- *Corresponding Author:
- N. M. Goudgaon
Department of studies and research in chemistry, Gulbarga university, Gulbarga-585 106, India
E-mail: naganna_g@yahoo.com
| Date of Submission | 27 February 2009 |
| Date of Revision | 24 August 2009 |
| Date of Acceptance | 25 November 2009 |
| Indian J Pharm Sci, 2009, 71 (6): 672-677 |
Abstract
4-Substituted-5-iodo-2-benzylthiopyrimidines were prepared efficiently in three steps. 2-Benzylthiopyrimidine on iodination in presence of base gave 5-iodo-2-benzylthiopyrimidine (1), which on chlorination with excess of POCl 3 furnished 4-chloro-5-iodo-2-benzylthiopyrimidine (2). Reaction of 2 with substituted aromatic amines, 2-aminopyridine and hydrazine hydrate yielded 4-amino-5-iodo-2-benzylthiopyrimidines 3(a-e), (3f) and (3g) respectively. Further, 4-hydrazino-5-iodo-2-benzylthiopyrimidine on condensation with substituted aromatic and heterocyclic aldehydes afforded the corresponding schiff bases 4(a-h). The structure of synthesized compounds have been established by spectral studies and elemental analysis. Synthesized compounds have been screened for antimicrobial activity. Compound 3f exhibited good antifungal activity against A. niger. The compounds 4a, 4c, 4d, 4g and 4h exhibited good antibacterial activity.
Keywords
Antibacterial, Antifungal, sSchiff bases, 5-Substituted pyrimidine
Pyrimidine derivatives have proved to be of great importance in exhibiting therapeutic applications [1]. A large number of pyrimidine nucleosides are clinically useful for the control of retroviral infections [2-5]. One of the important class of anti-herpetic nucleosides is series of 5-substituted uracil nucleosides such as (E)-5-(2-bromovinyl)-2'-deoxyuridine (BVdu) [6] showed specific antivaricella zoster virus (VZV) activity. In view of effect of 5-substitution on the activity of thiouracil, synthesis and antithyroid activity of several 5-substituted pyrimidine derivatives have been reported [7]. In addition, 5-alkyl or 5-aryl-substituted pyrimidine derivatives are useful intermediates in the synthesis of nucleosides [8]. Also, many 5-substituted pyrimidines have shown inhibitory activity against Streptococcus faecalis R growth [9] and some are evaluated as inhibitors of enzymes involved in the pyrimidine catabolism like dihydrouracil dehydrogenase and uridine phosphorylase [10]. Further, 5-iodo substituted pyrimidine analogs are known for their antimicrobial [11] and antiviral activity [12,13]. Synthesis and biological activities of several compounds derived from pyrimidine analogs were reported from our laboratory [10,14,15]. We report herein the synthesis and antimicrobial activities of 4-amino-5-iodo-2-benzylthiopyrimidines (3a-g) and 4-aryl/heteroarylidenehydrazino-5-iodo-2- benzylthiopyrimidines (4a-h).
Melting points were recorded by using Thomas- Hoover melting point apparatus and were uncorrected. IR spectra in KBr disc were recorded on Perkin- Elmer-Spectrum-one FT IR spectrophotometer (νmax in cm-1) and 1H NMR in DMSO-d6 and/or CDCl3 on amx 400, 400 MHz spectrophotometer using TMS as internal standard (chemical shift in δ ppm). Mass spectra were recorded on a Jeol SX 102 Mass spectrometer using argon/xenon (6kv, 10 mA) as the FAB gas. Purity of the compounds was checked by TLC using silica gel ‘G’ plates obtained from Whatman Inc, and a fl uorescent indicator. 5-Iodo-2- benzylthiouracil (1) was prepared by following the known literature method [7].
General procedure for the synthesis of 4-chloro-5- iodo-2-benzylthiopyrimidine (2) is as follows, to a mixture of 5-iodo-2-benzylthiouracil (1) (0.01 mol) and POCl3 (0.06 mol) was refluxed for 1 h. Excess POCl3 was distilled under reduced pressure and the reaction mixture was poured into 200 ml of ice cold water. The solid separated was extracted with ether (3 x 100 ml) and the ether extracts were washed with 5% aq. NaHCO3 solution (3 x 100 ml), followed by water (3 x 100 ml). Ether layer was dried over anhydrous MgSO4. Solvent was evaporated to produce pale yellow syrup. The syrup slowly solidified and recrystallized from ethanol; 2: IR (KBr, cm-1): 3026 (aromatic C-H str), 1599 (C=N). 1H NMR (CDCl3): δ 8.69 (1H, s, Ar-H, Pyrimidine), 7.44-7.26 (5H, m, Ar-H), 4.36 (2H, s, S-CH2-Ph).
