- Corresponding Author:
- V. F. Patel
Department of Pharmacy, School of Life and Medical Sciences, University of Hertfordshire, Hatfield AL10 9AB, UK
E-mail: v.patel5@herts.ac.uk
| Date of Submission | 11 September 2013 |
| Date of Revision | 04 September 2014 |
| Date of Acceptance | 10 September 2014 |
| Indian J Pharm Sci 2014;76(6):483-494 |
Abstract
The present study was aimed at investigating the effect of hydrotrope and surfactant on poor solubility of atorvastatin calcium. Excipients screening followed by factorial design was performed to study effect of excipients and manufacturing methods on solubility of drug. Three independent factors (carrier, surfactant and manufacturing method) were evaluated at two levels using solubility as a dependant variable. Solid-state characterisation was performed using Fourier transform infrared spectroscopy and differential scanning calorimetry. Optimised complex were incorporated into orally disintegrating micro tablets and in vitro dissolution test was performed. Nicotinamide, Plasdone and sodium dodecyl sulphate were emerged as promising excipients from excipient screening. General regression analysis revealed only the type of carrier has significantly enhanced (P<0.05) the solubility of drug while other factors were found to be nonsignificant. Ratio optimisation trial revealed that drug to nicotinamide ratio is more critical in enhancing the solubility of drug (40 fold increases in solubility compared to pure drug) in comparison to drug-surfactant ratio; however the presence of surfactant deemed essential. Significantly higher rate and extent of dissolution was observed from solid dispersion complex and tablets compared to dissolution of pure drug (P<0.05). Study revealed hydrotrope and surfactant have synergistic effect on solubility and dissolution of atorvastatin calcium and this can be explored further.
Keywords
Hydrotrope, surfactant, atorvastatin calcium, orally disintegrating microtablets, solid dispersion, factorial design
Use of medications either unlicensed or off-label is common in children and elderly population, either due to lack of availability of age appropriate formulations or available formulations may not have been approved for use in these age groups, ultimately pose a greater safety risk due to limited clinical evidence around the route of administration, drug posology and pharmacokinetic parameters [1,2].
Liquids are the most frequently used formulations for young children, as they are considered simple to administer and the dose can easily be manipulated if needed, as well as in elderly patients, especially when they have swallowing problems associated either with disease conditions or as a result of age or sometime due to psychological issues around refusal of solid formulations. However liquid formulations also possesses several problems such as short term stability in comparison to its solid counterpart, bulky in nature and often required controlled storage to maintain the shelf-life led to increase overall cost in logistics and palatability issues such as poor taste and flavours limits its wider acceptability [3]. Also limited availability of safe excipients such as solvents, preservatives and antioxidants can hinder the development of suitable liquid formulations [4]. Masking the unpleasant taste of a drug is much harder to achieve in liquid than in solid forms, and the unpleasant taste may affect the accuracy of dosing as chances of spitting formulation is higher if formulation is not palatable [5]. Solubility play important role in formulation design as poor solubility of the drug pose significant hurdle in product development on top of the other issues highlighted earlier. Majority of the newly developed drugs are poorly soluble and to overcome this, formulator often have to use excipients which are either not approved for use in certain population (e.g. children) or their prolong exposure in chronic disease may prove fatal.
Atorvastatin calcium, a selective competitive inhibitor of HMG-CoA reductase, is a white to off-white crystalline powder that is insoluble in aqueous solutions of pH 4 and below, very slightly soluble in distilled water, phosphate buffer pH 7.4 and acetonitrile, slightly soluble in ethanol, and freely soluble in methanol [6]. Atorvastatin is rapidly absorbed after oral administration, and reach peak concentrations (Tmax) within 1-2 h. The fraction of drug absorbed (%) and absolute bioavailability of atorvastatin are approximately 30 and 12%, respectively [7]. The low systemic availability is attributed to presystemic clearance in gastrointestinal (GI) mucosa and/or hepatic first-pass metabolism [7-9]. Currently conventional and chewable tablets are available in the market for use in children and adult from 10 y age but still healthcare professional often need to rely on ordering special liquid formulations [10], which are usually formulated with high level of alcohol to overcome poor solubility of atorvastatin calcium often results in a formulation with very short stability and poor palatability. All of these (cost and poor compliance) ultimately put greater economic burden on healthcare system as the drug needs to be taken on regular basis due to nature of the disease [10,11]. Orodispersible formulations emerged as an alternative formulation strategy for patients who are either suffering from dysphasia, or having swallowing difficulty either due to age or disease conditions or may have fear of choking conventional tablets. However as mentioned earlier, poor solubility of the drug can pose hurdle in development of orodisperisble formulations and hence needed to be overcome. Several approaches have been explored to increase the solubility and dissolution of atorvastatin calcium with limited success which include formation of inclusion complex with ß-cyclodextrin [12], fusion induced via microwave energy in presence of polyethylene glycol (PEG) 6000 [13], formation of nano-particles using supercritical antisolvent process [14] and spray drying using organic solvent [15].
Systematic optimization using design of experiment for evaluating effect of formulation and process variables is often preferred over traditional way of trial and error approach in formulation development as the former build quality in product development and provide assurance within the design space, and often proven as cost effective strategy in formulation development [16]. In this study, two step screening was employed, first to indentify few vital excipients suitable for solubility enhancement and second to use full factorial screening design to evaluate the effect of type of excipients (surfactant and carrier) and manufacturing methods (solvent evaporation and freeze drying) on solubility of atorvastatin calcium. Based on the outcome of the screening design, ratio optimization trials were performed to optimize the carrier and surfactant concentration required to achieve optimal solubility. Solid-state characterisation using Fourier transform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC) was performed for solid dispersion complex. The optimised complex was then incorporated into orally disintegrating micro tablets using direct compression technique and tested for in vitro disintegration and a non-sink in vitro dissolution test to check formulation performance.
