- *Corresponding Author:
- S. K. Sahu
University Department of Pharmaceutical Sciences, Utkal University, Vani Vihar, Bhubaneswar-751 004
E-mail: tutu_kh@yahoo.com
| Date of Submission | 21 March 2005 |
| Date of Revision | 18 July 2005 |
| Date of Acceptance | 05 June 2006 |
| Indian J Pharm Sci, 2006, 68 (3): 377-380 |
Abstract
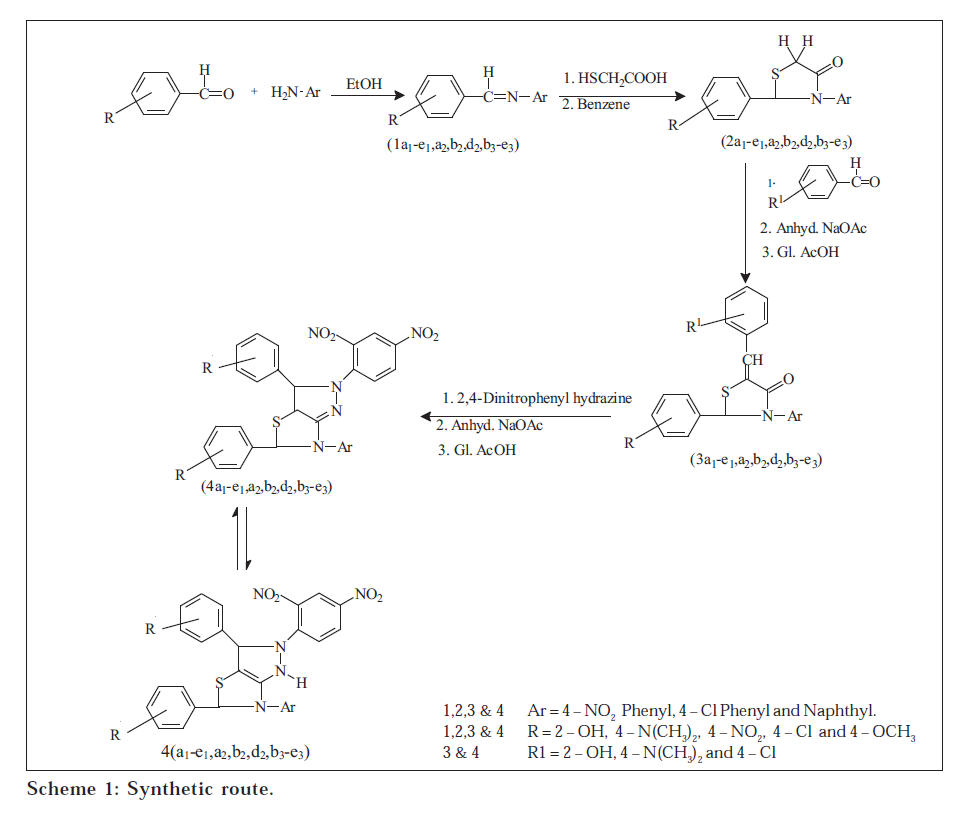
Condensation of substituted benzaldehydes with primary aryl amines gave a series of Schiff bases(1a 1 -e 1 ,a 2 ,b 2 ,d 2 ,b 3 -e 3 ) which, on reaction with thioglycolic acid, resulted in the formation of the corresponding 4-thiazolidinones(2a 1 -e 1 ,a 2 ,b 2 ,d 2 ,b 3 -e 3 ). These compounds, on condensation with substituted benzaldehydes in anhydrous sodium acetate, furnished 2-phenyl(substituted)-3-aryl-5-benzilidine(substituted)-thiazolidine-4-ones(3a 1 -e 1 ,a 2 ,b 2 ,d 2 , b 3 -e 3 ). The latter, on heating with 2,4-dinitrophenyl hydrazine in anhydrous sodium acetate, gave the title compounds(4a 1 -e 1 ,a 2 ,b 2 ,d 2 ,b 3 -e 3 ). The structures have been established on the basis of elemental analysis and spectral data. The title compounds have been screened in vitro for their possible antibacterial activity
Selected substituted thiazoles [1-3] as well as different heterocyclic systems containing pyrazole ring [4-6] possess potent biological activities. It is also believed that the presence of N-C-S linkage is responsible for the amoebicidal, anticonvulsant, fungicidal [7], and antiviral activities [8]. The present investigation deals with the development of a new series of nitrogen heterocyclic systems from easily available starting materials.