General procedure for the synthesis of 4-amino-5- iodo-2-benzylthiopyrimidines (3a-g) is as follows, to a solution of 4-chloro-5-iodo-2-benzylthiopyrimidine (0.001 mol) in methanol (20 ml) and pyridine (0.5 ml), appropriate primary amine (0.001 mol) was added. The reaction mixture was refl uxed for 4 h on a steam-bath. Excess of methanol was removed under reduced pressure and the residue triturated with a little crushed ice and aqueous layer was neutralized with 0.1N HCl. Solid separated was fi ltered and washed with cold water. Recrystallized the crude product from ethanol furnished the desired compounds (3a-g); 3a: IR (KBr, cm-1): 3379 (N-H), 1598 (C=N). 1H NMR (DMSO-d6): δ 8.27 (1H, s, Ar-H, pyrimidine), 7.68 (1H, s, N-H), 7.33-7.07 (9H, m, Ar-H), 4.17 (2H, s, S-CH2-Ph), 2.27 (3H, s, CH3). MS m/z: 433 (M+) and fragmented peaks at 341, 308, 214. Analysis calculated for C18H16IN3S: C, 50.0; H, 3.73; N, 9.72. Found: C, 49.98; H, 3.70; N, 9.65%; 3b: IR (KBr, cm-1): 3300 (N-H), 1580 (C=N). 1H NMR (CDCl3): δ 8.22 (1H, s, Ar-H, pyrimidine), 8.03 (1H, s, N-H), 7.7-6.78 (9H, m, Ar-H), 4.31 (2H, s, S-CH2-Ph), 3.7 (3H, s, OCH3). Analysis calculated for C18H16IN3OS: C, 48.21; H, 3.571; N, 9.372. Found: C, 48.18; H, 3.565; N, 9.365%; 3c: IR (KBr, cm-1): 3447 (N-H), 1603 (C=N), 1525 (NO2). 1H NMR (CDCl3): δ 8.5 (1H, s, Ar-H, pyrimidine), 7.4-7.26 (10H, m, Ar-H, N-H), 4.38 (2H, s, S-CH2-Ph). Analysis calculated for C17H13IN4O2S: C, 43.83; H, 2.281; N, 12.09. Found: C, 43.78; H, 2.275; N, 12.05%; 3d: IR (KBr, cm-1): 3356 (N-H), 1602 (C=N), 1539 (NO2). 1H NMR (DMSO-d6): δ 8.69 (1H, s, Ar-H, pyrimidine), 8.4 (1H, s, N-H) 7.98-7.22 (9H, m, Ar-H), 4.39 (2H, s, S-CH2-Ph); 3e: IR (KBr, cm-1): 3402 (NH2), 3303 (NH), 1616 (C=N). 1H NMR (CDCl3): δ 8.34 (1H, s, Ar-H, pyrimidine), 7.23-6.8 (12H, m, Ar-H, N-H), 4.12 (2H, s, S-CH2-Ph). MS m/z: 434 (M+) and fragmented ion peaks at 342, 214. Analysis calculated for C17H15IN4S: C, 47.11; H, 3.464; N, 12.93. Found: C, 47.08; H, 3.460; N, 12.88%; 3f: IR (KBr, cm-1): 3427 (N-H), 1602 (C=N). 1H NMR (CDCl3): δ 8.5 (1H, s, Ar-H, pyrimidine), 7.40-7.26 (10H, m, Ar-H, N-H), 4.38 (2H, s, S-CH2-Ph). Analysis calculated for C16H13IN4S: C, 45.73; H, 3.126; N, 13.33. Found: C, 45.71; H, 3.120; N, 13.28%; 3g: IR (KBr, cm-1): 3303 (NH2), 3239 (N-H), 1624 (C=N). 1H NMR (DMSO-d6): δ 7.82 (1H, s, Ar-H, pyrimidine), 7.29 (1H, s, N-H), 7.26-6.9 (5H, m, Ar-H), 4.04 (2H, s, S-CH2-Ph), 3.83 (2H, s, NH2). MS m/z: 358 (M+) and fragmented ion peak at 342, 231. Analysis calculated for C11H11IN4S: C, 36.97; H, 3.083; N, 15.68. Found: C, 36.90; H, 3.080; N, 15.64%.
General procedure for the synthesis of 4-aryl/heteroarylidinehydrazino-5-iodo-2- benzylthiopyrimidines (4a-h) as follows, to a solution of 4-hydrazino-5-iodo-2-benzylthiopyrimidine (3 g, 0.001 mol) in ethanol (20 ml) and catalytic amount of concentrated HCl, appropriate aromatic aldehyde (0.001 mol) was added. The reaction mixture was refluxed for 4 h. Excess ethanol was removed under reduced pressure, solid separated was fi ltrated and recrystalised from ethanol to get the desired compounds; 4a: IR (KBr, cm-1): 3419 (N-H), 1628 (C=N). 1H NMR (DMSO-d6): δ 8.69 (1H, s, Ar-H, pyrimidine), 8.27 (1H, s, N-H), 7.62 (1H, s, N=CH), 7.37-6.84 (10H, m, Ar-H, O-H), 4.41 (2H, s, S-CH2- Ph). Mass: m/z 462 (M+) and fragmented ion peak at 385, 336. Analysis calculated for C18H15IN4OS: C, 46.76; H, 3.271; N, 12.13. Found: C, 46.70; H, 3.270; N, 12.08%; 4b: IR (KBr, cm-1): 3414 (N-H), 1608 (C=N). 1H NMR (DMSO-d6): δ 8.42 (1H, s, Ar-H, pyrimidine), 8.28 (1H, s, NH), 7.72 (1H, s, N=CH), 7.69-6.83 (9H, m, Ar-H), 4.39 (2H, s, S-CH2-Ph), 3.7 (3H, s, OCH3). MS m/z: 476 (M+) and fragmented ion peak at 455, 349. Analysis calculated for C19H17IN4OS: C, 48.01; H, 3.57; N, 11.78. Found: C, 47.98; H, 3.5; N, 11.77%; 4c: IR (KBr, cm-1): 3380 (N-H), 1616 (C=N). 1H NMR (DMSO-d6): δ 8.32 (1H, s, Ar-H, pyrimidine), 8.25 (1H, s, N-H), 7.54 (1H, s, N=CH), 7.5-6.8 (9H, m, Ar-H, OH), 4.42 (2H, s, S-CH2-Ph), 3.59 (3H, s, OCH3). Analysis calculated for C19H17IN4O2S: C, 46.43; H, 3.462; N, 11.38. Found: C, 46.39; H, 3.460; N, 11.37%; 4d: IR (KBr, cm-1): 3453 (N-H), 1604 (C=N). 1H NMR (DMSO-d6): δ 8.49 (1H, s, Ar-H, pyrimidine), 8.3 (1H, s, N-H), 7.68-7.14 (10H, m, Ar-H, N=CH), 4.41 (2H, s, S-CH2-Ph). MS: m/z 480 (M+) and fragmented ion peak at 369, 353; 4e: IR (KBr, cm-1): 3400 (N-H), 1618 (C=N); 1H NMR (DMSO-d6): δ 8.73 (1H, s, Ar-H, pyrimidine), 8.04 (1H, s, N-H), 7.26 (1H, s, N=CH), 7.19-6.87 (9H, m, Ar-H), 4.16 (2H, s, S-CH2-Ph). Analysis calculated for C18H14ClIN4S: C, 45.04; H, 2.919; N, 11.67. Found: C, 45.01; H, 2.915; N, 11.65%; 4f: IR (KBr, cm-1): 3440 (N-H), 1616 (C=N). 1H NMR (DMSO-d6): δ 8.76 (1H, s, Ar-H, pyrimidine), 8.66 (1H, s, N-H), 8.36 (1H, s, N=CH), 8.05-7.04 (8H, m, Ar-H), 4.41 (2H, s, S-CH2-Ph). Analysis calculated for C16H13IN4S2: C, 42.57; H, 2.882; N, 12.41. Found: C, 42.54; H, 2.886; N, 12.42%; 4g: IR (KBr, cm-1): 3356 NH2, 1605 C=N. 1H NMR (DMSO-d6): δ 9.2 (1H, s, Ar-H, pyrimidine), 8.66 (1H, s, N-H), 8.39 (1H, s, N=CH), 8.39-6.82 (10H, m, Ar-H, NH2), 4.43 (2H, s, S-CH2- Ph). MS: m/z 462 (M+) and fragmented ion peak at 325, 275. Analysis calculated for C17H15IN6S: C, 44.27; H, 3.253; N, 18.22. Found: C, 44.24; H, 3.246; N, 18.20%; 4h: IR (KBr, cm-1): 3380 (N-H), 1616 (C=N); 1H NMR (DMSO-d6): δ 9.1 (s, 1H, Ar-H, pyrimidine), 9.01 (1H, s, N-H), 7.85-7.42 (17H, m, Ar-H, N=CH), 4.43 (2H, s, S-CH2-Ph). MS m/z: 588 (M+) and fragmented ion peak at 493, 295. Analysis calculated for C27H21IN6S2: C, 55.19; H, 3.577; N, 14.31. Found: C, 55.12; H, 3.569; N, 14.25%.