Materials and Methods
Atorvastatin calcium was purchased from Evershine Chemical Company (Hangzhou, China), Plasdone S630 and Polyplasdone XL10 were received from ISP Corporation (Covington, USA), Starch 1500 and Star Cap 1500 were received from Colorcon (Kent, UK), Polyox N10 was received from Dow Chemical Company (Edegem, Belgium), Ac-Di-Sol was received from FMC Corporation (Philadelphia, USA). Silicified microcrystalline cellulose (Prosolv® SMCC) was received from JRS Pharma (Slough, UK). Sodium stearyl fumarate (Lubripharm®) was received from SPI Pharma (Septemes-Les Vallons, France). Nicotinamide, Tween 80, pullulans, sodium dodecyl sulphate (SDS), maltodextrin, Poloxamer F68 and Span 80 were purchased from Sigma Aldrich (Dorset, UK). All other chemicals used were of analytical grade and purchased from Fisher Scientific Ltd (Loughborough, UK).
Excipient screening
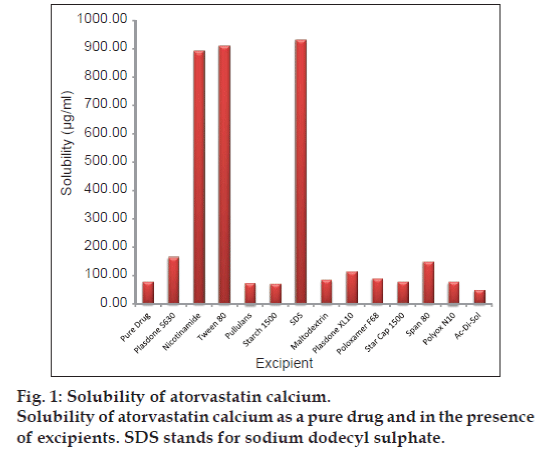
Initial screening was performed to screen few vital excipients, which can enhance the solubility of the atorvastatin calcium (AC) using solubility study. One hundred milligrams of each excipient listed in material section above except Prosolv® SMCC and Lurbipharm® was weighed and transferred into a screw cap vial containing 10 ml of artificial saliva [17] to make 1% w/v solution and then excess drug (equivalent to 50 mg) was added into each vial. The samples were then placed on a magnetic plate with water bath with controlled heating at 37° (RCT Basic IKA Larbortechnik, Germany) at 100 rpm for 24 h. The aliquots were filtered through 0.45 µm membrane filter and the filtrates were diluted subsequently using artificial saliva and analysed using double beam UV spectrophotometer (T80 UV/Vis Double Beam Spectrometer, PG Instruments Ltd, UK) at 241 nm. The amount of drug solubilised was calculated using developed calibration curve in artificial saliva with R2 of 0.998.
Preparation of solid dispersion complexes as per screening factorial design
Based on solubility data obtained from initial excipient screening, a randomised 23 full factorial design was created using MiniTab® 16 software (Coventry, UK) to study effect of three factors at two different levels producing total of 8 different combinations and the experimental design with corresponding formulations composition is outlined in Table 1. The independent variables were the type of carrier (A: Plasdone/nicotinamide), type of surfactant (B: Tween 80/SDS) and manufacturing method (C: Solvent evaporation/freeze drying). The solubility was performed in artificial saliva and used as a dependant variable. A statistical model was used to evaluate the response using the Eqn.: Y = a0+a1A+a2B+a3C+a4AB+a5AC+a6BC, where Y is the dependent variable solubility (µg/ml), a0 is the arithmetic mean response of the 8 samples and a1, a2, a3, a4, a5, a6 are the estimated coefficients for factors A, B, C and their interaction terms, respectively. Statistical analysis of the response generated according to factorial design was analysed using MiniTab® 16 statistical software.
| Batch No | Composition Coded value | Solubility (µg/ml) ± SD (n=3) | Fold increase* | ||
|---|---|---|---|---|---|
| A | B | C | |||
| Pure drug | - | - | - | 78.35 ± 6.05 | - |
| SD1 | -1 | -1 | -1 | 236.75 ± 13.20 | 3.08 |
| SD2 | -1 | -1 | +1 | 180.61 ± 10.75 | 2.16 |
| SD3 | -1 | +1 | -1 | 553.68 ± 20.55 | 6.74 |
| SD4 | -1 | +1 | +1 | 413.15 ± 9.62 | 5.25 |
| SD5 | +1 | -1 | -1 | 2085.44 ± 16.97 | 26.22 |
| SD6 | +1 | -1 | +1 | 1662.24 ± 39.60 | 20.43 |
| SD7 | +1 | +1 | -1 | 1545.84 ± 20.93 | 19.14 |
| SD8 | +1 | +1 | +1 | 1851.04 ± 6.79 | 23.20 |
| Actual value | |||||
| Factors | -1 | +1 | |||
| Type of carrier | Plasdone S630 | Nicotinamide | |||
| Type of surfactant | Tween 80 | SDS | |||
| Manufacturing method | Solvent evaporation | Freeze drying | |||
SD is solid dispersion. SDS stands for sodium dodecyl sulphate. *Calculated with reference to pure drug solubility
Table 1: Composition and solubility data for Solid dispersion complexes prepared as per factorial design
Ternary solid dispersion complexes were prepared at drug:carrier:surfactant ratio of 1:1:0.2. Briefly, 500 mg of drug, 500 mg of carrier and 100 mg of surfactant as per composition (Table 1) were weighed and added to 50 ml of purified water with continuous stirring for 30 min before transferred to oven or freeze drier for further process. For solvent evaporation method, the drug-carrier solutions were transferred on to a petri dish and placed in the oven at 60° to ensure complete evaporation of solvents. For freeze-drying method, the drug-carrier solutions were transferred on to a petri dish and transferred to freeze drier for removal of solvent. During freeze-drying process, the freeze-drier was maintained as -45° for 1 h, followed by - 30° for 3 h, - 10° for 3 h and finally at 20° for 12 h with constant compression pressure of 0.5 Torr (Micromodulyo 230 freeze dryer, UK). After complete drying, the containers were taken out, and the dried products were scraped from petri dishes. The solid dispersion complexes were passed through 20 mesh sieve and packaged in glass vials until further use.
