2.231154591.126888509.1695731800-1741928506.1695731800 https://www.metooo.io/u/6512d2c4dbe9bd71f3ca39e5 https://app.roll20.net/users/12465974/mavituronline12-m https://list.ly/mavituronline/activity https://giphy.com/channel/Mavituronline12 https://www.tumblr.com/mavituronline12 https://www.creativelive.com/student/https-mavitur-online?via=accounts-freeform_2 https://gab.com/Mavituronline12 https://sketchfab.com/Mavituronline12 https://www.flickr.com/people/199202082@N05/ https://profiles.wordpress.org/mavituronline12/ https://seedandspark.com/user/mavituronline12-01hb8vvn1x0tpyer92wwwam5xn https://os.mbed.com/users/mavituronline12/ https://calis.delfi.lv/blogs/lietotajs/316255-mavituronline12/ https://notionpress.com/author/929887# https://my.desktopnexus.com/Mavituronline12/ https://guides.co/a/mavituronline12-mavituronline12/ https://www.bahamaslocal.com/userprofile/1/246688/Mavituronline12.html http://molbiol.ru/forums/index.php?showuser=1300548 https://www.credly.com/users/mavituronline12-mavituronline12/badges https://ko-fi.com/mavituronline12#paypalModal https://devpost.com/mavituronline?ref_content=user-portfolio&ref_feature=portfolio&ref_medium=global-nav https://www.redbubble.com/people/Mavituronline12/shop?asc=u https://dzone.com/users/4999874/mavituronline12.html https://hub.docker.com/u/mavituronline12 http://onlineboxing.net/jforum/user/editDone/252908.page https://micro.blog/Mavituronline12 http://www.effecthub.com/user/3728420 http://hawkee.com/profile/5081670/ https://wefunder.com/mavituronline12 https://www.facer.io/user/qrNVyeaUK4 https://mastodon.online/@Maviyolculukonline12 https://www.mobafire.com/profile/bestbluecruises12-1124248?profilepage http://hawkee.com/profile/5122480/ https://www.youmagine.com/bestbluecruises12/designs https://www.myminifactory.com/users/Bestbluecruises12 http://qooh.me/Bestblue https://linktr.ee/bestbluecruises12 https://pubhtml5.com/homepage/mrus/ https://telegra.ph/Bestbluecruises12-10-04 https://www.diggerslist.com/651d773cbfbca/about https://allmyfaves.com/Bestbluecruises12 https://www.catchafire.org/profiles/2515198/ https://www.fimfiction.net/user/642880/Bestbluecruises12 https://www.hebergementweb.org/members/bestbluecruises12.561718/ https://www.sqlservercentral.com/forums/user/bestbluecruises12 https://www.twitch.tv/bestbluecruises12/about https://www.roleplaygateway.com/member/Bestbluecruises12/ https://www.provenexpert.com/bestbluecruises12/?mode=preview https://www.intensedebate.com/people/Bestbluee https://www.indiegogo.com/individuals/35426872 https://visual.ly/users/bestbluecruises/portfolio https://slides.com/bestbluecruises12 https://letterboxd.com/bestbluecruises/ https://fliphtml5.com/dashboard/public-profile/szzti https://community.windy.com/user/bestblue https://speakerdeck.com/bestbluecruises12 https://trello.com/u/bestbluecruises https://myanimelist.net/profile/Maviyolculukk https://3dwarehouse.sketchup.com/user/6f22bb61-99b2-48dc-939f-f42b9037d63a/Bestbluecruises12-B https://www.wattpad.com/user/Bestbluecruises12 https://www.goodreads.com/user/show/170441502-bestbluecruises12 https://gravatar.com/bestbluecruises https://tr.pinterest.com/bestbluecruises/ https://www.mapleprimes.com/users/Bestbluecruises12 https://medium.com/@bestbluecruises https://www.ted.com/profiles/45215119 https://www.infragistics.com/community/members/97e3a88b756cbc4c0db6d564997726d6beef80c7?