The antimicrobial activities were performed by cup plate method [14]. The sample was dissolved in DMF at the concentration of 1000 μg/ml. Compounds were screened for antibacterial activity against P. aeruginosa, E. coli, B. subtilis and S. aureus. Antifungal activity was carried out against A. terrus and A. niger under aseptic conditions. Gentamycine and fluconazole were used as standard drug for antibacterial and antifungal activities, respectively. The zone of inhibition was compared with standard drug after 24 h of incubation at 250 for antibacterial activity and 48 h at 300 for antifungal activity.
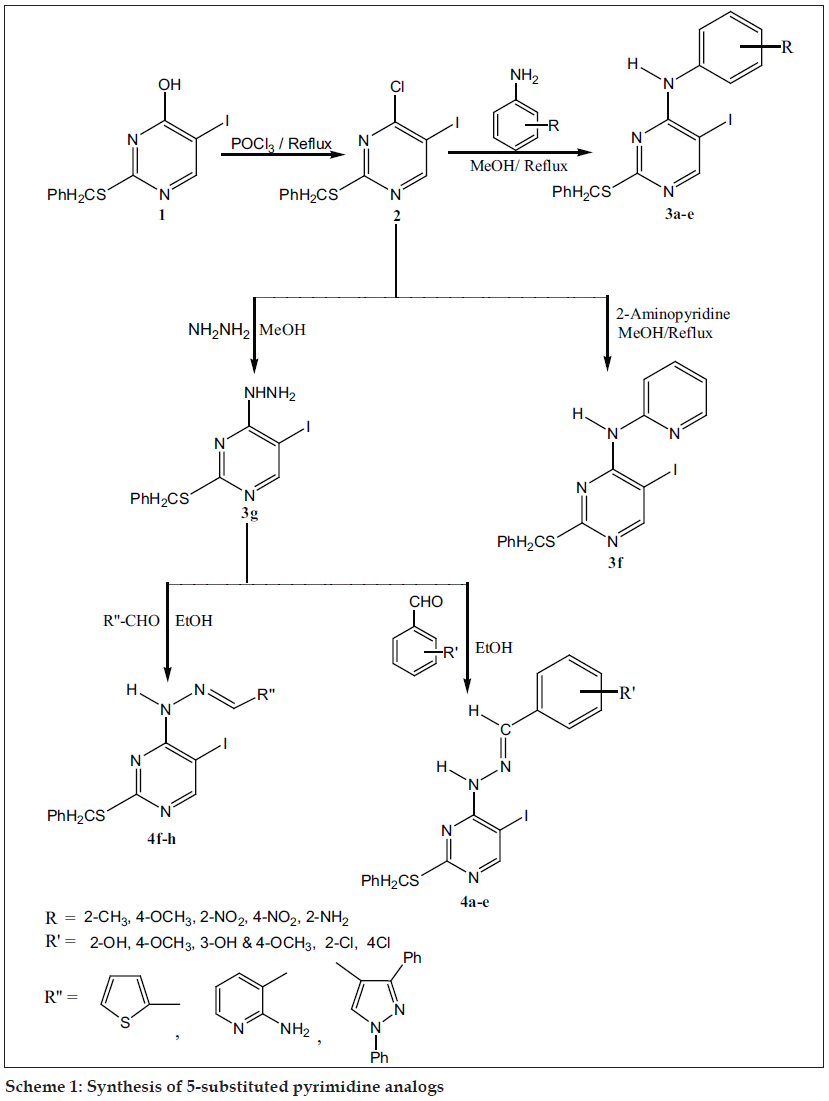
The starting material 4-chloro-5-iodo2- benzylthiopyrimidine (2) was prepared by chlorination of 5-iodo-2-benzylthiouracil with excess POCl3 (Scheme 1). Compound 2 obtained as yellow crystals having the melting point 90-910 in 80% yield. IR spectrum of compound 2 exhibited absorption at 3026 and 1599 cm-1 due to (aromatic C-H str), (C=N), respectively. 1H NMR signals are at δ 8.69 (1H, s, Ar- H, pyrimidine), 7.44-7.26 (5H, m, Ar-H), 4.36 (2H, s, S-CH2-Ph). Reaction of 2 with various substituted aromatic amines, 2-aminopyridine and hydrazine hydrate gave desired compounds 3a-g in 40-75% yield. Compound 3a obtained as yellow colored solid having melting point 80-820 in 55% yield. IR spectrum of compound 3a shows characteristic absorption of (N-H) at 3379, (C=N) at 1598 cm-1. 1H NMR signals at δ 8.27 (1H, s, Ar-H, pyrimidine), 7.68 (1H, s, N-H), 7.33-7.07 (9H, m, Ar-H), 4.17 (2H, s, S-CH2-Ph), 2.27 (3H, s, CH3). Further compound 3a was confi rmed by mass spectra analysis, the molecular ion peak at 433 (M+) and fragmented ion peaks at 342, 214. Compound 3g obtained as colorless crystals having the melting point 120-1210 in 65% yield. IR spectrum of compound 3g shows absorption of (NH2) at 3303, (N-H) at 3239 and (C=N) at 1624 cm-1. The 1H NMR of 3g shows signal at δ 7.82 (1H, s, Ar-H, pyrimidine), 7.29 (1H, s, NH), 7.26-6.9 (5H, m, Ar-H), 4.04 (2H, s, S-CH2-Ph), 3.83 (2H, s, NH2). Mass spectra of compound 3g shows molecular ion peak at 358 (M+) and fragmented ion peak at 342, 231. Reaction of compound 3g with substituted aromatic and heterocyclic aldehydes furnished desired compounds 4-(aryl/heteroarylidinehydrazino)-5-iodo- 2-benzylthiopyrimidines (4a-h) in 60-75% yield. The compound 4a was obtained as yellow colored solid in 75% yield, having m.p. 210-2120. The IR spectrum of compound 4a shows characteristic absorption of (O-H) at 3419, (N-H) at 3166 and (C=N) at 1621 cm-1. The 1H-NMR signals are at δ 8.6 (1H, s, Ar-H, pyrimidine), 8.2 (1H, s, NH), 7.6 (1H, s, N=CH), 7.3-6.8 (10H, m, Ar-H, OH),4.4 (2H, s, S-CH2-Ph). Further compound 4a was confi rmed by mass spectral analysis, the molecular ion peak at 462 (M+) and fragmented ion peak at 385, 336. Compound 4h was obtained as green colored solid in 52% yield, having m.p.210-2110. IR spectrum of compound 4h shows characteristic absorption of (N-H) at 3380, (C=N) at 1616 cm-1. 1H NMR signals are at δ 9.1 (1H, s, Ar-H, pyrimidine), 9.01 (1H, s, NH), 7.85-7.42 (17H, m, Ar-H, N = CH), 4.43 (2H, s, S-CH2-Ph). Further compound 4h was confi rmed by mass spectra analysis, the molecular ion peak at 588 (MM+), fragmented ion peak at 495, 293. The physical constant of all the compounds are given in Table 1.