Preparation of solid dispersion complexes for ratio optimisation
Based on the outcome of the screening design, ternary solid dispersion complexes consist of drug, nicotinamide and SDS at different ratios were prepared similarly as described above using solvent evaporation technique. The composition was shown as Table 2. After complete drying, the containers were taken out, and the dried products were scraped from petri dishes. The formulations were passed through 20 mesh sieve and packaged in glass vials until further use.
| Batch no | Quantity (mg) | Solubility (µg/ml) | Fold increase | ||
|---|---|---|---|---|---|
| Drug | Nicotinamide | SDS | |||
| Pure drug | - | - | - | 0.078 | 1.0 |
| SD9 | 1000 | - | 200 | 0.25 | 3.2 |
| SD10 | 1000 | 500 | 200 | 0.45 | 5.8 |
| SD11 | 1000 | 1000 | - | 0.86 | 11.0 |
| SD12 | 1000 | 1000 | 200 | 1.23 | 15.8 |
| SD13 | 1000 | 1000 | 600 | 1.48 | 19.0 |
| SD14 | 1000 | 2000 | 200 | 3.11 | 39.9 |
SDS stands for sodium dodecyl sulphate
Table 2: Composition and solubility data for ternary complexes prepared for ratio optimization
Solubility
Pure drug and its solid dispersion complexes in excess quantity (equivalent to 50 mg drug) were placed separately in screw cap glass vials containing 10 ml of artificial saliva [17] and the samples were shaken on thermostatically controlled water bath at 37±0.5° for 24 h (RCT Basic IKA Larbortechnik, Germany) at 100 rpm. The aliquots were filtered through 0.45 µm syringe filter. The filtrates were subsequently diluted using artificial saliva and analysed using double beam UV spectrophotometer (T80 UV/Vis double beam spectrometer, PG Instruments Ltd, UK) at 241 nm with corresponding excipient solution as blank. The amount of drug solubilised was calculated using developed calibration curve in artificial saliva with R2 of 0.998. This experiment was performed in triplicate.
FTIR spectroscopy
The FTIR spectra of pure drug, excipient and the solid dispersion complexes were obtained using Perkin Elmer Spectrum 100 FT-IR Spectrometer (Perkin Elmer, UK) via scanning of samples from 4000 cm-1 to 600 cm-1 using 32 scans per spectrum. A baseline correction was carried out before running the samples.
DSC analysis
The DSC measurements were performed using a DSC Q2000 (TA Instrument, UK). The instrument was calibrated with Indium before the start of the experiments. Samples weighing approximately 5 mg were sealed in aluminium pans and analyzed in an atmosphere of nitrogen at flow rate of 25 ml/min. A temperature range of 0 to 300° was used, and the heating rate was 10°/min. Thermograms of pure drug, excipients and the solid dispersion complexes were obtained.
In vitro dissolution study
The non-sink dissolution study was performed using 10 ml of artificial saliva as a dissolution medium in a glass vial. The solution was continuously stirred in glass vial with use of a magnetic flea at 400 rpm on magnetic stirrer. The pure drug and the solid dispersion complexes equivalent to 10 mg of drug were placed in separate vials and at predetermined time interval 1 ml of sample was withdrawn and replaced with fresh medium. After appropriate dilution, the samples were filtered and analysed using double beam UV spectrophotometer at 241 nm. The cumulative percentage drug release was calculated and plotted against time (n=3).
Preparation and characterisation of orally disintegrating micro tablets
The optimised complex comprising drug, nicotinamide and surfactant at ratio of 1:2:0.2 was used to produce orally disintegrating micro tablets using direct compression technique. Briefly, 320 mg of solid dispersion complex equivalent to 100 mg of drug was weighed and mixed with 420 mg of ProSolv® SMCC in small polybag for 5 min followed by lubrication of blend using 10 mg of sodium sterayl fumarate for another 2 min. The 75 mg of blend equivalent to 10 mg of drug was individually weighed and transferred into 6 mm die and manually compressed using standard concave punch on single punch machine at pressure of 1000 psi. Tablets were tested for in vitro disintegration and in vitro drug dissolution test. In vitro disintegration was carried out by placing one tablet in a petri dish filled with 5 ml of artificial saliva placed in water bath at 37°. The time required for tablet to disintegrate was noted (n=3). In vitro drug dissolution was performed in a similar manner as described earlier for dissolution test for solid dispersion complexes (n=3).
Statistical analysis
The factorial design was generated and analysed using MiniTab® 16 software (Coventry, UK) and the value at P less than 0.05 was considered as significant. IBM SPSS® 21 (Portsmouth, UK) was used to compare different groups either using one way ANOVA or t test.
Results
Solubility data for excipient screening
The solubility of atorvastatin calcium alone as well as in presence of 1% w/v excipient solution was shown as fig. 1. The highest solubility was observed in presence of SDS, nicotinamide and Tween 80 with approximately 11 fold enhancements in solubility compared to solubility of pure drug (78.35 µg/ml). Other excipients also have increased the solubility of drug but not to the extent as observed with excipients mentioned above.
Solubility data for solid dispersion complexes
The solubility of drug and its solid dispersion was performed in artificial saliva to closely mimic the oral cavity. The solubility results are shown as Table 1 along with the composition of prepared solid dispersion complexes as per factorial design. Solubility of the pure atorvastatin calcium was observed to be 78.35 µg/ml indicates poor solubility in aqueous medium. Incorporation of drug in solid dispersion complexes has enhanced the solubility by several folds (Table 1). Solubility value obtained from 180.61±10.75 µg/ml to 2085.44±16.97 µg/ml in solvent evaporated solid dispersion complex containing Plasdone S630 with Tween 80 (SD2) and nicotinamide with Tween 80 (SD5), respectively.
Statistical evaluation of factorial design
General regression analysis was performed to investigate the effect of three independent variables on solubility of atorvastatin calcium in artificial saliva and the summary of co-efficients and ANOVA are shown in Table 3. Overall, the model was found significant with high value of correlation coefficient indicates the solubility is significantly influenced by factors studied (P<0.05, P2=0.972). Type of carrier has significantly enhanced the solubility as evident from relatively high positive co-efficient with P value of 0.0011. Other two factors did influence the solubility of drug but the effect was nonsignificant (P<0.05). Overall positive co-efficient for type of surfactant indicates presence of SDS favours atorvastatin solubilisation compared to Tween 80. For manufacturing method changing from solvent evaporation to freeze drying has detrimental effect on solubility as shown by negative coefficient in regression model.
