- Corresponding Author:
- P. Mishra
Pharmaceutical Chemistry Division, Department of Pharmaceutical Sciences, Dr. H. S. Gour University, Sagar-470 003, India.
E-mail: pmishra51@rediffmail.com
| Date of Submission | 28 January 2005 |
| Date of Revision | 26 June 2005 |
| Date of Acceptance | 28 May 2006 |
| Indian J Pharm Sci, 2006, 68 (3): 360-363 |
Abstract
With a view to explore the versatile lead molecule 4(3H)-quinazolinones, a series of novel 2-methyl-3-(1'3'4'-thiadiazoyl)-4-(3H) quinazolinones have been synthesised by reacting 2-amino-5-aryl/alkyl-1'3'4'-thiadiazoyl with 2-substituted benzoxazin-2-one. The designed compounds (5a-f) were screened in vitro for antibacterial activity on Staphylococcus aureus , Bacillus subtilis , and Escherichia coli . Antifungal activity was screened against Candida albicans , Aspergillus niger , and Curvularia lunata . Synthesised compounds exhibited both antibacterial and antifungal activity.
Quinazolinone is a versatile lead molecule for designing potential bioactive agents. 4(3H)-Quinazolinone and its derivatives have been reported to exhibit analgesic, anaesthetic, antibacterial, anticancer, anticonvulsant, antimicrobial, antihypertensive, antiinflammatory, diuretic, muscle-relaxant, sedative, diuretic, CNS-depressant, and tranquilizer properties[1-6]. We have already reported the biological activities of 1, 3, 4-thiadiazoles, including antimicrobial activities[7-8]. This work was undertaken with a view to explore the possibility of antimicrobial activity in the present nucleus, which has both 4(3H)-quinazolinone and 1’3’4’-thiadiazoles in it.
Six new 2-methyl-3-(1’3’4’-thiadiazoyl)-4(3H)-quinazolinones were synthesised and evaluated for their antimicrobial and antifungal activity. Anthranilic acid reacts with acetic anhydride and acetic acid to form corresponding 2-methylbenzoxazin-2one by N-acylation, followed by dehydrative cyclization mechanism[9]. The 2-methylbenzoxazin-2-ones condensed with the primary amino groups of 1’3’4’-thiadiazolyl group (aromatic and aliphatic) to form 2-methyl-3-(1’3’4’-thiadiazoyl)-4(3H)quinazolinones. Melting points were determined in open glass capillary and are uncorrected. The purity was checked by TLC using silica gel G as stationary phase and hexane: ethanol: chloroform (1:3:6) as mobile phase. The structure of the synthesised compounds were elucidated by using Shimadzu 470 IRspectrophotometer in KBr disc (υ max in cm-1). 13C NMR spectra were recorded on a C13 Avance Bruker 300 MHz spectrometer. Mass spectra were obtained on a Varian Atlas CH-7 mass spectrometer at 70 eV. Nitrogen analysis was obtained from RSIC, CDRI, Lucknow.
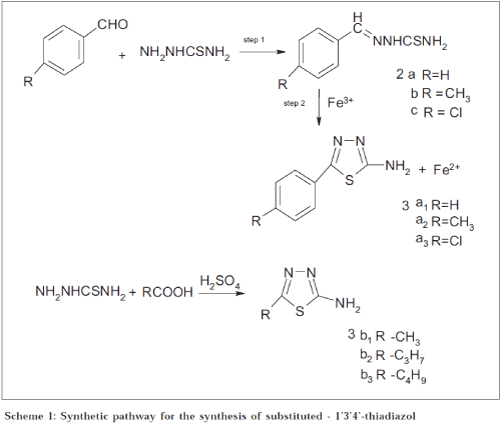
Synthesis of 2-amino-5-aryl 1’3’4’ thiadiazoles (3a) was achieved[10-12] usinga modification of the original procedure of Ranga Rao and Srinivasan[13] was modified and used. Aromatic aldehyde in warm alcohol (300 ml) was added to a solution of thiosemicarbazide (0.2 mol) in hot water (300 ml) slowly with continuous stirring. The products, which separated, were filtered off and recrystallised from 50% aqueous ethanol. 2a: R = -H, yield 92%, mp: 159-160°; 2b: R = -CH3, yield 95%, mp: 162°; 2c: R = -Cl, yield 90%, mp: 207-209°. Thiosemicarbazone (2) (0.05 mol) was suspended in 300 ml distilled water in a 1000 ml beaker. Ferric chloride (0.15 mol) in 300 ml distilled water was added to it. The contents were heated and maintained at 80-90° for an hour. Then it was filtered hot. A mixture of citric acid (0.11 mol) and sodium citrate (0.05 mol) was added to the solution and stirred. After cooling, the whole solution was taken in a bigger vessel (to account for the increase in volume) and neutralized with 10% aqueous ammonia. The precipitate that separated out was filtered and recrystallised from 25% aqueous ethanol except 3a3, which was recrystallised from 50% ethanol. 3a1: R = -H, yield 65%, mp: 224-225°; 3a2: R = -CH3, yield 62%, mp: 214-215°; 3a3: R = -Cl, yield 70%, mp: 227-229°.
