- *Corresponding Author:
- P. Gahtori
Department of Pharmacy, Kumaun University, Bhimtal-263 136, India
E-mail: pranioripharma@gmail.com
| Date of Submission | 20 March 2008 |
| Date of Revision | 04 August 2008 |
| Date of Acceptance | 07 February 2009 |
| Indian J Pharm Sci, 2009, 71 (1): 79-82 |
Abstract
In a wide search program towards new and efficient antibacterial agents, we assessed the extent to which physicochemical properties can be exploited to promote antibacterial activity associated with a series of substituted s-triazine. The synthesized compounds (1a-12b) were subsequently screened for their in vitro antibacterial activity against three gram positive ( Bacillus subtilis, Bacillus cereus, Staphylococcus aureus ) and three Gram-negative microorganism ( Salmonella typhi, Escherichia coli, Klebsiella aerogenes ) by the broth dilution technique, recommended by European Committee for antimicrobial susceptibility testing with reference to streptomycin.
Keywords
Bacterial resistance, physicochemical properties, s-Triazine, broth dilution technique, antibacterial activity
The extraordinary progress represented by the arrival of antibiotics has changed the medical prognosis of minor and major infections. However, over the years via several constantly changing mechanisms, many bacterial species acquired resistance to the most common classes of antibiotics. A relevant report on resistant antibacterial agents for human medicine is provided by World Health Organization. The panel agreed that the list of critically important antibacterial agents should be updated regularly as new information becomes available, including data on resistance patterns, new and emerging diseases and the development of new drugs[1].
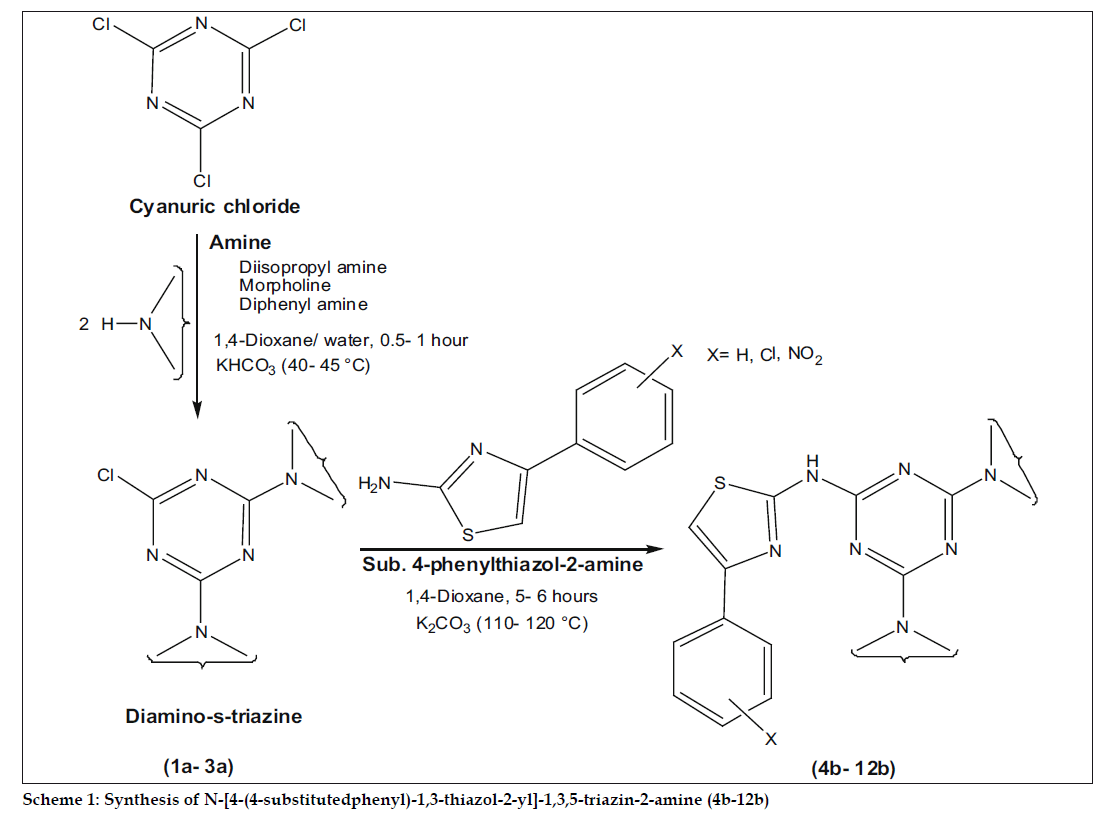
During the last few years the potential of s-triazine derivatives in molecular recognition, agrochemical and medicinal properties have been subjected to investigation. Literature survey reveals that substituted s-triazine derivatives are associated with number of pronounced antibacterial activities[2-4] against gram positive (B. subtilis, B. cereus and S. aureus) and gram negative organism (S. typhi, E. coli and K. aerogenes). The biological activity is a function of physicochemical properties of the targeted molecule and this assessment is made of the sorts of chemicals that might fit into an active site[5,6]. To randomly explore the novel compounds, our idea was to combine, cyanuric chloride with various amines possessing distinguish physicochemical properties. The substituted phenylthiazole-2-amine derivatives remain attractive, with their significant biological activities[7,8] and further incorporation of these derivatives giving access to a wide array of structures, which can be expected to show interesting antibacterial activities. In this wide search program, here we demonstrate various amines mediated substitution in cyanuric chloride and subsequently with higher bioactive, 4-sustituted phenylthiazole-2-amine to synthesize N-[4-(4-substituted phenyl)-1,3-thiazol-2-yl]-1,3,5- triazin-2-amine derivatives (scheme 1).