| Compd | R | R' | R'' | Yield (%) | M.P(°) |
|---|---|---|---|---|---|
| 3a | 4-CH3 | - | - | 55 | 80-82 |
| 3b | 4-OCH3 | - | - | 50 | 152-154 |
| 3c | 2-NO2 | - | - | 55 | 60-62 |
| 3d | 4-NO2 | - | - | 40 | 120-121 |
| 3e | 2-NH2 | - | - | 50 | 160-161 |
| 3f | - | - | - | 45 | Semi-solid |
| 3g | - | - | - | 65 | 120-121 |
| 4a | - | 2-OH | - | 75 | 170-172 |
| 4b | - | 4-OCH3 | - | 75 | 202-203 |
| 4c | - | 3-OH, 4-OCH3 | - | 70 | 190-191 |
| 4d | - | 4-Cl | - | 75 | 210-213 |
| 4e | - | 2-Cl | - | 70 | 182-183 |
| 4f | - | - | Thiophene-2-yl | 65 | 206-208 |
| 4g | - | - | 2-Aminopyridin-3-yl | 60 | 202-203 |
| 4h | - | - | 1,3-Diphenyl- | 52 | 210-211 |
| pyrazol-4-yl | |||||
Table 1: Physical constants of the synthesized compounds
All the compounds showed poor activity against gram (-)ve bacteria E. coli (Table 2). However compound 2 exhibited good antibacterial activity against gram (-) ve bacteria P. aeruginosa and good antifungal activity against both fungal strains A. niger and A. terrus. Compound 3d exhibited good antibacterial activity against gram (+)ve bacteria S. aureus. Compounds 3f exhibited good activity against A. niger. Compounds 4a, 4c and 4d showed good activity against P. aeruginosa. Compounds 4g and 4h exhibited good antibacterial activity against P. aeruginosa, S. aureus and B. subtilis and antifungal activity against both fungal strains. Other compounds have shown poor to moderate antibacterial and antifungal activity as compared to standard gentamycine and fl uconazole.
| Compd No | Dose | Antibacterial Activity | Antifungal Activity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (µg/ml) | Zone of Inhibition* (mm) | Zone of Inhibition* (mm) | |||||||
| P. aeruginosa | E. coli | B. subtilis | S. aureus | A. niger | A. terrus | ||||
| 2 | 1000 | 19 | 05 | 15 | 10 | 12 | 11 | ||
| 3a | 1000 | 07 | 09 | 10 | 11 | 17 | 17 | ||
| 3b | 1000 | 06 | 08 | 12 | 13 | 10 | 10 | ||
| 3c | 1000 | 07 | 10 | 10 | 11 | 12 | 11 | ||
| 3d | 1000 | 10 | 05 | 14 | 16 | 15 | 13 | ||
| 3e | 1000 | 08 | 15 | 11 | 14 | 13 | 12 | ||
| 3f | 1000 | 05 | 10 | 09 | 15 | 19 | 14 | ||
| 3g | 1000 | 19 | 04 | 10 | 11 | 10 | 10 | ||
| 4a | 1000 | 18 | 07 | 13 | 15 | 13 | 12 | ||
| 4b | 1000 | 15 | 05 | 12 | 12 | 11 | 15 | ||
| 4c | 1000 | 19 | 04 | 13 | 12 | 12 | 11 | ||
| 4d | 1000 | 18 | 08 | 10 | 10 | 10 | 12 | ||
| 4e | 1000 | 19 | 06 | 08 | 09 | 14 | 11 | ||
| 4f | 1000 | 10 | 05 | 15 | 12 | 15 | 10 | ||
| 4g | 1000 | 18 | 07 | 17 | 18 | 18 | 15 | ||
| 4h | 1000 | 16 | 10 | 20 | 18 | 16 | 15 | ||
| Control (DMF) | - | Nil | Nil | Nil | Nil | Nil | Nil | ||
| Gentamycine | 1000 | 22 | 19 | 24 | 22 | - | - | ||
| Flucanazole | 1000 | - | - | - | - | 22 | 20 | ||
*Zone of inhibition excluding well size 6 mm
Table 2: Antimicrobial activities of synthesized compounds
From the above results, it can be concluded that introduction of NO2, OH, Cl and heterocyclic moieties like pyridine and pyrazole to the pyrimidine analogs enhanced the antibacterial and antifungal activities.
References
- Jain KS, Chitre TS, Maniyar PB, Kathiravan MK, Bindre VS, Veer VS, et al. Biological and medicinal significance of pyrimidine. Current Sci 2006;90:793-803.
- Mitsuya H, Weinhold KJ, Furman PA, St.Clair MH, Nusinoff-Lehman S, Gallo RC, et al. 3'-Azido-3'-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. ProcNatlAcadSci USA 1985;82:7096-100.
- Lin TS, Guo JY, Schinazi RF, Chu CK, Xiang JN, Prusoff WH. Synthesis and antiviral activity of various 3-azido analogues of pyrimidine deoxyribonucleosides against human immunodeficiency virus (HIV-1, HTLV-III/LAV). J Med Chem 1988;31:336-40.
- Choo H, Chong Y, Choi Y, Mathew J, Schinazi RF, Chu, CK. Synthesis, anti-HIV activity and molecular mechanism of drug resistance of L-2'3'-didehydro-2',3'-dideoxy-2'-fluoro-4'-thionucleosides. J Med Chem 2003;46:389-98.
- Balzarini J, Pannecouque C, De Clercq E, Aquaro S, Perno CF, Egberink H, et al. Antiretrovirus activity of a novel class of acyclic pyrimidine nucleoside phosphonates. Antimicrob Agents Chemother 2002;46:2185-93.