Substituted benzaldehydes on condensation with primary aryl amines gave Schiff bases (1a1-e1,a2,b2,d2,b3-e3) which, on reaction with thioglycolic acid in refluxing benzene, furnished the corresponding 4-thiazolidinones(2a1-e1,a2,b2,d2,b3-e3). The latter, on condensation with substituted benzaldehydes in the presence of anhydrous sodium acetate and glacial acetic acid, afforded the formation of 2-phenyl(substituted)-3-aryl-5-benzilidine (substituted)-thiazolidine-4-ones(3a1-e1,a2,b2,d2,b3-e3) which, in turn, heated with 2,4-dinitrophenyl hydrazine in the presence of anhydrous sodium acetate and glacial acetic acid, furnished the bridgehead nitrogen heterocyclic system, 2-(2,4-dinitrophenyl)-3,5-diphenyl(substituted)-6aryl-3,3a,5,6-tetrahydro-2H-pyrazolo[3,4-d]thiazoles(4a1-e1,a2,b2,d2,b3-e3) (Scheme 1). Newly synthesised compounds were characterised by IR, NMR spectral data and elemental analysis. The title compounds were evaluated for their possible antibacterial activity.
Melting points were determined in open capillaries and were uncorrected. Purity of the compounds was checked by TLC. IR spectra were recorded on a Jasco FT/IR 410 spectrophotometer in KBr disc. 1H NMR spectra were taken on a Bruker DPX, 300 MHz, spectrometer using TMS as internal reference. C, H, and N analyses were carried out on a Euro EA analyzer (Italy). The bacteria used in the antibacterial activity study were procured from the Department of Bacteriology and Virology, Faculty of Veterinary Science and Animal Husbandry, Orissa University of Agriculture and Technology, Bhubaneswar.
Schiff bases(1a1-e1,a2,b2,d2,b3-e3) and the corresponding 4-thiazolidinones(2a1-e1,a2,b2,d2,b3-e3) were synthesised following the reported method [9].
Synthesis of 2-phenyl (substituted)-3-aryl-5-benzilidine (substituted)-thiazolidine-4-ones(3a1-e1,a2,b2,d2,b3-e3) was achieved by refluxing an equimolar (0.001 mol) mixture of 4-thiazolidinone derivatives (2a1-e1,a2,b2,d2,b3-e3), substituted benzaldehydes, and anhydrous sodium acetate (0.082 g) in glacial acetic acid (20 ml) for 3 h. The reaction mixture was concentrated, cooled, and poured into ice-cold water. The solid thus separated was filtered, washed with water, dried, and recrystallised from glacial acetic acid. The yield and melting points are given in Table 1. (3c1): IR( KBr, vmax in cm-1): 3290(OH), 3045(CHarom.),1736(C=O), 1538(asym.NO2), 1337 (sym.NO2). (3a2): IR(KBr, vmax in cm-1): 3286(OH), 3026(CH-arom.), 1722(C=O), 754(C-Cl). (3b3): IR (KBr, vmax in cm-1): 3063(CH-arom.), 1742(C=O), 743(C-Cl). The NMR spectra of the synthesised compounds (3) of the series revealed peaks around 5.1-5.8 δ (s, 1H, C=CH) and 6.5-8.0 δ due to bulk aromatic protons.