Phenyl, 4-chlorophenyl and 4-nitrophenyl thiazole- 2-amine derivatives were synthesized according to literature procedures[9,10], with the help of corresponding acetophenone, thiourea, thionyl chloride and bromine. The designed compounds were prepared in two step reactions, first step consists of nucleophilic substitution of two chlorines in cyanuric chloride in presence of aq. dioxane[11] with various amines like diisopropylamine, morpholine and diphenylamine to synthesize (1a-3a) diaminos- triazines (0.5-1 h) and second step involves further substitution of third chlorine in presence of 1,4-dioxane[12] with synthesized thiazole-2-amine to obtain a series (4b-12b) of N-[4-(4-substituted phenyl)-1,3-thiazol-2-yl]-1,3,5-triazin-2-amine (5-6 h).
Subsequently synthesized compounds were screened for their in vitro antibacterial activity (MIC) against three gram positive (Bacillus subtilis, Bacillus cereus, Staphylococcus aureus) and three gram negative microorganism (Salmonella typhi, Escherichia coli, Klebsiella aerogenes) by the broth dilution technique, recommended by European Committee for antimicrobial susceptibility testing (EUCAST) E. Dis 5.1[13] with reference to streptomycin. Nutrient agar (M090) and nutrient broth (M002) were procured from Himedia Laboratories, Mumbai. A set of sterilized test tubes with nutrient broth medium capped with cotton plugs (1–8). The test compound is dissolved in dimethylsulfoxide (512 μg/ml), which are serially diluted from 1 to 8. One millilitre of the standardized broth culture was added to 1 ml of each serially diluted test tube. The capped tubes with cotton plugs were incubated at 35-37° for 20 h and compared with standardized 0.5 McFarland reagent.
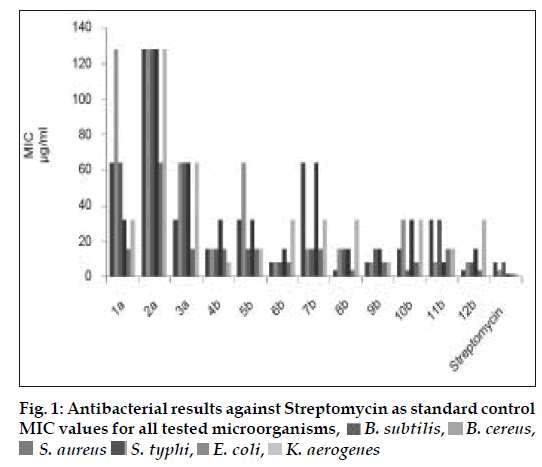
The MIC values (Table 1) of this class of synthesized compounds against tested organism displayed a significant activity with wide degree of variation (4-128 μg/ml). The preliminary antibacterial results showed that diamino-s-triazine (1a-3a) derivatives were significantly less active, among them diphenylamine substituted s-triazine were more efficient substituent for the entire tested microorganisms. Further a considerable difference in antibacterial activity between (1a-3a) disubstituted, diamino-s-triazine (MIC= 16-128 μg/ml) and (4b-12b) trisubstituted, N-[4-(4-substitutedphenyl)-1,3-thiazol- 2-yl]-1,3,5-triazin-2-amine (MIC= 4-64 μg/ml), emphasized the importance of incorporating bulky groups. All of the biologically active substituted s-triazine derivatives show lipophilic traits. Possibly, this is because of the enhanced ability of compound to penetrate the cell permeability barrier. The substituted s-triazine derivatives (6b, 9b and 12b have shown significant antibacterial activity, suggesting that the 4-nitrophenyl thiazole-2-amino group was the all-important functional moiety (fig. 1).