- De Clerecq E, Descamps J, De Somer P, Barr PJ, Jones AS, Walker RT. (E)-5-(2-bromovinyl)-2-deoxyuridine: a potent and selective anti-herpes agents. ProtNatlAcadSci USA 1979;76:2947-51.
- Barrett HW, Goodman I, Dittmer K. The synthesis of 5-halogeno-2-thiouracil and 6-methyl-5-halogeno-2-thiouracil derivatives. J Am ChemSoc 1948;70:1753-6.
- Schinazi RF, Arbiser J, Lee J, Kalman, T, Prusoff W. Synthesis and biological activity of 5-phenylselenenyl-substituted pyrimidine. J Med Chem 1986;29:1293-5.
- Puleston HS, Charles FP, Norman FW. Inhibition studies with pyrimidines on Streptococcus faecalis R. J BiolChem 1955;212:319-23.
- Goudgaon NM, Naguib FNM, El-Kouni, MH, Schinazi, RF. Phenylselenenyl and phenylthio-substituted pyrimidines as inhibitors of dihydrouracil dehydrogenase and uridinephosphorylase. J Med Chem 1993;36:4250-4.
- Melvin DT, Anthony BN, Kyoichi AW, Jack JF. Evaluation of the antiherpetic activity of 2-fluoro-5-iodo-Ara-c in rabbit eyes and cell cultures. Invest Opthlmol Vis Sci 1981;21:826-32.
- Kaufman HE, Martola EL, Dolman H. Use of 5-iodo-2'-deoxyuridine in the treatment of herpes simplex keratitis. Arch Opthalmol 1962;68:235-8.
- Kuafman HE, Nesburn AB, Maloney ED. IDU therapy of experimental herpes simplex keratitis. Arch Opthalmol 1962;67:583-8.
- Goudgaon NM, Macmillan A, Schinazi RF. 1-(Ethoxymethyl)-6-(phenylselenenyl)pyrimidines with activity against human immunodeficiency viruses types 1 and 2. Antiviral ChemChemother 1992;3:263-6.
- Goudgaon NM, Vijayalaxmi A. Antimicrobial activity and structure-activity relationship of acyclic nucleosides. Indian J Pharm Sci 2003;65:545-9.
Keywords
Antibacterial, Antifungal, sSchiff bases, 5-Substituted pyrimidine
Pyrimidine derivatives have proved to be of great importance in exhibiting therapeutic applications [1]. A large number of pyrimidine nucleosides are clinically useful for the control of retroviral infections [2-5]. One of the important class of anti-herpetic nucleosides is series of 5-substituted uracil nucleosides such as (E)-5-(2-bromovinyl)-2'-deoxyuridine (BVdu) [6] showed specific antivaricella zoster virus (VZV) activity. In view of effect of 5-substitution on the activity of thiouracil, synthesis and antithyroid activity of several 5-substituted pyrimidine derivatives have been reported [7]. In addition, 5-alkyl or 5-aryl-substituted pyrimidine derivatives are useful intermediates in the synthesis of nucleosides [8]. Also, many 5-substituted pyrimidines have shown inhibitory activity against Streptococcus faecalis R growth [9] and some are evaluated as inhibitors of enzymes involved in the pyrimidine catabolism like dihydrouracil dehydrogenase and uridine phosphorylase [10]. Further, 5-iodo substituted pyrimidine analogs are known for their antimicrobial [11] and antiviral activity [12,13]. Synthesis and biological activities of several compounds derived from pyrimidine analogs were reported from our laboratory [10,14,15]. We report herein the synthesis and antimicrobial activities of 4-amino-5-iodo-2-benzylthiopyrimidines (3a-g) and 4-aryl/heteroarylidenehydrazino-5-iodo-2- benzylthiopyrimidines (4a-h).
Melting points were recorded by using Thomas- Hoover melting point apparatus and were uncorrected. IR spectra in KBr disc were recorded on Perkin- Elmer-Spectrum-one FT IR spectrophotometer (νmax in cm-1) and 1H NMR in DMSO-d6 and/or CDCl3 on amx 400, 400 MHz spectrophotometer using TMS as internal standard (chemical shift in δ ppm). Mass spectra were recorded on a Jeol SX 102 Mass spectrometer using argon/xenon (6kv, 10 mA) as the FAB gas. Purity of the compounds was checked by TLC using silica gel ‘G’ plates obtained from Whatman Inc, and a fl uorescent indicator. 5-Iodo-2- benzylthiouracil (1) was prepared by following the known literature method [7].
General procedure for the synthesis of 4-chloro-5- iodo-2-benzylthiopyrimidine (2) is as follows, to a mixture of 5-iodo-2-benzylthiouracil (1) (0.01 mol) and POCl3 (0.06 mol) was refluxed for 1 h. Excess POCl3 was distilled under reduced pressure and the reaction mixture was poured into 200 ml of ice cold water. The solid separated was extracted with ether (3 x 100 ml) and the ether extracts were washed with 5% aq. NaHCO3 solution (3 x 100 ml), followed by water (3 x 100 ml). Ether layer was dried over anhydrous MgSO4. Solvent was evaporated to produce pale yellow syrup. The syrup slowly solidified and recrystallized from ethanol; 2: IR (KBr, cm-1): 3026 (aromatic C-H str), 1599 (C=N). 1H NMR (CDCl3): δ 8.69 (1H, s, Ar-H, Pyrimidine), 7.44-7.26 (5H, m, Ar-H), 4.36 (2H, s, S-CH2-Ph).