Synthesis of 2-amino-5-alkyl-1’3’4’-thiadiazoles (3b) was completed as shown in Scheme 1[14]. Required fatty acid (0.15 mol), concentrated sulphuric acid (Analar, sp. gr. 1.84, 25 ml), and thiosemicarbazide (0.125 mol) were slowly heated to 80-90° on a thermostatically controlled water bath for 7 h. After cooling, the content was poured on crushed ice. The acid was neutralised with the help of 10% ammonia solution. The crude precipitate that got separated was filtered and washed several times with distilled water and dried. Final products were recrystallised from hot water except 3b3 which was, recrystallised from 50% ethanol. 3b1: R = -H, yield 65%, mp: 231°; 3b2: R = -C3H7, yield 85%, mp: 204-205°; 3b3: R = -C4H9, yield 80%, mp: 195°.
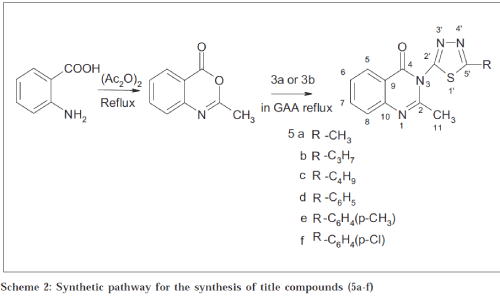
Synthesis of the title compounds 5a-f[15] is shown in Scheme 2. A mixture of recrystallised anthranilic acid (0.01 mol), acetic anhydride (0.02 mol), and acetic acid (0.01 mol) was taken in a round-bottomed flask and refluxed for 4 h. The acetic acid together with acetic anhydride was distilled off under reduced pressure. The compound 3(a and b) (0.01 mol) in glacial acetic acid was added to the benzoxazine-4-one (4) slowly and refluxed for 4-5 h. The mixture so obtained was added to crushed ice and the separated precipitate was filtered off. Recrystallisation was carried out from 25% aqueous ethanol, except (5a) in hot water; (5f) with 50% ethanol; (5b) and (5c) with ethanol. All the synthesised compounds (5a-f) were characterised by nitrogen analysis. IR and NMR spectra were consistent with the assigned structure. Melting point, nitrogen analysis, IR and NMR study is presented in Table 1.