| Parameter | Coefficients of regression parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| a0 | a1 | a2 | a3 | a4 | a5 | a6 | R2 | P | |
| Solubility (µg/ml) | 1066.09 | 720.05 | 24.83 | -39.33 | -112.53 | 9.83 | 80.5 | 0.972 | 0.005 |
| P values | 0.0002 | 0.0011 | 0.787* | 0.671* | 0.467* | 0.938* | 0.573* | ||
| ANOVA | |||||||||
| Source of variation | DF | SS | MS | F | P | ||||
| Regression | 3 | 4165043.689 | 1388347.896 | 23.480 | 0.005 | ||||
| Residual | 4 | 236506.438 | 59126.609 | ||||||
| Total | 7 | 4401550.128 | |||||||
DF is degree of freedom; SS is sum of square; MS is mean sum of square; and F is Fischer’s ratio, *indicate co-efficient is insignificant at P<0.05. ANOVA: Analysis of variance
Table 3: Results of general regression analysis and anova for atorvastatin calcium solubility
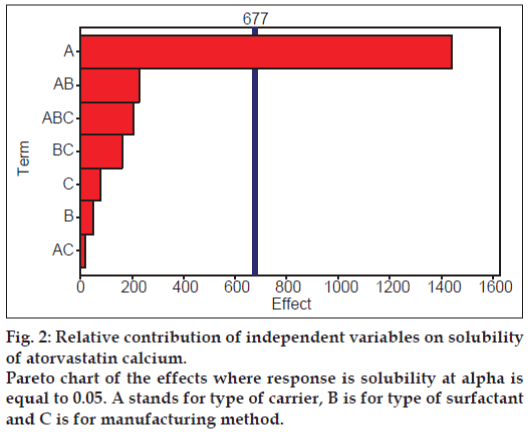
To check the significance of the relative contribution of all the factors alone as well as in combination with each other’s, the Pareto chart was constructed and shown as fig. 2. It was clearly evident that only factor A which is type of carrier has significant influence on solubility of drug as the only bar for type of carrier has crossed the significance line and this result is also complemented by P value of 0.0011 in regression model. The second most influencing factor was the combination of type of carrier and surfactant but statistically, it is of little significance as clearly seen from Pareto chart.
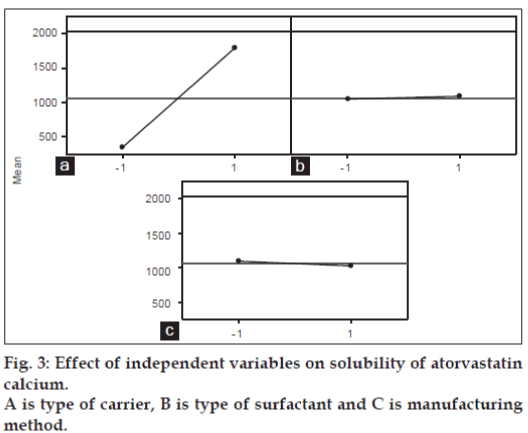
Main effect and interaction plots also constructed to visually analyse the data in greater details and presented as figs. 3 and 4, respectively. Main effect plot also showed that the solubility was increased when type of carrier changed from plasdone to nicotinamide regardless of other two factors. Almost flat lines for type of surfactant shows regardless of type of surfactant used, solubility did not change significantly. The same is also true for manufacturing method but with little decline in solubility was observed when manufacturing method changed from solvent evaporation to freeze-drying.
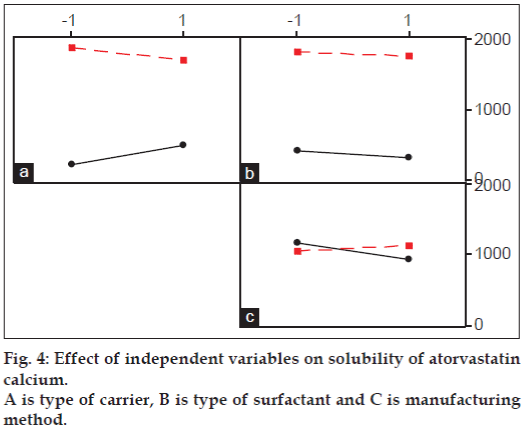
Little interaction was evident between type of carrier and surfactant as well as between type of carrier and manufacturing method. Solubility has declined slightly when surfactant changed from Tween 80 to SDS in presence of nicotinamide while the effect was opposite when Plasdone S630 used as a carrier. Manufacturing method did not show any significant effect on solubility regardless of carrier used as seen by parallel lines in interaction plot. However, crossover interaction was seen between type of surfactant and manufacturing method. The solubility was slightly decreased when Tween 80 was used as a surfactant and manufacturing method was changed from solvent evaporation to freezedryingbut it is of negligible significance. When SDS was used as a surfactant, changing method from solvent evaporation to freeze drying has little positive influence on solubility enhancement but again of little significance. Overall it was observed that only type of carrier, i.e changing Plasdone S630 to nicotinamide had significantly positive influence on enhancing solubility of atorvastatin calcium while other factors did affect the solubility but are of little importance.
Solubility data for ratio optimisation trials
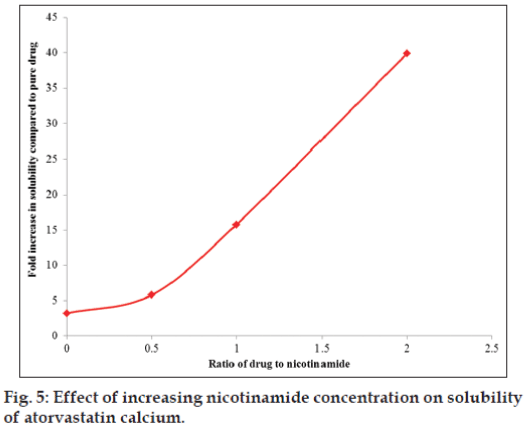
Solubility data for solid dispersion complexes prepared for ratio optimization trial using nicotinamide as a carrier and SDS as a surfactant via solvent evaporation method was shown in Table 3. The plot showing relationship between drug to nicotinamide ratio (SDS at constant level) and fold increase in solubility of drug is presented as fig. 5. Solubility of drug was increased with increasing concentration of nicotinamide keeping SDS at constant level, and also this relationship was found linear between drug to nicotinamide ratio of 1:0.5 to 1:2. Surfactant alone at drug to surfactant ratio of 1:0.2 did increase the solubility of drug to approximately 3 fold, while nicotinamide alone at drug to carrier ratio of 1:1 increased the solubility by 11 fold with reference to solubility of pure drug. Solubility was further increased to 15 and 40 fold in presence of nicotinamide at drug to carrier ratio of 1:1 and 1:2, respectively keeping surfactant at constant level which clearly shows dominant role of nicotinamide in increasing the solubility of atorvastatin calcium. Solubility enhancement was more pronounced in presence of nicotinamide owing to its hydrotropic solubilisation effect, which was further escalated in presence of surfactant.