General procedure for the synthesis of 4-amino-5- iodo-2-benzylthiopyrimidines (3a-g) is as follows, to a solution of 4-chloro-5-iodo-2-benzylthiopyrimidine (0.001 mol) in methanol (20 ml) and pyridine (0.5 ml), appropriate primary amine (0.001 mol) was added. The reaction mixture was refl uxed for 4 h on a steam-bath. Excess of methanol was removed under reduced pressure and the residue triturated with a little crushed ice and aqueous layer was neutralized with 0.1N HCl. Solid separated was fi ltered and washed with cold water. Recrystallized the crude product from ethanol furnished the desired compounds (3a-g); 3a: IR (KBr, cm-1): 3379 (N-H), 1598 (C=N). 1H NMR (DMSO-d6): δ 8.27 (1H, s, Ar-H, pyrimidine), 7.68 (1H, s, N-H), 7.33-7.07 (9H, m, Ar-H), 4.17 (2H, s, S-CH2-Ph), 2.27 (3H, s, CH3). MS m/z: 433 (M+) and fragmented peaks at 341, 308, 214. Analysis calculated for C18H16IN3S: C, 50.0; H, 3.73; N, 9.72. Found: C, 49.98; H, 3.70; N, 9.65%; 3b: IR (KBr, cm-1): 3300 (N-H), 1580 (C=N). 1H NMR (CDCl3): δ 8.22 (1H, s, Ar-H, pyrimidine), 8.03 (1H, s, N-H), 7.7-6.78 (9H, m, Ar-H), 4.31 (2H, s, S-CH2-Ph), 3.7 (3H, s, OCH3). Analysis calculated for C18H16IN3OS: C, 48.21; H, 3.571; N, 9.372. Found: C, 48.18; H, 3.565; N, 9.365%; 3c: IR (KBr, cm-1): 3447 (N-H), 1603 (C=N), 1525 (NO2). 1H NMR (CDCl3): δ 8.5 (1H, s, Ar-H, pyrimidine), 7.4-7.26 (10H, m, Ar-H, N-H), 4.38 (2H, s, S-CH2-Ph). Analysis calculated for C17H13IN4O2S: C, 43.83; H, 2.281; N, 12.09. Found: C, 43.78; H, 2.275; N, 12.05%; 3d: IR (KBr, cm-1): 3356 (N-H), 1602 (C=N), 1539 (NO2). 1H NMR (DMSO-d6): δ 8.69 (1H, s, Ar-H, pyrimidine), 8.4 (1H, s, N-H) 7.98-7.22 (9H, m, Ar-H), 4.39 (2H, s, S-CH2-Ph); 3e: IR (KBr, cm-1): 3402 (NH2), 3303 (NH), 1616 (C=N). 1H NMR (CDCl3): δ 8.34 (1H, s, Ar-H, pyrimidine), 7.23-6.8 (12H, m, Ar-H, N-H), 4.12 (2H, s, S-CH2-Ph). MS m/z: 434 (M+) and fragmented ion peaks at 342, 214. Analysis calculated for C17H15IN4S: C, 47.11; H, 3.464; N, 12.93. Found: C, 47.08; H, 3.460; N, 12.88%; 3f: IR (KBr, cm-1): 3427 (N-H), 1602 (C=N). 1H NMR (CDCl3): δ 8.5 (1H, s, Ar-H, pyrimidine), 7.40-7.26 (10H, m, Ar-H, N-H), 4.38 (2H, s, S-CH2-Ph). Analysis calculated for C16H13IN4S: C, 45.73; H, 3.126; N, 13.33. Found: C, 45.71; H, 3.120; N, 13.28%; 3g: IR (KBr, cm-1): 3303 (NH2), 3239 (N-H), 1624 (C=N). 1H NMR (DMSO-d6): δ 7.82 (1H, s, Ar-H, pyrimidine), 7.29 (1H, s, N-H), 7.26-6.9 (5H, m, Ar-H), 4.04 (2H, s, S-CH2-Ph), 3.83 (2H, s, NH2). MS m/z: 358 (M+) and fragmented ion peak at 342, 231. Analysis calculated for C11H11IN4S: C, 36.97; H, 3.083; N, 15.68. Found: C, 36.90; H, 3.080; N, 15.64%.
General procedure for the synthesis of 4-aryl/heteroarylidinehydrazino-5-iodo-2- benzylthiopyrimidines (4a-h) as follows, to a solution of 4-hydrazino-5-iodo-2-benzylthiopyrimidine (3 g, 0.001 mol) in ethanol (20 ml) and catalytic amount of concentrated HCl, appropriate aromatic aldehyde (0.001 mol) was added. The reaction mixture was refluxed for 4 h. Excess ethanol was removed under reduced pressure, solid separated was fi ltrated and recrystalised from ethanol to get the desired compounds; 4a: IR (KBr, cm-1): 3419 (N-H), 1628 (C=N). 1H NMR (DMSO-d6): δ 8.69 (1H, s, Ar-H, pyrimidine), 8.27 (1H, s, N-H), 7.62 (1H, s, N=CH), 7.37-6.84 (10H, m, Ar-H, O-H), 4.41 (2H, s, S-CH2- Ph). Mass: m/z 462 (M+) and fragmented ion peak at 385, 336. Analysis calculated for C18H15IN4OS: C, 46.76; H, 3.271; N, 12.13. Found: C, 46.70; H, 3.270; N, 12.08%; 4b: IR (KBr, cm-1): 3414 (N-H), 1608 (C=N). 1H NMR (DMSO-d6): δ 8.42 (1H, s, Ar-H, pyrimidine), 8.28 (1H, s, NH), 7.72 (1H, s, N=CH), 7.69-6.83 (9H, m, Ar-H), 4.39 (2H, s, S-CH2-Ph), 3.7 (3H, s, OCH3). MS m/z: 476 (M+) and fragmented ion peak at 455, 349. Analysis calculated for C19H17IN4OS: C, 48.01; H, 3.57; N, 11.78. Found: C, 47.98; H, 3.5; N, 11.77%; 4c: IR (KBr, cm-1): 3380 (N-H), 1616 (C=N). 1H NMR (DMSO-d6): δ 8.32 (1H, s, Ar-H, pyrimidine), 8.25 (1H, s, N-H), 7.54 (1H, s, N=CH), 7.5-6.8 (9H, m, Ar-H, OH), 4.42 (2H, s, S-CH2-Ph), 3.59 (3H, s, OCH3). Analysis calculated for C19H17IN4O2S: C, 46.43; H, 3.462; N, 11.38. Found: C, 46.39; H, 3.460; N, 11.37%; 4d: IR (KBr, cm-1): 3453 (N-H), 1604 (C=N). 1H NMR (DMSO-d6): δ 8.49 (1H, s, Ar-H, pyrimidine), 8.3 (1H, s, N-H), 7.68-7.14 (10H, m, Ar-H, N=CH), 4.41 (2H, s, S-CH2-Ph). MS: m/z 480 (M+) and fragmented ion peak at 369, 353; 4e: IR (KBr, cm-1): 3400 (N-H), 1618 (C=N); 1H NMR (DMSO-d6): δ 8.73 (1H, s, Ar-H, pyrimidine), 8.04 (1H, s, N-H), 7.26 (1H, s, N=CH), 7.19-6.87 (9H, m, Ar-H), 4.16 (2H, s, S-CH2-Ph). Analysis calculated for C18H14ClIN4S: C, 45.04; H, 2.919; N, 11.67. Found: C, 45.01; H, 2.915; N, 11.65%; 4f: IR (KBr, cm-1): 3440 (N-H), 1616 (C=N). 1H NMR (DMSO-d6): δ 8.76 (1H, s, Ar-H, pyrimidine), 8.66 (1H, s, N-H), 8.36 (1H, s, N=CH), 8.05-7.04 (8H, m, Ar-H), 4.41 (2H, s, S-CH2-Ph). Analysis calculated for C16H13IN4S2: C, 42.57; H, 2.882; N, 12.41. Found: C, 42.54; H, 2.886; N, 12.42%; 4g: IR (KBr, cm-1): 3356 NH2, 1605 C=N. 1H NMR (DMSO-d6): δ 9.2 (1H, s, Ar-H, pyrimidine), 8.66 (1H, s, N-H), 8.39 (1H, s, N=CH), 8.39-6.82 (10H, m, Ar-H, NH2), 4.43 (2H, s, S-CH2- Ph). MS: m/z 462 (M+) and fragmented ion peak at 325, 275. Analysis calculated for C17H15IN6S: C, 44.27; H, 3.253; N, 18.22. Found: C, 44.24; H, 3.246; N, 18.20%; 4h: IR (KBr, cm-1): 3380 (N-H), 1616 (C=N); 1H NMR (DMSO-d6): δ 9.1 (s, 1H, Ar-H, pyrimidine), 9.01 (1H, s, N-H), 7.85-7.42 (17H, m, Ar-H, N=CH), 4.43 (2H, s, S-CH2-Ph). MS m/z: 588 (M+) and fragmented ion peak at 493, 295. Analysis calculated for C27H21IN6S2: C, 55.19; H, 3.577; N, 14.31. Found: C, 55.12; H, 3.569; N, 14.25%.