| Compound | MIC (µg/ml) | |||||
|---|---|---|---|---|---|---|
| B. subtilis | B. cereus | S. aureus | S. typhi | E. coli | K. aerogenes | |
| 1a | 64 | 128 | 64 | 32 | 16 | 32 |
| 2a | 128 | 128 | 128 | 128 | 64 | 128 |
| 3a | 32 | 64 | 64 | 64 | 16 | 64 |
| 4b | 16 | 16 | 16 | 32 | 16 | 8 |
| 5b | 32 | 64 | 16 | 32 | 16 | 16 |
| 6b | 8 | 8 | 8 | 16 | 8 | 32 |
| 7b | 64 | 16 | 16 | 64 | 16 | 32 |
| 8b | 4 | 16 | 16 | 16 | 4 | 32 |
| 9b | 8 | 8 | 16 | 16 | 8 | 8 |
| 10b | 16 | 32 | 4 | 32 | 8 | 32 |
| 11b | 32 | 8 | 32 | 8 | 16 | 16 |
| 12b | 4 | 8 | 8 | 16 | 4 | 32 |
| Streptomycin | 8 | 4 | 8 | <2 | <2 | <2 |
| *DMSO as negative control | ||||||
Table 1: In Vitro Antibacterial Activity of the Synthesized Compounds.
Today up to 75% of antibiotic use is said to be of questionable therapeutic value, antibiotic use is the key driver of resistance. We have presented a new economical synthesis of N-[4-(4-substitutedphenyl)- 1,3-thiazol-2-yl]-1,3,5-triazin-2-amine. The operational simplicity, significant short reaction times and relevant antibacterial results can impose this procedure as a useful and attractive alternative.
Acknowledgements
The authors acknowledge the valuable contributions made to their work by the AICTE, New Delhi for grating a scholarship and Lonza, Switzerland for arranging gift sample of cyanuric chloride for the work presented in this report.
References
- Critically important antibacterial agents for human medicine for risk management strategies of non-human use, a report of a WHO working group consultation, Canberra, Australia, February 2005. p. 15-18.
- Nishigaki S, Yoneda UH, Tsumoto H, Jiorinaga AI. Synthetic Antibacterials. I. Nitrofurylvinyl-s-triazine Derivatives. J Am Chem Soc 1969;12:39-42.
- Zhou Y, Sun Z, Froelich JM, Hermann T, Wall D. Structureactivity relationships of novel antibacterial translation inhibitors; 3,5-Diamino-piperidinyl triazines. Bioorg Med Chem Lett 2006;16:5451-6.
- Srinivas K, Srinivas U, Bhanuprakash K, Harakishore K, Murthy US, Rao VJ. Synthesis and antibacterial activity of various substituted s-triazines. Eur J Med Chem 2006;41:1240-6.
- Gubernator K, Bohm HJ. Structure-based ligand design, methods and principles in medicinal chemistry. Weinheim: Wiley-VCH Publishers; 1998.
- Weiner DB, Williams WV. Chemical and structural approaches to rational drug design. Boca Raton, FL: CRC; 1994.
- Metwally MA, Abdel-Latif E, Amer FA, Kaupp G. Versatile 2-amino-4-substituted 1,3-thiazoles: Synthesis and reactions. J Sulfur Chem 2004;25:63-85.
- Yamaguchi K, Yada M, Tsuji T, Hatanaka Y, Goda K, Kobori T. 4-Phenylthiazole derivatives inhibit IL-6 secretion in osteoblastic cells and suppress bone weight loss in ovariectomized mice. Bioorg Med Chem Lett 1999;9:957-60.
- Tarayre JP, H. Cousse H, Mouzin G, Lauressergues H, Casadio S, Milan. Immunoactivators derived from amino thiazoles. US patent 4,225,610. 1980.
- Dickey JB, Torne EB, Bloom MS, Moore WH, Heynemann H, Hedberg DG, et al. Azo Dyes from Substituted 2-Aminothiazoles. J Org Chem 1959;24:187-96.
- Thruston JT, Dudley JR, Kaiser DW, Henbleikner I, Schaefer FC, Holm-Hensen. D. Cyanuric Chloride Derivatives. I. Aminochloro-striazines. J Am Chem Soc 1951;73:2981-3.
- Modha JJ, Datta N, Parekh H. Synthesis and biological evaluation of some new 3,4-dihydropyrimidin-4-ones. IL Farmaco 2001;56:641-6.
- Eucast definitive document E. Def. 3.1, Clinical Microbiology and Infection August 2003;9:1-7.
 B. subtilis,
B. subtilis,  B. cereus,
B. cereus,  S. aureus
S. aureus  S. typhi,
S. typhi,  E. coli,
E. coli,  K. aerogenes
K. aerogenes