FTIR analysis
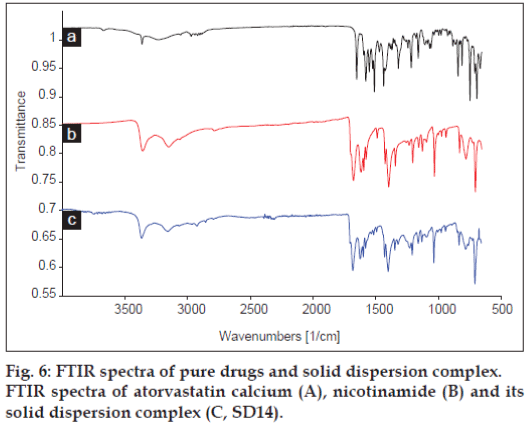
Fig. 6 shows FTIR spectra of atorvastatin calcium, nicotinamide and its solid dispersion complex. The FTIR spectra of solid dispersion complex exhibited significant differences in the observed vibrational transitions and the bands in the spectrum of pure drug were cleared and sharper than the bands of the solid dispersion complex of atorvastatin calcium. The most significant characteristic peaks were observed between 3700 and 3000 cm-1 (3363, 3234, 3056 cm-1) due to the stretching of the –OH group associated to a hydrogen bond. Also, three bands at 3363 cm-1 (N-H stretching), 3234 cm-1 (asymmetric O-H stretching) and 3056 cm-1 (symmetric O-H stretching) were observed. The intensity at 3363 and 3056 cm-1 were reduced, while band at 3234 cm-1 disappeared in the solid dispersion complex. Also other bands at 1650 cm-1 (asymmetric C=O stretching), 1578 cm-1 (symmetric C=O stretching), 1550-1468 cm-1 (bands related to C-C ring stretching), 1317 cm-1 (CH3/ CH2deformation), 1243 cm-1 (C-N stretching) and 1213 cm-1 (C-N stretching/C-O stretching) were observed in the FTIR spectra of the pure drug while in case of solid dispersion complex, all the characteristic peaks for atorvastatin calcium were broad with reduced intensity indicating molecular interaction of drug with carriers. Also a characteristic band of nicotinamide at 3148 cm-1 (-OH stretching) shows reduced intensity in solid dispersion complex further support molecular interaction between drug and carrier.
DSC analysis
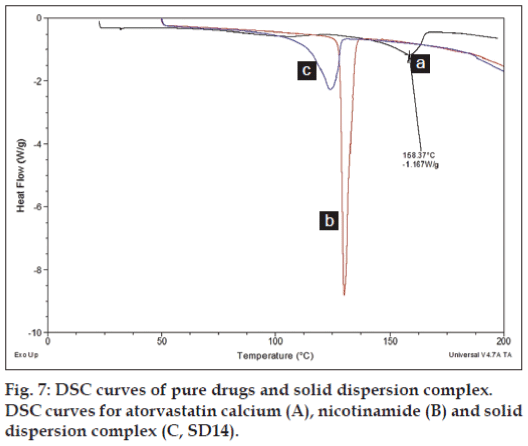
The DSC curve of atorvastatin calcium showed as fig. 7. A small broad endotherm onset at 50° was possibly due to the loss of water as it contains three water molecules and the sharp endothermic peak at 158.37° was attributed to atorvastatin calcium corresponding to its melting point. DSC curve for nicotinamide shows a sharp characteristic peak at 130.35° at its melting point. DSC curve for the solid dispersion showed endothermic peak corresponding to nicotinamide but the peak was broaden with reduced intensity, while atorvastatin calcium peak was completely disappear. Overall there is a slight shifting of nicotinamide peak towards lower melting point with complete absence of crystalline drug peak was observed in DSC curve for solid dispersion.
Characterisation of micro tablets
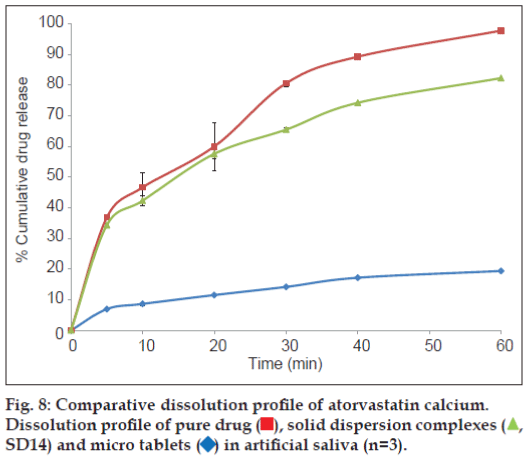
The prepared tablets were evaluated for in vitro disintegration test in 5 ml of artificial saliva to mimic the oral cavity. All the tablets were disintegrated within few seconds with average disintegration time was found to be 20.97±0.12 sec. This relatively quicker disintegration matches the requirement for orodisperisble formulation criteria. The tablets were also evaluated for the in vitro drug dissolution to see the process impact on the dissolution behaviour of solid dispersion on incorporation into a formulation, and compared against dissolution profile of solid dispersion and pure drug. Non-sink dissolution was performed to see the formulation performance in small volume of dissolution media. The dissolution profile of atorvastatin calcium as pure drug, solid dispersion complex and micro tablet is shown as fig. 8.
Significantly higher rate and extent of drug dissolution was observed from both solid dispersion complex and micro tablet compared to dissolution of pure drug (P<0.05) with approximately 35-40% drug was dissolved in 5 min from complex and tablets compared to less than 10% drug was dissolved when presented as pure drug. The slower dissolution in case of pure drug was attributed to poor wettability and poor solubility of atorvastatin calcium in aqueous medium. The dissolution profile of pure drug was stagnant later on which revealed the drug saturation was achieved rapidly in small volume of dissolution medium, and hence further dissolution was retarded due to poor solubility of drug. Stagnant dissolution was not evident with solid dispersion complex and micro tablets due to solubility advantage of prepared solubilised complex and presence of hydrotrope and surfactant within the formulation. Extent of dissolution was slightly lower from tablet formulation in later time points. In the current study, although slight decline in extent of dissolution was observed when solid dispersion was incorporated into tablet formulation, it will be of little significance as more than 80% drug was released within 30 min.