The antimicrobial activities were performed by cup plate method [14]. The sample was dissolved in DMF at the concentration of 1000 μg/ml. Compounds were screened for antibacterial activity against P. aeruginosa, E. coli, B. subtilis and S. aureus. Antifungal activity was carried out against A. terrus and A. niger under aseptic conditions. Gentamycine and fluconazole were used as standard drug for antibacterial and antifungal activities, respectively. The zone of inhibition was compared with standard drug after 24 h of incubation at 250 for antibacterial activity and 48 h at 300 for antifungal activity.
The starting material 4-chloro-5-iodo2- benzylthiopyrimidine (2) was prepared by chlorination of 5-iodo-2-benzylthiouracil with excess POCl3 (Scheme 1). Compound 2 obtained as yellow crystals having the melting point 90-910 in 80% yield. IR spectrum of compound 2 exhibited absorption at 3026 and 1599 cm-1 due to (aromatic C-H str), (C=N), respectively. 1H NMR signals are at δ 8.69 (1H, s, Ar- H, pyrimidine), 7.44-7.26 (5H, m, Ar-H), 4.36 (2H, s, S-CH2-Ph). Reaction of 2 with various substituted aromatic amines, 2-aminopyridine and hydrazine hydrate gave desired compounds 3a-g in 40-75% yield. Compound 3a obtained as yellow colored solid having melting point 80-820 in 55% yield. IR spectrum of compound 3a shows characteristic absorption of (N-H) at 3379, (C=N) at 1598 cm-1. 1H NMR signals at δ 8.27 (1H, s, Ar-H, pyrimidine), 7.68 (1H, s, N-H), 7.33-7.07 (9H, m, Ar-H), 4.17 (2H, s, S-CH2-Ph), 2.27 (3H, s, CH3). Further compound 3a was confi rmed by mass spectra analysis, the molecular ion peak at 433 (M+) and fragmented ion peaks at 342, 214. Compound 3g obtained as colorless crystals having the melting point 120-1210 in 65% yield. IR spectrum of compound 3g shows absorption of (NH2) at 3303, (N-H) at 3239 and (C=N) at 1624 cm-1. The 1H NMR of 3g shows signal at δ 7.82 (1H, s, Ar-H, pyrimidine), 7.29 (1H, s, NH), 7.26-6.9 (5H, m, Ar-H), 4.04 (2H, s, S-CH2-Ph), 3.83 (2H, s, NH2). Mass spectra of compound 3g shows molecular ion peak at 358 (M+) and fragmented ion peak at 342, 231. Reaction of compound 3g with substituted aromatic and heterocyclic aldehydes furnished desired compounds 4-(aryl/heteroarylidinehydrazino)-5-iodo- 2-benzylthiopyrimidines (4a-h) in 60-75% yield. The compound 4a was obtained as yellow colored solid in 75% yield, having m.p. 210-2120. The IR spectrum of compound 4a shows characteristic absorption of (O-H) at 3419, (N-H) at 3166 and (C=N) at 1621 cm-1. The 1H-NMR signals are at δ 8.6 (1H, s, Ar-H, pyrimidine), 8.2 (1H, s, NH), 7.6 (1H, s, N=CH), 7.3-6.8 (10H, m, Ar-H, OH),4.4 (2H, s, S-CH2-Ph). Further compound 4a was confi rmed by mass spectral analysis, the molecular ion peak at 462 (M+) and fragmented ion peak at 385, 336. Compound 4h was obtained as green colored solid in 52% yield, having m.p.210-2110. IR spectrum of compound 4h shows characteristic absorption of (N-H) at 3380, (C=N) at 1616 cm-1. 1H NMR signals are at δ 9.1 (1H, s, Ar-H, pyrimidine), 9.01 (1H, s, NH), 7.85-7.42 (17H, m, Ar-H, N = CH), 4.43 (2H, s, S-CH2-Ph). Further compound 4h was confi rmed by mass spectra analysis, the molecular ion peak at 588 (MM+), fragmented ion peak at 495, 293. The physical constant of all the compounds are given in Table 1.
| Compd | R | R' | R'' | Yield (%) | M.P(°) |
|---|---|---|---|---|---|
| 3a | 4-CH3 | - | - | 55 | 80-82 |
| 3b | 4-OCH3 | - | - | 50 | 152-154 |
| 3c | 2-NO2 | - | - | 55 | 60-62 |
| 3d | 4-NO2 | - | - | 40 | 120-121 |
| 3e | 2-NH2 | - | - | 50 | 160-161 |
| 3f | - | - | - | 45 | Semi-solid |
| 3g | - | - | - | 65 | 120-121 |
| 4a | - | 2-OH | - | 75 | 170-172 |
| 4b | - | 4-OCH3 | - | 75 | 202-203 |
| 4c | - | 3-OH, 4-OCH3 | - | 70 | 190-191 |
| 4d | - | 4-Cl | - | 75 | 210-213 |
| 4e | - | 2-Cl | - | 70 | 182-183 |
| 4f | - | - | Thiophene-2-yl | 65 | 206-208 |
| 4g | - | - | 2-Aminopyridin-3-yl | 60 | 202-203 |
| 4h | - | - | 1,3-Diphenyl- | 52 | 210-211 |
| pyrazol-4-yl | |||||
Table 1: Physical constants of the synthesized compounds
All the compounds showed poor activity against gram (-)ve bacteria E. coli (Table 2). However compound 2 exhibited good antibacterial activity against gram (-) ve bacteria P. aeruginosa and good antifungal activity against both fungal strains A. niger and A. terrus. Compound 3d exhibited good antibacterial activity against gram (+)ve bacteria S. aureus. Compounds 3f exhibited good activity against A. niger. Compounds 4a, 4c and 4d showed good activity against P. aeruginosa. Compounds 4g and 4h exhibited good antibacterial activity against P. aeruginosa, S. aureus and B. subtilis and antifungal activity against both fungal strains. Other compounds have shown poor to moderate antibacterial and antifungal activity as compared to standard gentamycine and fl uconazole.