_ga=2.57077487.4540886.1696435593-1559734635.1696435593 https://www.metooo.io/u/651d8e14119b751a01c959a9 https://app.roll20.net/users/12496984/bestbluecruises12-b https://list.ly/bestbluecruises/activity https://giphy.com/channel/Bestbluecruises12 https://www.tumblr.com/bestbluecruises12 https://www.creativelive.com/student/bestbluecruises12?via=accounts-freeform_2 https://gab.com/Bestbluecruises12 https://sketchfab.com/Bestbluecruises12 https://www.flickr.com/people/199269439@N03/ https://seedandspark.com/user/bestbluecruises12-01hbzzqmzcr9f9k2dpwfh46sjx https://os.mbed.com/users/bestbluecruises12/ https://calis.delfi.lv/blogs/posts/215055-httpsbestbluecruisescom/lietotajs/317705-bestbluecruises12/ https://notionpress.com/author/933125 https://my.desktopnexus.com/Bestbluecruises12/ https://guides.co/a/bestbluecruises12-bestbluecruises12/ https://www.bahamaslocal.com/userprofile/1/249561/Bestbluecruises12.html http://molbiol.ru/forums/index.php?showuser=1302017 https://www.credly.com/users/bestbluecruises12-bestbluecruises12/badges https://ko-fi.com/bestbluecruises12#paypalModal https://devpost.com/bestbluecruises?ref_content=user-portfolio&ref_feature=portfolio&ref_medium=global-nav https://www.redbubble.com/people/Bestbluee/shop?asc=u https://dzone.com/users/5002347/bestbluecruises12.html https://hub.docker.com/u/bestbluecruises12 http://onlineboxing.net/jforum/user/editDone/254077.page https://micro.blog/Bestbluecruises12 http://www.effecthub.com/user/3729339 http://hawkee.com/profile/5122480/ https://wefunder.com/bestbluecruises12 https://www.facer.io/user/L7ivlMN2QG https://mastodon.online/@Bestbluecruises12Fo https://profiles.wordpress.org/mavituronline12/ https://myseoblog.blogdon.net/ https://myseoblog.blogaaja.fi/ https://myseoblog.jimdosite.com/ https://myseoblog.edublogs.org/ https://myseoblog.websites.co.in/ https://myseoblog47.wordpress.com/ https://myseoblog.blogger.ba/ https://myseoblog.waarbenjij.nu/ https://myseoblog.jigsy.com/ https://szeith-rhounds-kliagy.yolasite.com/ https://myseoblog-40.webselfsite.net/ https://myseoblog.mystrikingly.com/ https://myseoblog.splashthat.com/ https://myseoblog.webnode.com.tr/ https://myseoblog.odoo.com/ https://myseoblog.creatorlink.net/ https://www.geocities.ws/myseoblog/ https://whiteseotr1-s-site.thinkific.com/ https://artistecard.com/myseoblog https://myseoblog.estranky.cz/ https://65390c7d9a166.site123.me/ https://myblogseoooo.blogspot.com/ https://myseoblog.hashnode.dev/ https://whiteseotr1.wixsite.com/myseoblog https://myseoblogg.weebly.com/ https://sites.google.com/view/myseoblogg/ https://codepen.io/myseoblog/pens/public https://myseoblogg.livejournal.com/ https://wakelet.com/@myseoblog87204 https://www.homify.com/users/9537482/myseoblog/ https://theomnibuzz.com/author/myseoblog/ https://lessons.drawspace.com/profile/323508/myseoblog/ https://my.desktopnexus.com/myseoblog/ https://writeupcafe.com/profile/myseoblog/ https://www.pearltrees.com/myseoblog https://www.easyfie.com/myseoblog https://pharmahub.org/members/27544 https://www.zupyak.com/u/myseoblog/posts https://www.metroflog.co/myseoblog https://www.fuzia.com/fz/myseoblog-myseoblog https://tr.pinterest.com/whiteseotr1/ https://my.getjealous.com/myseoblog https://myseoblog.contently.com/ https://micro.blog/myseoblog https://www.tumblr.com/blog/myseobloggsblog https://hub.docker.com/u/myseoblog https://fire.blogfree.net/?act=Profile&MID=1342100 https://myseoblog.pixnet.net/blog https://myseoblogg.seesaa.net/ https://www.threadless.com/@myseoblog/activity https://neocities.org/site/myseoblog https://myseoblog.amebaownd.com/ https://teletype.in/@myseoblog https://ubl.xml.org/users/myseoblog https://educatorpages.com/site/myseoblog/ https://myseoblog.onlc.fr/ https://myseoblog.gumroad.com/ https://blogum.blogdon.net/ https://blogum.blogaaja.fi/ https://blogum-1.jimdosite.com/ https://blogummm.edublogs.org/ https://blogummm.websites.co.in/ https://blogum18.wordpress.com/ https://blogum.blogger.ba/ https://blogum.waarbenjij.nu/ https://benim-blogum.jigsy.com/ https://fuiegs-symbeaurds-build.yolasite.com/ https://blogum-03.webselfsite.net/ https://blogummm.mystrikingly.com/ https://blogum.splashthat.com/ https://blogum3.webnode.com.tr/ https://blogum.odoo.com/ https://blogum.creatorlink.net/ https://whiteseotr1-s-site.thinkific.com/enrollments https://artistecard.com/blogum https://blogum.estranky.cz/ https://653ba4fbb538c.site123.me/ https://blogum12m.blogspot.com/ https://blogum.hashnode.dev/ https://whiteseoturkey1.wixsite.com/blogum https://sites.google.com/view/blogummm/ https://codepen.io/blogum https://blogumm.livejournal.com/ https://wakelet.com/@blogum82816 https://www.homify.com/users/9538383/blogum https://theomnibuzz.com/author/blogum/ https://lessons.drawspace.com/profile/323613/blogum https://my.desktopnexus.com/blogum/ https://writeupcafe.com/profile/BLOGUM/ https://www.pearltrees.com/blogum https://www.easyfie.com/blogum https://pharmahub.org/members/27615/profile https://www.zupyak.com/u/blogum/posts https://www.metroflog.co/blogum https://www.fuzia.com/fz/blogum-blogum https://tr.pinterest.com/blogum12/ https://my.getjealous.com/blogum https://blogum.contently.com/ https://micro.blog/blogum https://www.tumblr.com/blog/blogummm https://hub.docker.com/u/blogum https://fire.blogfree.net/?act=Profile&MID=1342323 https://blogum.pixnet.net/blog https://blog.seesaa.jp/ https://www.threadless.com/@blogum/activity https://neocities.org/site/blogum https://blogum12.amebaownd.com/ https://teletype.in/@blogum https://ubl.xml.org/users/blogum https://educatorpages.com/site/blogum/ https://blogum.onlc.fr/ https://whiteseoturkey.gumroad.com/
Keywords
Embelia ribes, embelin, stress degradation
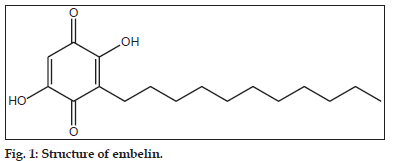
Embelin (2,5–dihydroxy–3–undecyl–1,4–benzoquinone) (fig. 1), is a naturally occurring benzoquinone is isolated from Embelia ribes. The fruits of this plant contain 2.5–3.1% of embelin on dry weight basis and finds its use in several Ayurvedic preparations like Sanjivani Vati, Pippalyasavam, Dhanwantara ghritham, Vidangarishta, Kaisoraguggulu vatica[1]. Embelin shows diverse pharmacological activities including chemo prevention in hepato–carcinogenesis observed in Wistar rats[2], antifertility effects[3], wound healing[4], antibacterial[5], free radical scavenging[6] and in vitro cytotoxic activity against B16 and XC cell lines[7]. It has been shown that embelin is a fairly potent, nonpeptidic, cell–permeable inhibitor of X–linked inhibitor of apoptosis protein[8,9] and hence can be considered as a potential drug candidate in modern system of medicine. Ammonium salt of embelin (ammonium embelate) is already marketed as an anthelmentic agent[10]. Considering its future applications in modern medicine, the objective of the current work was to carry out stress degradation studies to establish the inherent or intrinsic stability characteristics of the molecule and ascertain the degradation pathways. The parent drug stability guidelines by International Conference on Harmonisation (Q1AR) requires that the stress testing of drug substance should include the effect of elevated temperature, humidity, light, oxidizing agents, as well as the susceptibility across a range of pH values[11]. Stress degradation studies of various phytoconstituents such as forskolin and guggulosterone already been reported[12,13].