Discussion
Currently available formulations of atorvastatin calcium suffer from poor bioavailability due to its poor aqueous solubility and extensive first pass metabolism on oral GI administration [6,7]. Some attempts to increase the solubility and dissolution of atorvastatin calcium have been reported in literature with varied success [12-15]. In the current study, an attempt was made to improve the solubility and dissolution of atorvastatin calcium using GRAS listed excipients. Excipient screening revealed that the surfactants such as Tween 80 and SDS; a hydrotrope, nicotinamide; and a hydrophilic polymer Plasdone S630 were found to enhance the solubility of drug with SDS has shown highest increase in solubility. SDS being a surfactant owing to its micelle forming nature entrapped the hydrophobic drug molecule inside its micelle and increases the solubility of the drug [18,19]. Nicotinamide being a hydrotrope also have shown similar potential as of surfactant but like surfactant hydrotrope do not form micelle to solubilise the drug [20,21]. Several theories have proposed to show how hydrotrope enhances the solubility of poorly soluble drug. Among them, formations of complexes via stacking complexation and/or molecular aggregation are widely accepted mechanisms in literature [22,23]. The initial experiment, which is kind of phase solubility study, only gave information about few vital excipients useful for increasing solubility of drug but to use as a formulation components, these excipients needs to be evaluated further in presence of other factors that also affects formulation such as manufacturing method and the interaction of different excipients amongst each other. Full factorial screening design was employed to further evaluate the effect of different excipients and manufacturing methods on enhancing solubility of drugfrom its solid dispersion complexes.
Solubility has increased significantly from solid dispersion complexes (P<0.05) compared to pure drug solubility (Table 1). The highest increase in solubility was observed in solid dispersion complex comprising Tween 80 and nicotinamide prepared via solvent evaporation method. General regression analysis revealed only the type of carrier has significant influence (P<0.05) on enhancing solubility of drug while other factors such as type of surfactant and manufacturing method did not influence the solubility significantly (P<0.05, Table 2). A straight line in main effect plot (fig. 3) further support this outcome by indicating the changes in levels from lower to higher do not have significant contribution. Also relatively less magnitude of the bars in Pareto chart representing these two factors (surfactant and manufacturing method) demonstrated little contribution in enhancing solubility of atorvastatin calcium in comparison to carrier. Two types of surfactants were used in this study, Tween 80 being a non-anionic while SDS being an anionic surfactant. Overall SDS containing complex have shown higher solubility compared to its counterpart, Tween 80 (fig. 3). Similar observation was also reported by Park and Choi where solubility of three model drugs mefenamic acid, nimesulide and ibuprofen was higher in presence of SDS compared to Tween 80 but the difference was not statistically significant amongst each other [24] which support the finding in the current study. It reveals that the presence of surfactant is essential but type of surfactant does not make significant influence and also considering the process aspects (Tween 80 being a liquid pose significant problem in solid dispersion formulation when incorporated in solid dosage form), SDS was chosen as a surfactant for ratio optimisation study.
Overall slight decrease in solubility was observed when complexes were prepared using freeze drying method compared to solvent evaporation method but the differences are of little significance as clearly evident in main effect plot and Pareto chart (figs. 2 and 3). This could be due to relatively less time for drug, carrier and surfactant molecules to interact with each other when freeze drying method was employed. In freeze drying technique, drugcarrier solution being frozen followed by removal of solvent in form of a vapour allows little opportunity for these molecules to interact with each other compared to solvent evaporation technique where presence of all components in liquid medium would have relatively more time for the interaction to occur until all the solvent being removed to form solid dispersion. He and co-workers [25] compared freeze drying and solvent evaporation method for preparation of baicalein solid dispersion and no significant difference was observed between these two methods on improving the dissolution of baicalein [25]. Although not significant but contrast to the results reported above were published by Dontireddy and Crean [26], where freeze drying method found to be more effective in improving the dissolution of hydrocortisone using PVP as carrier than spray drying method. Relatively higher surface area and the orientation of drug and carrier particles within the matrices thought to be the primary reasons for such differences observed, but the observed effect was obvious as Dontireddy and Crean [26] have used spray drying method where drug and carrier may not have enough time for interaction before being spray dried compared to freeze drying method as explained earlier. Therefore, in the current study, keeping in mind no significant difference observed between solvent evaporation and freeze drying methods and relatively higher cost associated with using freeze drying method, it was decided to use solvent evaporation method for ratio optimization study.
Comparing carriers, the increase in solubility was more evident in case of nicotinamide compared to Plasdone S630 as seen by relatively higher solubility value for complexes comprising nicotinamide as a carrier (Table 1). Plasdone S630 have been reported in literature to increase the solubility of ketoconazole but the solubility enhancement was merely 1.17 fold measured in terms of dissolution efficiency [27]. In another study, although equilibrium solubility was not measured, kinetic solubility represented by resembling in vitro drug dissolutiontest was performed with co-ground mixture of paliperidone and Plasdone S630 [28]. Paliperidone to Plasdone S630 at ratio of 1:2 has slower the dissolution of paliperidone compared to 1:1 ratio. This was attributed to leaching out of the polymer at higher concentration formed concentrated barrier surrounding the drug particles preventing faster drug dissolution [28]. Solubility enhancement was relatively higher with Plasdone S630 in the current study compared to the reported literature [27,28]. This could be due to additional presence of surfactant, which also contributed towards increasing the solubility of the drug. As stated earlier, surfactant owing to its micelle formation entrapped the hydrophobic drug molecule and this along with presence of hydrophilic polymeric carrier such as Plasdone S630, can stabilised the system via increasing drug solubility and homogeneity in carrier polymer [29]. On another side, nicotinamide has significantly increased the solubility of atorvastatin calcium owing to its hydrotropic solubilisation either via formation of stacking complexation and/or via molecular aggregation [22,23] and hence chosen as a carrier for further ratio optimisation trials.