| C. No. | Mol. formula | Mol. formula | % nitrogen calc/found | MP0 | IR (KBr) cm-1 | 13NMR benzene+ methanol δppm | |
|---|---|---|---|---|---|---|---|
| 5a | CH3 1” | C12 H10 N4 SO | 21.7/20.87 | 168-170 | C-H Str(aro)-3020, C-H def 4H atom-760, C-H str (alkyl) 2850, C-H def (alkyl)-1450, C=Cstr-1650, C=O str-1690, C-N str –1310, C=N str-1630, C-S str-650 | C-11 (13.5), C-1” (21), C-8 (118.5), C-6 (121), C-9(128), C-5 (130), C-7 (133.7), C-10 (142), C-5’ (140), C-2 (158), C-5 (168), C-4 (170) | |
| 5b | CH2 CH2 CH31” 2” 3” | C14 H14 N4 SO | 19.5/18.95 | 158-160 | C-H Str (aro)-3020, C-H def 4H atom-760, C-H str (alkyl) 2850, C-H def (alkyl)-1450, C=Cstr-1650, C=O str-1690, C-N str –1310, C=N str-1630, C-S str-650 | C-11 (13.5), C-1” (21), C-8 (118.5), C-6 (121), C-9(128), C-5 (130), C-7 (133.7), C-10 (142),C-5’ (140), C-2 (158), C-5 (168), C-4 (170), C-2” (32.1), C-3” (22.5) | |
| 5c | CH2 CH2 CH2 CH3 1” 2” 3” 4” | C15 H16 N4 SO | 18.6/18 | 160-162 | C-H Str (aro)-3020, C-H def 4H atom-760, C-H str (alkyl) 2850, C-H def (alkyl)-1450, C=Cstr-1650, C=O str-1690, C-N str –1310, C=N str-1630, C-S str-650 | C-11 (13.5), C-1” (21), C-8 (118.5), C-6 (121), C-9(128), C-5 (130), C-7 (133.7), C-10 (142), C-5’ (140), C-2 (158), C-5 (168), C-4 (170), C-2” (32.1), C-3” (22.5), C-4”(15.0) | |
| 5d | 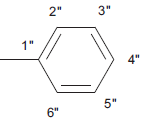 |
C17H12 N4 SO | 17.5/17.3 | 172-174 | C-H Str (aro)-3020, C-H def 4H atom-760, C-H str (alkyl) 2850, C-H def (alkyl)-1450, C=Cstr-1650, C=O str-1690, C-N str –1310, C=N str-1630, C-S str-650, mono substituted phenyl ring-760 | C-11 (13.5), C-1” (21), C-8 (118.5), C-6 (121), C-9(128), C-5 (130), C-7 (133.7), C-10 (142), C-5’ (140), C-2 (158), C-2’ (168, C-4 (170), C-1” (134), C-2” and C-6” (130.3), C-3” and C-5” (138.2), C-4” (127.7). | |
| 5e | 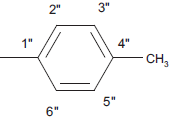 |
C18 H12 N4 SO | 16.7/16.1 | 180-182 | C-H Str (aro)-3020, C-H def 4H atom-760, C-H str (alkyl) 2850, C-H def (alkyl)-1450, C=Cstr-1650, C=O str-1690, C-N str –1310, C=N str-1630, C-S str-650, 1,4-substituted phenyl ring 830-815 | C-11 (13.5), C-1” (21), C-8 (118.5), C-6 (121), C-9(128), C-5 (130), C-7 (133.7), C-10 (142), C-5’ (140), C-2 (158), C-2’ (168, C-4 (170), C-1” (134), C-2” and C-6” (130.3), C-3” and C-5” (138.2), C-4” (127.7), C-7” (21.5) | |
| 5f | 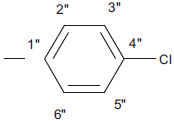 |
15.7/15 | 189-190 | C-H Str (aro)-3020, C-H def 4H atom-760, C-H str (alkyl) 2850, C-H def (alkyl)-1450, C=Cstr-1650, C=O str-1690, C-N str –1310, C=N str-1630, C-S str-650, 1,4-substituted phenyl ring 830-815 | C-11 (13.5), C-1” (21), C-8 (118.5), C-6 (121), C-9(128), C-5 (130), C-7 (133.7), C-10 (142), C-5’ (140), C-2 (158), C-2’ (168, C-4 (170), C-1” (134), C-2” and C-6” (130.3), C-3” and C-5” (138.2), C-4’ (132.4) | ||
Table 1: Molecular Formula, Nitrogen Estimation, Melting Point, Ir And Nmr Study of the Synthesised Molecules
All the compounds were screened for antibacterial and antifungal activity. Liquid dilution technique was used for determining minimum inhibitory concentration (MIC) of the synthesised compounds. Bacterial strains of Staphylococcus aureus, Escherichia coli (Gram-negative), and Bacillus subtilis (Gram-positive); and fungal strains of Candida albicans, Aspergillus niger, and Curvularia lunata obtained from Institute of Microbial Technology, Chandigarh, were used in the present study. Nutrient broth (beef extract 1 g, yeast extract 2 g, peptone 5 g, sodium chloride 5 g, distilled water q.s. 1000 ml) was used as growth medium for bacteria, and Sabouraund’s medium (dextrose 40 g, peptone 10 g, distilled water q.s. 1000 ml) was used for growth of fungi. Cook’s procedure of serial dilution and observation for presence of growth was used in the determining of the MIC. The MIC was taken as the lowest concentration (highest dilution) without visible growth. Dimethylformamide was used as solvent for the compounds. The activity was compared with known standard drug norfloxacin and clotrimazole for antibacterial and antifungal activity, respectively. A blank test was done to check the antimicrobial activity of DMF. The results have been presented in Table 2.