In this study, the effect of different stress conditions on the stability of the drug was observed using high–performance liquid chromatography (HPLC) analysis. The endeavor was to quantify degradation of embelin under various stress conditions. There is no report yet on these aspects for embelin.
The dried roots of E. ribes were obtained from local market of Mumbai, India and at Khalsa College, Mumbai; a voucher specimen was deposited in Medicinal Natural Products Research Laboratory, Institute of Chemical Technology, Mumbai. All the chemicals and solvents employed were of Analytical grade and procured from Merck Ltd., Mumbai or S. D. Fine Chemicals Ltd., Mumbai. Embelin was isolated from dried berries of E. ribes by an earlier reported method and its identity was confirmed with the aid of spectroscopic data[14]. The isolated compound was analyzed using a reported reverse phase high–performance liquid chromatography method comprising of a mobile phase of water containing 0.1% v/v ortho–phosphoric acid and acetonitrile (10:90) at a flow rate of 1.0 ml/min and employing ultraviolet (UV) detection at 286 nm[15]. Embelin shows a retention time of 7.3 min and the percent purity on the basis of area under curve was found to be 98.3% w/w. Embelin thus obtained was further used as working standard and subjected to stress degradation studies.
For quantification purpose, a stock solution of 1.0 mg/ml of embelin was prepared in methanol and stored in refrigerator. A set of standard solutions was prepared by diluting aliquots of the stock solution, with methanol to give concentrations ranging from 10.0 to 100.0 μg/ml. The calibration graphs were obtained in triplicate by plotting the peak areas against concentration of analyte and were found to be linear (equation of linearity: y=82559x−86459 with r2=0.994).
To study the stability of embelin in solution, two different concentration solutions of 10 and 100 μg/ml were prepared from stock solution and stored at room temperature in tightly capped volumetric flasks protected from light for 6.0, 12.0, 24.0, 48.0 and 72.0 h, respectively. They were then analysed by HPLC and the chromatograms obtained were analysed for additional peaks, if any. The percentage relative standard deviation (%RSD) for the samples analysed at different elapsed assay times was found to be <2%, which proved the stability of the drug in solution state. In order to determine its susceptibility to different stress conditions embelin, was subjected to forced degradation studies under severe conditions of acid, base, oxidation, heat and light.
For acid and base degradation studies, accurately weighed 10 mg embelin was dissolved in 100 ml of methanol. 1 ml of this solution was incubated in dark (in order to exclude the possible effects of light on degradation) with 1 ml of methanol solution of 0.01, 0.1 and 1 N HCl for acid induced degradation and with 1 ml of methanol solution of 0.01, 0.1 and 1 N NaOH for base induced degradation studies. After 2 h the solutions were neutralized and volume made upto 10 ml. The resultant solutions were analysed by HPLC in triplicate.
For oxidative degradation studies accurately weighed 10 mg of embelin was dissolved in 100 ml of methanol. Subsequently, to each ml of the resultant solution 1 ml of hydrogen peroxide of concentrations 0.3, 3.0 and 30.0% v/v were added to three different tubes containing 1 ml of the stock solution and incubated in dark for 2 h and then the solution was heated in boiling water bath for 1 h to remove the excess hydrogen peroxide. The volume of the resultant solution was made up to 10 ml with methanol and analysed by HPLC in triplicate for measuring oxidative degradation.