Table 3 shows the composition of solid dispersion complexes prepared for ratio optimisation trials along with its solubility data. Increasing drug to nicotinamide ratio has enhanced the solubility of drug linearly between the ratios studied. This suggest stacking complexation where at lower ratio drug and nicotinamide forms 1:1 complex; while at higher concentration sandwich type 1:2 complexation is likely to occur [22]. Similar observation was also reported by Aggarwal and Jain where nicotinamide formed complex with ketoconazole in a similar fashion as observed in the current study but with ketoconazole, the increase in solubility was non-linear at higher concentration which could be due to relatively higher concentration of nicotinamide used by Aggarwal and Jain [30]. The magnitude at which nicotinamide improve the solubility is also dependant on physicochemical properties of the molecules. For example, nicotinamide has increased the solubility of benzophenyl urea derivative by 4000 fold compared to merely 1.3 fold increased in solubility of phenobarbitone [22]. A study by Kim and co-workers [31] also revealed that physicochemical properties of the solutes such as presence of aromatic ring, hydrophobicity, hydrogen bonding ability plays significant role in solubilisation by hydrotrope and among them hydrophobicity of drug play vital role regardless of the presence or absence of aromatic ring but the presence of aromatic ring found to favour solubilisation between two equally hydrophobic solutes [31]. Also structure dependant interaction between nicotinamide and drug molecule contribute towards solubilisation effect where degree of hydrotropic solubilisation is depends upon magnitude of total energy drop due to inclusion of the hydrophobic solute in nicotinamide aggregate where change in total energy of the system is related to hydrogen bonds and hydrophobic interactions between nicotinamide and hydrophobic solute [32]. The broad crystalline peak for drug was disappeared in DSC curve (fig. 7) for solid dispersion complex suggest conversion of drug from crystalline to amorphous state. An additional broad crystalline peak appeared around 125° and this could be either due to presence of free nicotinamide in solid dispersion complex or may be a formation of impure co-crystal of atorvastatin calcium-nicotinamide. Reduced intensity of the major characteristic peaks in FTIR spectrum of solid dispersion complex for atorvastatin calcium (fig. 6) suggest molecular interaction of drug with carrier via formation of hydrogen bonds and possible conversion of drug from its crystalline and amorphous. Molecular interaction via formation of hydrogen bond is more likely to occur as atorvastatin calcium has 6 hydrogen bond donor and 12 acceptor site which can form a bond with nicotinamide who has 1 donor and two acceptor site. Therefore it suggests atorvastatin calcium is no longer present in its original crystalline form but exists either in amorphous state or molecularly dispersed with nicotinamide and therefore increase in solubility was observed. Further investigation is required to establish whether the drug is present in amorphous state or has formed co-crystal with nicotinamide using other techniques such as X-ray diffraction, nuclear magnetic resonance (NMR) and Raman Spectroscopy and will be subject of subsequent report.
Increasing the ratio of drug to surfactant and keeping nicotinamide constant at 1:1 level, has increased the solubility of drug from 11 fold (no surfactant was present) to 15.8 and 19 fold when drug to surfactant ratio of 1:0.2 and 1:0.6 was used, respectively. But in absence of nicotinamide (SD9), presence of surfactant merely increased the solubility by 3.2 fold, which is very less as compared to solubilisation effect in presence of nicotinamide alone without surfactant (SD11, 11 fold). Further increase in nicotinamide at drug to carrier ratio of 1:2 and keeping SDS constant (SD14), has significantly improved the solubility with 39.9 fold increase was seen with reference to solubility of pure drug. This remarkable enhancement in solubility was achieved despite using relatively low concentration of hydrotrope (~0.08 M in this study) than usually used in the literature [30,31,33] in presence of SDS as a surfactant. The further increase in nicotinamide concentration could enhance the solubility of the drug linearly or non-linearly but considering the toxicity potential of hydrotrope in formulation on administration and keeping in mind benefit to risk potential further increase in nicotinamide was not considered [31,33].
Solid dispersion complex comprising drug, nicotinamide and surfactant in ratio of 1:2:0.2 was incorporated into a tablet formulation with the aim to deliver the drug via orally disintegrating formulation and also to check whether incorporation of solid dispersion complex maintain high solubilisation power or not. Rapid disintegration (~21 sec) of formulated orally disintegrating tablets was observed in condition simulating oral cavity meeting the criteria laid out by regulatory agencies for orodispersible formulation. Rapid disintegration also enabled rapid dissolution of drug (fig. 8) from prepared tablets, which was brought about via local solubilisation effect of nicotinamide and surfactant as described earlier. Delivering drug at absorption site above its supersaturation would promote the drug absorption and hence bioavailability can be improved due to enhanced drug absorption [34] and therefore, non-sink dissolution was performed in the current study to evaluate the formulation performance. Solid dispersion complex and tablets comprising solid dispersion complex has delivered the drug rapidly in non-sink condition simulating the oral cavity. Therefore it can be assumed that pregastric absorption can be improved in oral cavity before drug reach to GI tract. Atorvastatin calcium belongs to BCS Class II with high permeability could ensure complete absorption as soon as drug is available in solution form to get absorbed. The permeability study would be a subject of subsequent investigation along with toxicity potential of the prototype formulation.
This study revealed that combining hydrotrope and surfactant have enhanced the solubility of atorvastatin calcium and has potential to overcome toxicity concern as combination of these required less amount of individual components to get desired increase in solubility, and could be explored further with other poorly soluble drugs. Incorporation of solid dispersion complex into microtablets has maintained its solubilisation power, which is essential for formulation performance. Development of such orally disintegrating micro tablets not only enhance the compliance in the patient suffering from swallowing difficulty but also hold great potential to increase the bioavailability due to rapid intra-oral dissolution which can provide supersaturated system at delivery site leading to rapid drug permeation via highly vascularised buccal mucosa before reaching into GI tract.
Acknowledgements
Authors would like to thank excipient suppliers and University of Hertfordshire for providing support to carry out this research.
References
- Nahata MC, Allen LV Jr. Extemporaneous drug formulations. ClinTher 2008;30:2112-9.
- Blumer JL. Off-label uses of drugs in children. Pediatrics 1999;104:598-602.
- Breitkreutz J, Wessel T, Boos J. Dosage forms for peroral administration to children. PaediatrPerinat Drug Ther 1999;3:25-33.
- Breitkreutz J, Boos J. Paediatric and geriatric drug delivery. Expert Opin Drug Deliv 2007;4:37-45.