| Compd. | Substituents | mp (°) | Yield (%) | Molecular formulaa | |||
|---|---|---|---|---|---|---|---|
| A r | R | R1 | |||||
| 3a1 | 4-NO2Ph | 2-OH | 2-OH | 152 | 47 | C22 H16N2 O5 S | |
| 3b1 | 4-NO2Ph | 4-N(CH3)2 | 2-OH | 88 | 45 | C24 H21N3 O4 S | |
| 3c1 | 4-NO2Ph | 4-NO2 | 2-OH | 132 | 44 | C22 H15N3 O6 S | |
| 3d1 | 4-NO2Ph | 4-Cl | 2-OH | 202 | 42 | C22 H15 N2 O4SCl | |
| 3e1 | 4-NO2Ph | 4-OCH3 | 2-OH | 190 | 46 | C23 H18N2 O5 S | |
| 3a2 | 4-Cl Ph | 2-OH | 4-N(CH3)2 | 108 | 48 | C24 H21 N2 O2SCl | |
| 3b2 | 4-Cl Ph | 4-N(CH3)2 | 4-N(CH3)2 | 86 | 58 | C26 H26N3OSCl | |
| 3d2 | 4-Cl Ph | 4-Cl | 4-N(CH3)2 | 118 | 48 | C24H20 N2 OSCl2 | |
| 3b3 | Naphthyl | 4-N(CH3)2 | 4-Cl | 206 | 64 | C28H23N2OSCl | |
| 3c3 | Naphthyl | 4-NO2 | 4-Cl | 98 | 65 | C26H17N2O3SCl | |
| 3d3 | Naphthyl | 4-Cl | 4-Cl | 80 | 49 | C26H17NOSCl2 | |
| 3e3 | Naphthyl | 4-OCH3 | 4-Cl | 95 | 69 | C27H20NO2SCl | |
Table 1: Physical Data Of The Synthesised Compounds (3a1-e1,a2,b2,d2,b3-e3)
Synthesis of 2-(2,4-dinitrophenyl)-3,5-diphenyl(substituted)6-aryl-3,3a,5,6-tetrahydro-2H-pyrazolo[3,4-d]thiazoles(4a1e1,a2,b2,d2,b3-e3) was performed by heating an equimolar (0.001 mol) mixture of 2-phenyl(substituted)-3-aryl-5benzilidine(substituted)-thiazolidine-4-ones (3a1-e1,a2,b2,d2, b3-e3), 2,4-dinitrophenyl hydrazine (0.198 g) and anhydrous sodium acetate (0.082 g) in glacial acetic acid (20 ml) under reflux for 6 h, which was then cooled to room temperature. The solid thus separated was filtered, washed thoroughly with water, dried, and recrystallised from glacial acetic acid. The yield and melting points are given in Table 2. (4c1):IR(KBr, vmax in cm-1): 3294(OH), 3031(CH-arom.), 1617(C=N), 1520(asym.NO2), 1346 (sym.NO2); 1H NMR(CDCL3) δ ppm: 3.18(s,1H,CH), 5.82 (s, 1H, CH), 6.60-8.75 (m, 15H, Ar-H), 11.20 (s, 1H, OH). (4a2): IR (KBr, vmax in cm-1): 3285 (OH), 3078 (CH-arom.), 1611 (C=N), 1539(asym.NO2), 1358 (sym.NO2), 751 (C-Cl); 1H NMR (CDCl3) δ ppm: 2.02 (s, 6H, 2×CH3), 3.16 (s, 1H, CH), 5.75 (s, 1H, CH), 5.90 – 7.97(m, 15H, Ar-H), 10.99 (s, 1H, OH). (4b3): IR(KBr, vmax in cm-1): 3059 (CH-arom.), 1614 (C=N), 1518 (asym. NO2), 1337 (sym. NO2), 756 (C-Cl); 1 H NMR (CDCl3) δ ppm: 1.98 (s, 6H, 2×CH3), 3.22 (s, 1H, CH), 5.83 (s, 1H, CH), 6.39- 8.88 (m, 18H, Ar – H).
| Compd. | Substituents | mp (°) | Yield (%) | Molecular formulab | |||
|---|---|---|---|---|---|---|---|
| A r | R | R1 | |||||
| 4a1 | 4-NO2Ph | 2-OH | 2-OH | 238 | 52 | C28H20N6O8 S | |
| 4b1 | 4-NO2Ph | 4-N(CH3)2 | 2-OH | 216 | 54 | C30 H25N7 O7 S | |
| 4c1 | 4-NO2Ph | 4-NO2 | 2-OH | 236 | 68 | C28H19N7O9S | |
| 4d1 | 4-NO2Ph | 4-Cl | 2-OH | 222 | 65 | C28H19N6O7SCl | |
| 4e1 | 4-NO2Ph | 4-OCH3 | 2-OH | 196 | 62 | C29H22N6O8S | |
| 4a2 | 4-Cl Ph | 2-OH | 4-N(CH3)2 | 180 | 60 | C30H24N6 O5SCl | |
| 4b2 | 4-Cl Ph | 4-N(CH3)2 | 4-N(CH3)2 | 200 | 42 | C32H30N7O4SCl | |
| 4d2 | 4-Cl Ph | 4-Cl | 4-N(CH3)2 | 138 | 68 | C30H24N6O4 SCl2 | |
| 4b3 | Naphthyl | 4-N(CH3)2 | 4-Cl | 160 | 64 | C34H27N6 O4SCl | |
| 4c3 | Naphthyl | 4-NO2 | 4-Cl | 244 | 63 | C32H21N6 O6SCl | |
| 4d3 | Naphthyl | 4-Cl | 4-Cl | 199 | 61 | C32H21N5O4 SCl2 | |
| 4e3 | Naphthyl | 4-OCH3 | 4-Cl | 220 | 65 | C33H24N5O5SCl | |
Table 2: Physical data of the synthesised compounds (4a1-e1,a2,b2,d2,b3-e3)
The IR spectra of the title compounds exhibited prominent peaks around 3080-3030 cm-1(CH-arom), 16201600 cm-1(C=N), 1550-1510 cm-1(asym. NO2) and 1360-1330 cm-1(sym. NO2). The NMR spectra of the final compounds (4) of the series revealed peaks around 3.16-3.22 δ (s, 1H, CH), 5.75-5.83 δ (s, 1H, CH) and 5.90-8.88 δ due to bulk aromatic protons. No doublet was seen in the NMR spectrum of any compound of the series, thus indicating that the initial structure got rapid transformation through the tautomeric shift of H-atom to the more stable structure, as indicated in the Scheme 1.