| (MIC in µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Comp. No. | R | Antibacterial activity | Antifungal activity | |||||
| E. coli | B. subtils | S. aureus | A. niger | C. albican | C. lunata | |||
| 5a | -CH3 | 95 | 91 | 92 | 67 | 73 | 77 | |
| 5b | -C3H7 | 92 | 90 | 89 | 76 | 65 | 78 | |
| 5c | -C4H9 | 88 | 86 | 93 | 54 | 59 | 61 | |
| 5d | -(C6 H5) | 93 | 95 | 90 | 74 | 76 | 69 | |
| 5e | -(C6H4) pCH3 | 90 | 98 | 97 | 70 | 68 | 74 | |
| 5f | -(C6H4) p-Cl | 85 | 88 | 86 | 50 | 58 | 52 | |
| Norfloxacin | 10 | 12 | 11 | - | - | - | ||
| Clotrimazole | - | - | - | 13 | 15 | 14 | ||
Table 2: Antibacterial And Antifungal Activity of The Prepared Molecules (5a-F)
Synthesised new chemical entities were screened for antibacterial potential against Staphylococcus aureus, Bacillus subtilis, and Escherichia coli; and for antifungal potential against Candida albicans, Aspergillus niger, and Curvularia lunata by liquid dilution technique. It has been observed that all the compounds tested showed mild to moderate activity against tested bacteria and fungi. Overall results showed that compounds possess better fungitoxic properties than bacterotoxic. Compound no. 5f emerged as potent antibacterial and antifungal. Escherichia coli and Aspergillus niger were the most susceptible bacteria and fungi, respectively. Compound no. 5c exhibited comparable (in comparison to 5f) activity for Bacillus subtilis and Curvularia lunata.
Acknowledgements
The authors are thankful to RSIC, CDRI, Lucknow; and Sophisticated Instruments Division of NIPER, Chandigarh, for spectral and nitrogen analysis, respectively. Two of the authors, VJ and SKJ, are thankful to UGC for financial assistance.
References
- Ossman, A.R.E. and Barakat, S.E., Arzneim. Forsch-Drug Res., 1994, 44, 915.
- Kacker, I.K. and Zaheer, S.H., J. Indian Chem. Soc., 1951, 28, 344.
- Kaussi, A.A., Egypt. J. Pharm. Sci., 1979, 20, 383.
- Mishra, P., Gupta, P.N. and Shakya, A.K., J. Indian Chem. Soc., 1991, 68, 618.
- Mishra, P., Sanmati, J.K. and Jain, S., J. Indian Chem. Soc., 1997, 74, 816.
- Mishra, P., Paneerselvum, P. and Jain, S., J. Indian Chem. Soc., 1995, 72, 559.
- Shakya, A.K., Patnaik, G.K. and Mishra, P, Eur. J. Med. Chem., 1992, 27, 1.
- Mishra, P., Shakya, A.K. and Agarwal, R.K., J. Indian Chem.Soc., 1990, 67, 520.
- Zentmyer, D.T. and Kangner, E.C., J. Org. Chem., 1949, 14, 967.
- Young, G. and Eyre, W.J., J. Indian Chem. Soc., 1901, 54.
- Bernotein, J., Yale, H.L., Losee, K., Holsing, M., Martins, J. and Lott, W.A. J. Amer. Chem. Soc., 1957, 73, 906.
- Maffii, G., Testa, E., Ettorre, R., Farmaco Sci., 1958, 13, 187.
- Range, V.R. and Srinivasan, V.R., Indian J. Chem., 1970, 8, 509.
- Funatsukuri, G. and Veda, M., Japan Pat., 1966, 20, 944 through Chem. Abst., 1969, 66, 46430.
- Bogert, M.T. and Seil, H.A., J. Amer. Chem. Soc., 1905, 1305.