- Cram A, Breitkreutz J, Desset-Brèthes S, Nunn T, Tuleu C. European Paediatric Formulation Initiative (EuPFI). Challenges of developing palatable oral paediatric formulations.Int J Pharm 2009;365:1-3.
- Lennernäs H. Clinical pharmacokinetics of atorvastatin.ClinPharmacokinet 2003;42:1141-60.
- Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins.PharmcolTher 1999;84:413-28.
- Cilla DD, Whitfield JL, Gibson DM, Sedman AJ, Posvar EL. Multipledose pharmacokinetics, pharmacodynamics, and safety of atorvastatin, an inhibitor of HMG-CoA reductase, in healthy subjects. ClinPharmacolTher 1996;60:687-95.
- Shayanfar A, Ghavimi H, Hamishekar H, Jouyban A. Coamorphous atorvastatin calcium to improve its physicochemical and pharmacokinetic properties. J Pharm PharmSci 2013;16:577-87.
- Options for patients unable to take solid oral dosage forms (tablets and capsules). Stock on Trent NHS. c2011. Available from: http://www.medicinesmanagementstoke.nhs.uk/documents/Crushing_Tabs_FAQs_ December_2011_FINAL.PDF. [Last accessed on 2013 May 3].
- Prescribing and Clinical effectiveness bulletin. Linconshire NHS. 2012;6:1-16. Available from: http://www.lincolnshire.nhs.uk/policies/ doc./390-vol-6-no-11-pace-bulletin. [Last accessed on 2013 May 3].
- Ajmera A, Deshpande S, Kharadi S, Rathod K, Patel K, Patel P. Dissolution rate enhancement of atorvastatin, fenofibrate and ezetimibe by inclusion complex with β-cyclodextrin. Asian J Pharm Clin Res 2012;5:73-6.
- Maurya D, Belgamwar V, Tekade A. Microwave induced solubility enhancement of poorly water soluble atorvastatin calcium. J Pharm Pharmacol 2010;62:1599-606.
- Kim M, Jin S, Kim J, Park HJ, Song H, Neubert RH, et al. Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent (SAS) process. Euro J Pharm Biopharm 2008;69:454-65.
- Kim J, Kim M, Park HJ, Jin S, Lee S, Hwang S. Physicochemical properties and oral bioavailability of amorphous atorvastatin hemi-calcium using spray-drying and SAS process. Int J Pharm 2008;359:211-9.
- Shamma RN, Basha M. Soluplus®: A novel polymeric solubilizer for optimization of carvedilol solid dispersions: Formulation design and effect of method of preparation. Powder Tech 2013;237:406-14.
- Okamoto H, Taguchi H, Iida K, Danjo K. Development of polymer film dosage forms of lidocaine for buccal administration I. Penetration rate and release rate. J Control Release 2001;77:253-60.
- Shalviri A, Sharma AC, Patel D, Sayani A. Low-surfactant microemulsions for enhanced topical delivery of poorly soluble drugs. J Pharm PharmSci 2011;14:315-24.
- Seedher N, Kanojia M. Micellarsolubilisation of some poorly soluble antidiabetic drugs: A technical note. AAPS PharmSciTech 2008;9:431-6.
- Suzuki H, Sunada H. Mechanistic studies on hydrotropic solubilisation of nifedipine in nicotinamide solution. Chem Pharm Bull 1998;46:125-30.
- Booth JJ, Abbott S, Shimizu S. Mechanism of hydrophobic drug solubilisation by small molecule hydrotropes. J PhysChem B 2012;116:14915-21.
- Sanghvi R, Evans D, Yalkowsky SH. Stacking complexation by nicotinamide: A useful way of enhancing drug solubility. Int J Pharm 2007;336:35-41.
- Coffman RE, Kildsig DO. Effect of nicotinamide and urea on the solubility of riboflavin in various solvents. J Pharm Sci 1996;85:951-4.
- Park S, Choi H. The effects of surfactants on the dissolution profiles of poorly water-soluble acidic drugs.Int J Pharm 2006;321:35-41.
- He X, Pei L, Tong HH, Zheng Y. Comparison of spray freeze drying and the solvent evaporation method for preparing solid dispersions of baicalein with Pluronic F68 to improve dissolution and oral bioavailability. AAPS PharmSciTech 2011;12:104-13.
- Dontireddy R, Crean A. A comparative study of spray-dried and freezedried hydrocortisone/polyvinyl pyrrolidone solid dispersions. Drug DevInd Pharm 2011;37:1141-9.
- Kamlesh K, Sharma V, Philip B, Pathak K. Preparation and evaluation of binary and ternary inclusion complex of Itraconazole. Der Pharmacia Lettr 2010;2:144-55.
- Pandey A, Rath B, Dwivedi A. Dissolution rate and bioavailability enhancement of co-ground mixtures of paliperidone with different hydrophilic carriers.IntCurr Pharm J 2013;2:70-7.
- Ghebremeskel AN, Vemavarapu C, Lodaya M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: Stability testing of selected solid dispersions. Pharm Res 2006;23:1928-36.
- Aggrawal AK, Jain S. Physicochemical characterisation and dissolution study of solid dispersions of ketoconazole and nicotinamide. Chem Pharm Bull 2011;59:629-38.
- Kim JY, Kim S, Papp M, Park K, Pinal R. Hydrotropic solubilisation of poorly water-soluble drugs. J Pharm Sci 2010;99:3953-65.
- Cui Y, Xing C, Ran Y. Molecular dynamics simulations of hydrotropic solubilisation and self-aggregation of nicotinamide. J Pharm Sci 2010;99:2048-59.
- Kim JY, Kim S, Pinal R, Park K. Hydrotropic polymer micelles as versatile vehicles for delivery of poorly water-soluble drugs. J Control Release 2011;152:13-20.
- Miller JM, Beig A, Carr RA, Spence JK, Dahan A. A win-win solution in oral delivery of lipophilic drugs: Supersaturation via amorphous solid dispersions increases apparent solubility without sacrifice of intestinal membrane permeability. Mol Pharm 2012;9:2009-16.
 ), solid dispersion complexes (
), solid dispersion complexes (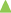 , SD14) and micro tablets (
, SD14) and micro tablets ( ) in artificial saliva (n=3).
) in artificial saliva (n=3).