The photochemical stability of the drug was studied by exposing the drug solution (1 mg/ml) to direct sunlight and UV radiation at 254 nm for 24 h. The resultant solutions were diluted to obtain a concentration of 10 μg/ml and analysed by HPLC in triplicate for degradation studies. Embelin (10 mg) was stored at 80°, 90° and 100° for 1 h under dry heat conditions in a hot air oven for analysis of thermal degradation when subjected to dry heat. It was then dissolved in methanol to obtain a concentration of 10 μg/ml and analysed by HPLC in triplicate to study the degradation products. In all degradation studies, the average peak area of embelin, after application of three replicates was obtained and the content of residual embelin was calculated.
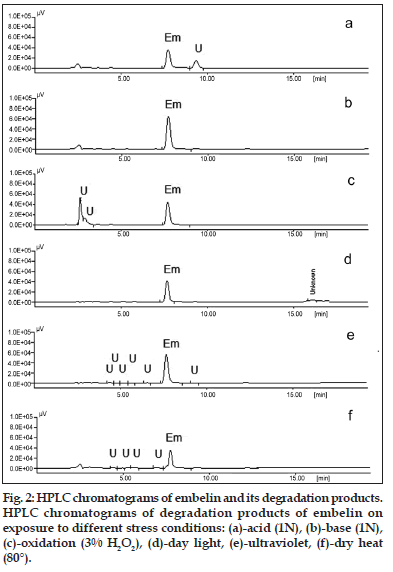
On treating the solution with 1, 0.1 and 0.01 N HCl for 2 h, the area of the peak corresponding to embelin decreased and an additional peak was observed at Rt 8.9 min (fig. 2a), indicating that embelin undergoes degradation under acidic conditions. However, the drug was found to be less susceptible to alkaline medium. On treating the solution with 1, 0.1 and 0.01 N NaOH for 2 h, the area of the drug peaks shows decrease in concentration when treated with 1N NaOH, however, no significant decrease was observed when treated with lower concentrations of alkali (fig. 2b). Embelin also undergoes oxidative degradation in presence of 0.3, 3.0 and 30.0% w/v of hydrogen peroxide when exposed for 2 h and the HPLC chromatogram shows an additional peak at Rt 2.5 min (fig. 2c). Exposure to dry heat indicated that the sample degrades completely when heated to a temperature of 90° for 15 min, degrades slowly when kept at 80° for 1 h and remains stable when kept at 60° for 24 h. Embelin was stable when exposed to sunlight and UV–254 nm for 24 h (fig. 2d and e). Based on the area under curve the concentration of embelin unaffected was calculated under various stress conditions (Table 1).
After these studies it was found out that embelin was more susceptible to oxidative and acid degradation. Since oxidative degradation using peroxide follows a free radical mechanism, the degradation products so formed, can be nonspecific and hence emphasis was given on separation and identification of the acid degradation product. In order to isolate the acid degradation product, about 1 g of embelin was dissolved in 200 ml of methanol and 50 ml of hydrochloric acid was added to the above solution, this mixture was stored in dark for 24 h. The mixture was neutralized by alkali, filtered and concentrated in vacuum. The residue thus obtained was loaded on silica gel (60#) and subjected to column chromatography using light petroleum ether and ethyl acetate as eluent. A 5% fraction of ethyl acetate in petroleum ether yielded orange coloured needles of the acid degradation product (235 mg) which was subjected to spectroscopic studies.