| Compd No | Dose | Antibacterial Activity | Antifungal Activity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (µg/ml) | Zone of Inhibition* (mm) | Zone of Inhibition* (mm) | |||||||
| P. aeruginosa | E. coli | B. subtilis | S. aureus | A. niger | A. terrus | ||||
| 2 | 1000 | 19 | 05 | 15 | 10 | 12 | 11 | ||
| 3a | 1000 | 07 | 09 | 10 | 11 | 17 | 17 | ||
| 3b | 1000 | 06 | 08 | 12 | 13 | 10 | 10 | ||
| 3c | 1000 | 07 | 10 | 10 | 11 | 12 | 11 | ||
| 3d | 1000 | 10 | 05 | 14 | 16 | 15 | 13 | ||
| 3e | 1000 | 08 | 15 | 11 | 14 | 13 | 12 | ||
| 3f | 1000 | 05 | 10 | 09 | 15 | 19 | 14 | ||
| 3g | 1000 | 19 | 04 | 10 | 11 | 10 | 10 | ||
| 4a | 1000 | 18 | 07 | 13 | 15 | 13 | 12 | ||
| 4b | 1000 | 15 | 05 | 12 | 12 | 11 | 15 | ||
| 4c | 1000 | 19 | 04 | 13 | 12 | 12 | 11 | ||
| 4d | 1000 | 18 | 08 | 10 | 10 | 10 | 12 | ||
| 4e | 1000 | 19 | 06 | 08 | 09 | 14 | 11 | ||
| 4f | 1000 | 10 | 05 | 15 | 12 | 15 | 10 | ||
| 4g | 1000 | 18 | 07 | 17 | 18 | 18 | 15 | ||
| 4h | 1000 | 16 | 10 | 20 | 18 | 16 | 15 | ||
| Control (DMF) | - | Nil | Nil | Nil | Nil | Nil | Nil | ||
| Gentamycine | 1000 | 22 | 19 | 24 | 22 | - | - | ||
| Flucanazole | 1000 | - | - | - | - | 22 | 20 | ||
*Zone of inhibition excluding well size 6 mm
Table 2: Antimicrobial activities of synthesized compounds
From the above results, it can be concluded that introduction of NO2, OH, Cl and heterocyclic moieties like pyridine and pyrazole to the pyrimidine analogs enhanced the antibacterial and antifungal activities.
References
- Jain KS, Chitre TS, Maniyar PB, Kathiravan MK, Bindre VS, Veer VS, et al. Biological and medicinal significance of pyrimidine. Current Sci 2006;90:793-803.
- Mitsuya H, Weinhold KJ, Furman PA, St.Clair MH, Nusinoff-Lehman S, Gallo RC, et al. 3'-Azido-3'-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. ProcNatlAcadSci USA 1985;82:7096-100.
- Lin TS, Guo JY, Schinazi RF, Chu CK, Xiang JN, Prusoff WH. Synthesis and antiviral activity of various 3-azido analogues of pyrimidine deoxyribonucleosides against human immunodeficiency virus (HIV-1, HTLV-III/LAV). J Med Chem 1988;31:336-40.
- Choo H, Chong Y, Choi Y, Mathew J, Schinazi RF, Chu, CK. Synthesis, anti-HIV activity and molecular mechanism of drug resistance of L-2'3'-didehydro-2',3'-dideoxy-2'-fluoro-4'-thionucleosides. J Med Chem 2003;46:389-98.
- Balzarini J, Pannecouque C, De Clercq E, Aquaro S, Perno CF, Egberink H, et al. Antiretrovirus activity of a novel class of acyclic pyrimidine nucleoside phosphonates. Antimicrob Agents Chemother 2002;46:2185-93.
- De Clerecq E, Descamps J, De Somer P, Barr PJ, Jones AS, Walker RT. (E)-5-(2-bromovinyl)-2-deoxyuridine: a potent and selective anti-herpes agents. ProtNatlAcadSci USA 1979;76:2947-51.
- Barrett HW, Goodman I, Dittmer K. The synthesis of 5-halogeno-2-thiouracil and 6-methyl-5-halogeno-2-thiouracil derivatives. J Am ChemSoc 1948;70:1753-6.
- Schinazi RF, Arbiser J, Lee J, Kalman, T, Prusoff W. Synthesis and biological activity of 5-phenylselenenyl-substituted pyrimidine. J Med Chem 1986;29:1293-5.
- Puleston HS, Charles FP, Norman FW. Inhibition studies with pyrimidines on Streptococcus faecalis R. J BiolChem 1955;212:319-23.
- Goudgaon NM, Naguib FNM, El-Kouni, MH, Schinazi, RF. Phenylselenenyl and phenylthio-substituted pyrimidines as inhibitors of dihydrouracil dehydrogenase and uridinephosphorylase. J Med Chem 1993;36:4250-4.
- Melvin DT, Anthony BN, Kyoichi AW, Jack JF. Evaluation of the antiherpetic activity of 2-fluoro-5-iodo-Ara-c in rabbit eyes and cell cultures. Invest Opthlmol Vis Sci 1981;21:826-32.
- Kaufman HE, Martola EL, Dolman H. Use of 5-iodo-2'-deoxyuridine in the treatment of herpes simplex keratitis. Arch Opthalmol 1962;68:235-8.
- Kuafman HE, Nesburn AB, Maloney ED. IDU therapy of experimental herpes simplex keratitis. Arch Opthalmol 1962;67:583-8.
- Goudgaon NM, Macmillan A, Schinazi RF. 1-(Ethoxymethyl)-6-(phenylselenenyl)pyrimidines with activity against human immunodeficiency viruses types 1 and 2. Antiviral ChemChemother 1992;3:263-6.
- Goudgaon NM, Vijayalaxmi A. Antimicrobial activity and structure-activity relationship of acyclic nucleosides. Indian J Pharm Sci 2003;65:545-9.