The antibacterial activity of the title compounds was determined by agar cup-plate method [10] against Staphylococcus aureus, Actinomycus pyoginus, Escherichia coli, and Klebsiella aeruginosa. The medium was prepared as per the instructions of the manufacturer of dry Mueller Hinton agar powder (Hi-Media). The test samples were dissolved in dimethyl sulphoxide (DMSO) at a concentration of 100 μg/ml. Ampicillin trihydrate (100 μg/ml) in DMSO was used as reference standard, and the solvent control (only DMSO) was also maintained throughout the experiment. The zones of inhibition are reported in Table 3.
| Compd. | Zone of inhibition (mm*) | |||
|---|---|---|---|---|
| S.a. | A.p. | E.c. | K.a. | |
| 4a1 | 17 | 15 | 16 | 18 |
| 4b1 | 16 | 19 | 18 | 17 |
| 4c1 | 18 | 16 | 15 | 19 |
| 4d1 | 21 | 18 | 19 | 20 |
| 4e1 | 16 | 15 | 17 | 18 |
| 4a2 | 19 | 20 | 21 | 17 |
| 4b2 | 22 | 21 | 20 | 21 |
| 4d2 | 21 | 22 | 23 | 22 |
| 4b3 | 21 | 20 | 22 | 23 |
| 4c3 | 20 | 22 | 23 | 24 |
| 4d3 | 22 | 23 | 24 | 22 |
| 4e3 | 20 | 18 | 19 | 17 |
| Ampicilintrihydrate | 29 | 30 | 32 | 31 |
| DMSO | 00 | 00 | 00 | 00 |
Table 3: Antibacterial activity data of the synthesised compounds (4a1-e1,a2,b2,d2,b3-e3)
Synthesised compounds were screened for antibacterial activity by agar cup-plate method, the results of which revealed promising activity for most of the test compounds. Among the compounds, 4b2, 4d2, 4b3, 4c3, and 4d3 were found to be most active against all the microbes tested. Even though the test compounds are less active with reference to the standard drug ampicillin trihydrate, the data reported in this article may be a helpful guide for the medicinal chemists who are working in the area.
Acknowledgements
The authors wish to thank the authorities of Utkal University, Bhubaneswar, for providing laboratory facilities.
References
- Sanfilippo, P.J., Urbanski, M.J., Beers, K.N., Eckardt, A., Falotico, R., Ginsberg, M.H., Offord, S., Press, J.B., Tighe, J. and Tomoko, K., J.Med. Chem., 1995, 38, 34.
- Pattan, S.R., Maste, M. and Angadi, J., Indian Drugs, 2002, 39, 429.
- Javed, S.A., Siddiqui, N. and Drabu, S., Indan J. Heterocycl.Chem., 2004, 13, 287.
- Solanki, P.R. and Wadodkar, K.N., Indan J. Heterocycl. Chem., 2003,13, 135.
- Budzisz, E. ,Krajewska, U., Rozalski, M., Szulawska, A., Czyz, M. and Nawrot, B., Eur. J. Pharm., 2004, 502, 59.
- Gaikawad, M.S., Mane, A.S., Shingare, B.B. and Shingare, M.S., Indian J. Heterocycl. Chem., 2004,13, 279.
- Joshi, H.D. ,Upadhyay, P.S. and Baxi, A.J., Indian J. Chem., 2000, 39B, 967.
- Al-khamees, H.A., Bayomi, S.M., Kandil, H.A. and El-Thahir, K., Eur. J. Med. Chem., 1990, 25, 103.
- Shrama, R.C. and Kumar, D., J. Indian Chem. Soc., 2000, 77, 492.
- Anonymous, British Pharmacopoeia, University Press, Cambridge, London, 1953, 796.