| Condition | Time | % recovery | RT of degradation products |
|---|---|---|---|
| Acid (1 N) | 2 h | 70.07±0.21 | 8.9 |
| Acid (0.1 N) | 2 h | 83.47±0.39 | 8.9 |
| Acid (0.01 N) | 2 h | 99.41±0.19 | 8.9 |
| Base (1 N) | 2 h | 94.09±0.27 | ? |
| Base (0.1 N) | 2 h | 99.09±0.43 | ? |
| Base (0.01 N) | 2 h | 99.30±0.32 | ? |
| Hydrogen peroxide (30% v/v) | 2 h | 37.00±0.25 | 2.5 |
| Hydrogen peroxide (3% v/v) | 2 h | 73.17±0.21 | 2.5 |
| Hydrogen peroxide (0.3% v/v) | 2 h | 90.63±0.19 | 2.5 |
| Day light | 3 weeks | 85.76±0.16 | 4.4, 5.9, 6.5, 16.4 |
| UV light (254 nm) | 24 h | 96.23±0.29 | 4.4, 5.2, 5.9, 6.5, 9.1 |
| Heat (80°) | 1 h | 40.65±0.34 | 4.4, 5.9, 6.5 |
| RT=Retention time |
Table 1: Forced Degradation Of Embelin.
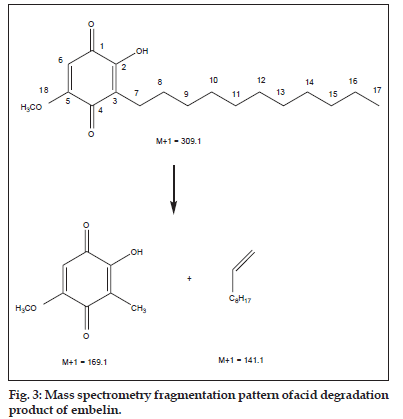
The spectroscopic data of the acid degradation product (Table 2) shows base peak of acid degradation product to be M+15 of the parent compound indicating addition of a methyl group to the parent structure which remains unaltered as observed from mass spectrometry fragmentation peaks at 169.1 and 141.1 (fig. 3). The proton nuclear magnetic resonance spectroscopy and carbon nuclear magnetic resonance spectra shows a peak at δ 3.86 and δ 56.5 respectively, corresponding to formation of a methoxy group, indicating that one of the two hydroxyl groups of embelin has undergone etherification. Since the hydroxyl group present at C–2 of embelin is sterically hindered by the undecyl chain present on adjacent C–3 carbon, it was concluded that the 5–hydroxyl group of embelin must have undergone etherification to form 2–hydroxy–5–methoxy–3–undecyl–1,4–benzoquinone (methyl ether of embelin).
Stress degradation studies on embelin were carried out and it was found that embelin was susceptible to oxidative, acid and to lesser extent thermal degradation. Embelin when treated with 1 N HCl for 2 h undergoes 30% degradation as compared to 1 N NaOH which shows only 6% degradation. It also undergoes 63% degradation when stored in presence of oxidizing agents such as 30% H2O2 indicating its liability to oxidizing agents. When stored in dry heat at a temperature of 80° for 1h the compound undergoes 60% degradation (fig. 2f). The HPLC technique employed for its estimation is a good measure for indicating stability of embelin, since it resolves the degradation products under all stress conditions. It was observed that embelin etherifies when stored in alcohol (methanol or ethanol) in presence of acid to form corresponding ethers of embelin. (spectroscopic data for the ethyl ether is not mentioned). These findings may help in formulation studies of the product considering its applications in modern as well as traditional medicine. When stored under acidic condition care should be taken to use a solvent other than alcohols to prevent etherification. The relevance of these studies in predicting the stability of embelin in Ayurvedic formulations is that they are known to contain self–generated alcohol which under acidic conditions may undergo etherification to form corresponding ether of embelin. In conclusion, the stress degradation studies on embelin have been carried out, which may provide an insight for its stability and storage considering the formulation aspects of modern medicine.
| Characterization method | Values obtained |
|---|---|
| IR υ max (KBr) cm−1 | 3353 (Ar-OH), 2920 (Ar-H), 2852 (C-H), 1638, 1599 (C=O), 1205 (−OCH3) |
| Mass spectra | [M+1]+309 |
| MS-MS | [M+1]+169.1, 141.1 |
| 1H-NMR (CDCl3, 400 MHz) | δ 0.5 (t, 3H, R-CH3), 1.25 (s, 20H,– CH2), 3.86 (s, 3H, O-CH3), 7.23 (d, 1H, Ar-H) |
| 13C-NMR (CDCl3, 125 MHz) | 14 (C-17), 22 (C-16), 21 (C-7), 27 (C-8) 31 (C-15), 30 (C-14, C-13, C-12, C-11, C-10, C-9), 56.5 (C-18), 102 (C-6), 119 (C-3), 151 (C-2), 161 (C-5), 181 (C-4), 182 (C-1) |
| Melting point | 118° |
Table 2: Spectroscopic Data Of Aciddegradation Product Of Embelin.
Acknowledgements
The authors thanks to the University Grants Commission- Special Assistance Program for providing financial assistance for this project and to Prof. H. M. Pandit, Khalsa College, Mumbai for plant material authentication.
References
- Ayurvedic Formulary of India.Government of India, Ministry of Health and Family Welfare. Part. I. New Delhi: Department of Health, Controller of Publications; 1989. p. 154.
- Podolak I, Galanty A, Janeczko Z. Cytotoxic activity of embelin from Lysimachiapunctata. Fitoterapia 2005;76:333-5.
- Githui EK, Makawiti DW, Midiwo JO. Changes in the concentrations of testosterone, luteinising hormone and progesterone associated with administration of embelin. Contraception 1991;44:311-7.
- Kumara Swamy HM, Krishna V, Shankarmurthy K, Abdul Rahiman B, Mankani KL, Mahadevan KM, et al. Wound healing activity of embelin isolated from the ethanol extract of leaves of EmbeliaribesBurm. J Ethnopharmacol 2007;109:529-34.
- Chitra M, Devi CS, Sukumar E. Antibacterial activity of embelin. Fitoterapia 2003;74:401-3.
- Joshi R, Kamat JP, Mukherjee T. Free radical scavenging reactions and antioxidant activity of embelin: Biochemical and pulse radiolytic studies. ChemBiol Interact 2007;167:125-34.
- Jime'nez-Alonso S, Cha'vez H, Este'vez-Braun A, Ravelo AG, Feresin G, Tapia A. An efficient synthesis of embelin derivatives through domino Knoevenagel hetero Diels–Alder reactions under microwave irradiation. Tetrahedron 2008;64:8938-42.
- Mori T, Doi R, Kida A, Nagai K, Kami K, Ito D, et al. Effect of the XIAP inhibitor Embelin on TRAIL-induced apoptosis of pancreatic cancer cells. J Surg Res 2007;142:281-6.
- Chen J, Nikolovska-Coleska Z, Wang G, Qiu S, Wang S. Design, synthesis, and characterization of new embelin derivatives as potent inhibitors of X-linked inhibitor of apoptosis protein. Bioorg Med ChemLett 2006;16:5805-8.
- Windholz M, The Merck Index: An encyclopedia of chemicals, Drugs and Biologicals. 10th ed. USA: Merck Research Laboratories; Merck and Co. Inc.; 1983. p. 513.
- ICH. Q1A Stability Testing of New Drug Substances and Products: Proceedings of the International Conference on Harmonization, IFPMA, Geneva, 2000.
- Mohd SA. Rizwan RP, Mohd M, Mohd A. A validated stability-indicating tlc method for determination of forskolin in crude drug and pharmaceutical dosage form. Chromatographia 2008;67:441-7.
- Agrawal H, Kaul N, Paradkar AR, Mahadik KR. HPTLC method for guggulsterone. II. Stress degradation studies on guggulsterone. J Pharm Biomed Anal 2004;36:23-31.
- Ferreira G, Laddha KS. Synthesis of ether derivatives of Embelin. Indian Drugs 2011;48:40-3.
- Podolak I, Strza?ka M. Qualitative and quantitative LC profile of embelin and rapanone in selected lysimachia species. Chromatographia 2008;67:471-5.